Tongue Abnormalities Are Associated to a Maternal Folic Acid Deficient Diet in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Diets
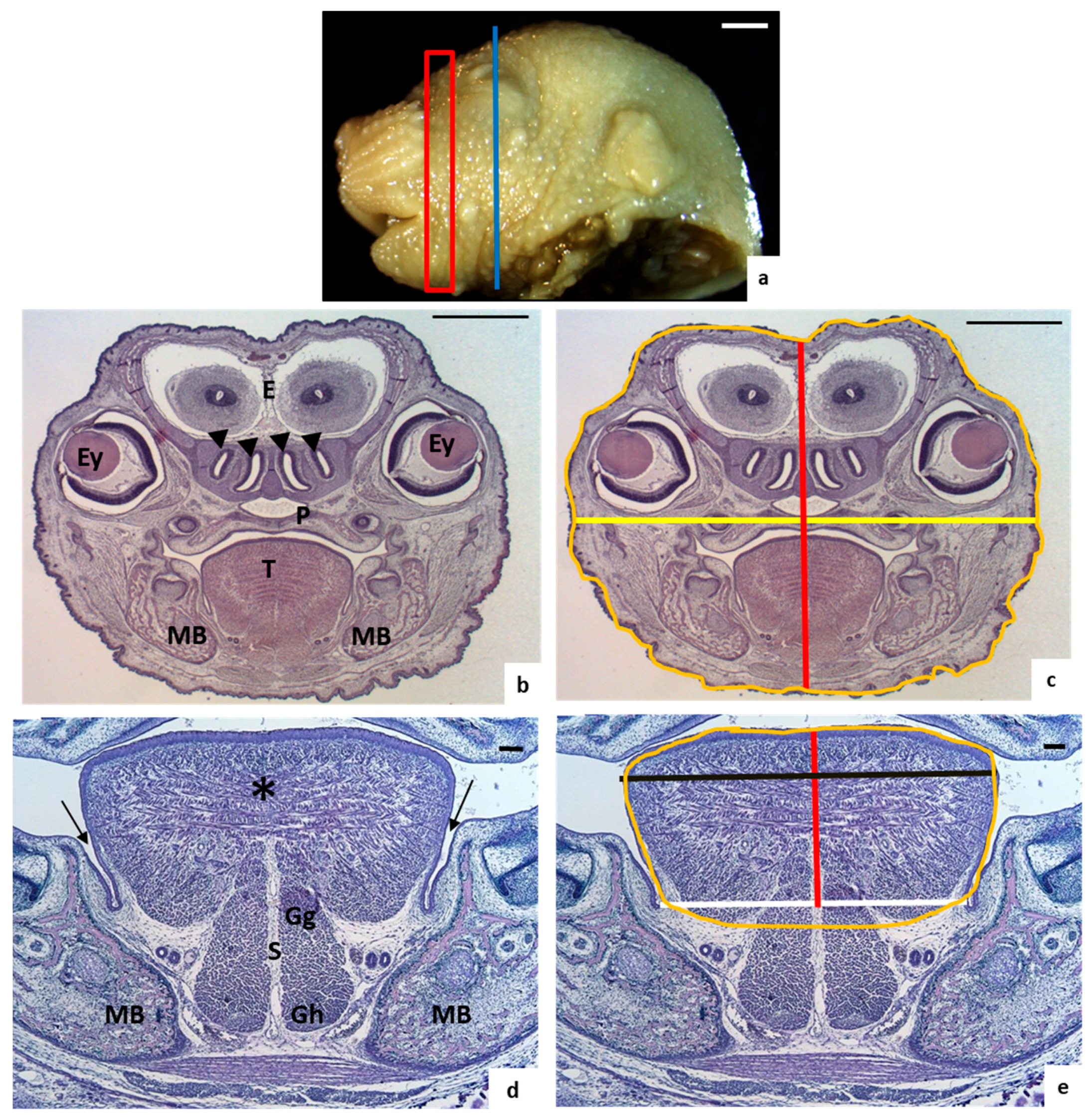
2.2. Morphological Study
2.3. Tongue and Head Measurements
2.4. Statistical Analysis
3. Results
3.1. Morphological Analysis
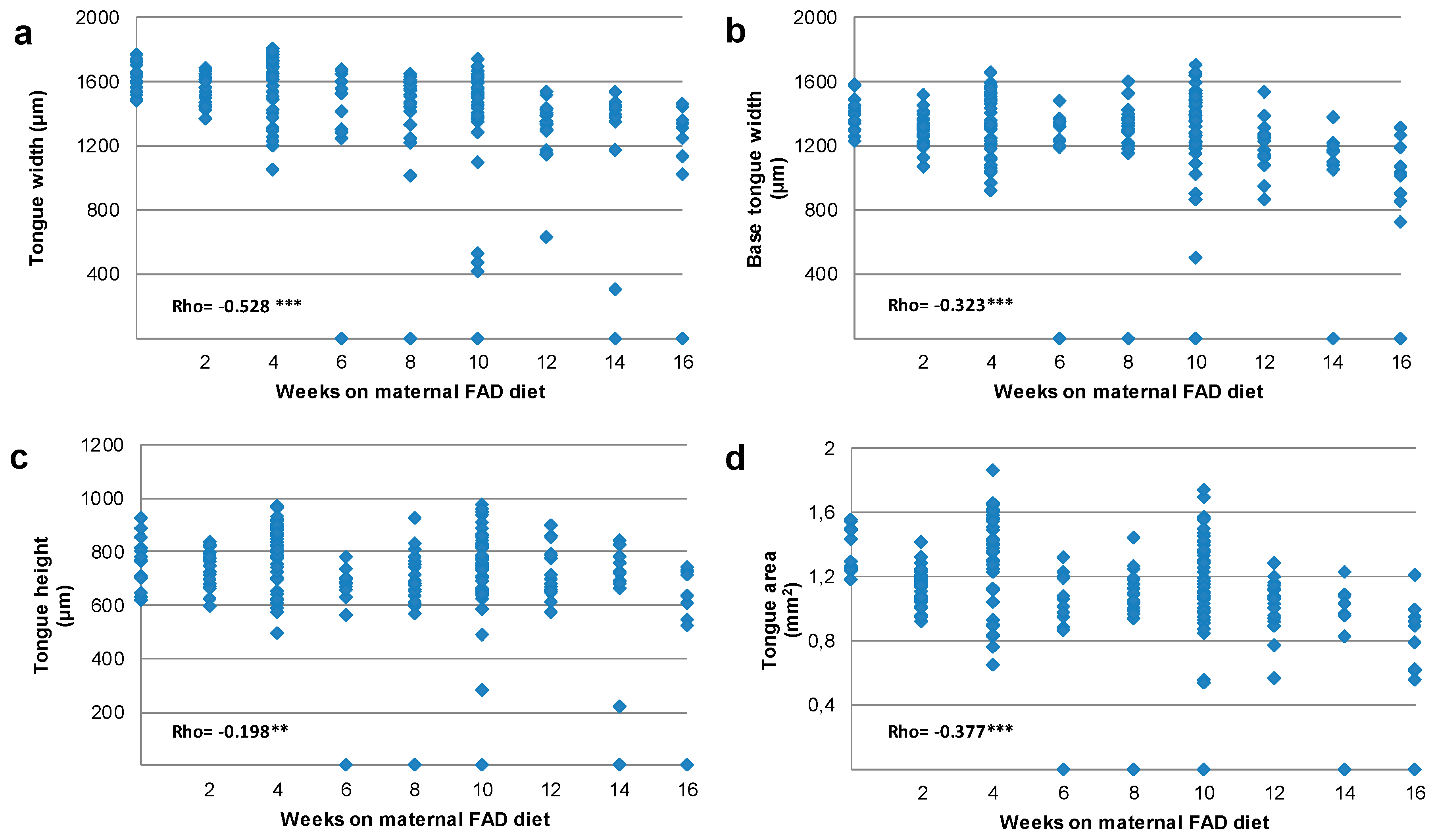
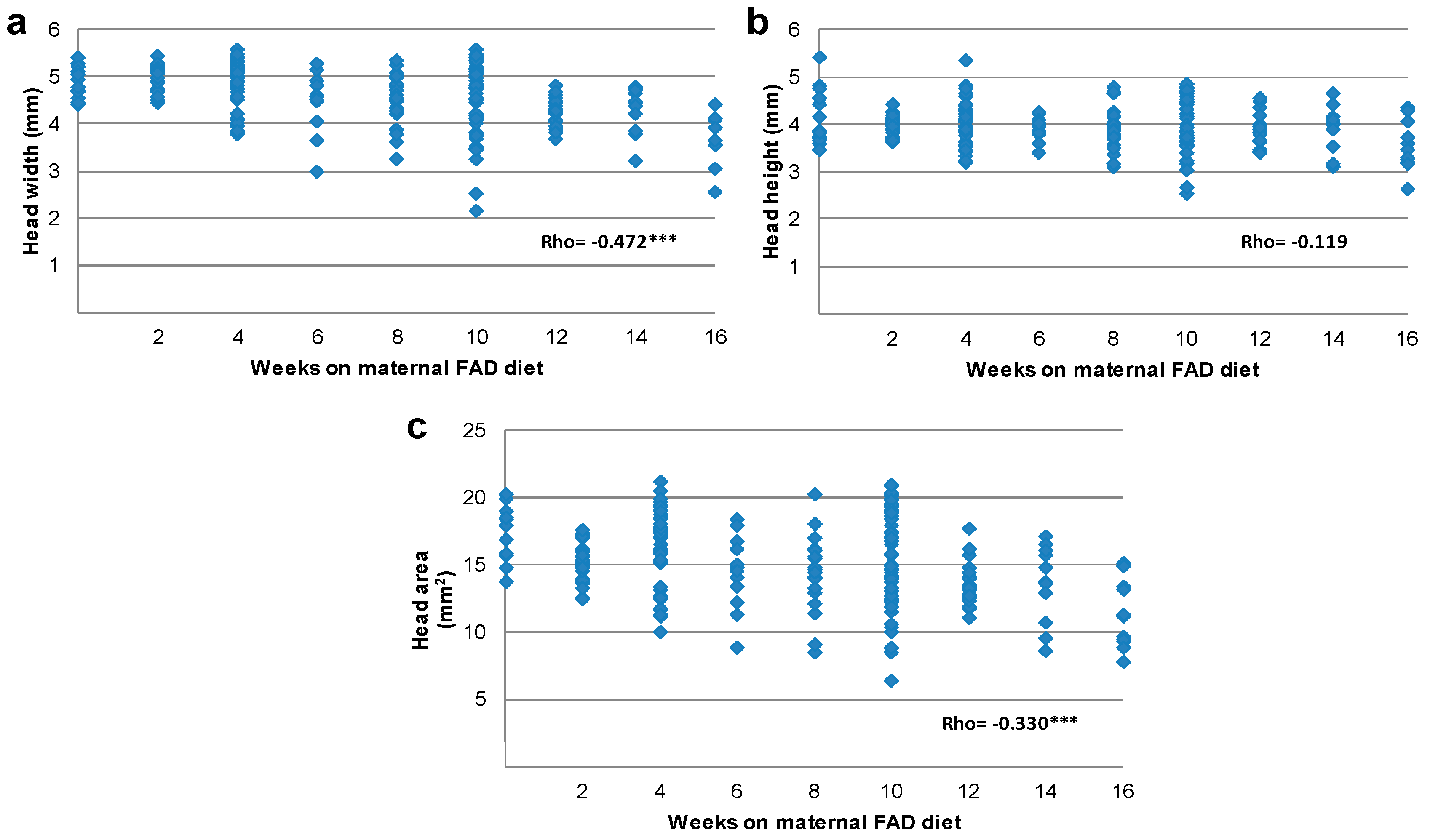
3.2. Tongue and Head Measurements
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kaufman, M.H.; Kaufman, M. The Atlas of Mouse Development, 1st ed.; Academic Press: Edinburgh, UK, 1992; pp. 291–337, 421–425. ISBN 9780124020351. [Google Scholar]
- Parada, C.; Chai, Y. Mandible and Tongue Development. Curr. Top. Dev. Biol. 2015, 115, 31–58. [Google Scholar] [CrossRef] [PubMed]
- Czajkowski, M.T.; Rassek, C.; Lenhard, D.C.; Bröhl, D.; Birchmeier, C. Divergent and conserved roles of Dll1 signaling in development of craniofacial and trunk muscle. Dev. Biol. 2014, 395, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Zhi, Q.; Izpisua-Belmonte, J.C.; Christ, B.; Patel, K. Origin and development of the avian tongue muscles. Anat. Embryol. 1999, 200, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Noden, D.M.; Francis-West, P. The differentiation and morphogenesis of craniofacial muscles. Dev. Dyn. 2006, 235, 1194–1218. [Google Scholar] [CrossRef] [PubMed]
- Shuler, C.F.; Dalrymple, K.R. Molecular regulation of tongue and craniofacial muscle differentiation. Crit. Rev. Oral Biol. Med. 2001, 12, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Botto, L.D.; Olney, R.S.; Erickson, J.D. Vitamin supplements and the risk for congenital anomalies other than neural tube defects. Am. J. Med. Genet. C Semin. Med. Genet. 2004, 125, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Zhao, H.; Parada, C.; Hacia, J.G.; Bringas, P., Jr.; Chai, Y. A TGFβ-Smad4-Fgf6 signaling cascade controls myogenic differentiation and myoblast fusion during tongue development. Development 2012, 139, 1640–1650. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Liu, C.; Iwata, J.; Gu, S.; Suzuki, A.; Sun, C.; He, W.; Shu, R.; Li, L.; Chai, Y.; Chen, Y. Mice with Tak1 deficiency in neural crest lineage exhibit cleft palate associated with abnormal tongue development. J. Biol. Chem. 2013, 288, 10440–10450. [Google Scholar] [CrossRef] [PubMed]
- Yamane, A. Embryonic and postnatal development of masticatory and tongue muscles. Cell Tissue Res. 2005, 322, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Okano, J.; Udagawa, J.; Shiota, K. Roles of retinoic acid signaling in normal and abnormal development of the palate and tongue. Congenit. Anom. (Kyoto) 2014, 54, 69–76. [Google Scholar] [CrossRef] [PubMed]
- De Wals, P.; Tairou, F.; Van Allen, M.I.; Uh, S.; Lowry, R.B.; Sibbald, B.; Evans, J.A.; Van den Hof, M.C.; Zimmer, P.; Crowley, M.; et al. Reduction in Neural-Tube Defects after Folic Acid Fortification in Canada. N. Engl. J. Med. 2007, 357, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Wald, N.; Sneddon, J.; Densem, J.; Frost, C.; Stone, R. MRC Vitamin Study Research Croup. Prevention of neural tube defects: Results of the Medical Research Council Vitamin Study. Lancet 1991, 338, 131–137. [Google Scholar]
- Pitkin, R.M. Folate and neural tube defects. Am. J. Clin. Nutr. 2007, 85, 285S–288S. [Google Scholar] [PubMed]
- Rieder, M.J. Prevention of neural tube defects with periconceptional folic acid. Clin. Perinatol. 1994, 21, 483–503. [Google Scholar] [PubMed]
- Crider, K.S.; Yang, T.P.; Berry, R.J.; Bailey, L.B. Folate and DNA methylation: A review of molecular mechanisms and the evidence for folate’s role. Adv. Nutr. 2012, 3, 21–38. [Google Scholar] [CrossRef] [PubMed]
- Bailey, L.B.; Stover, P.J.; McNulty, H.; Fenech, M.F.; Gregory, J.F., 3rd; Mills, J.L.; Pfeiffer, C.M.; Fazili, Z.; Zhang, M.; Ueland, P.M.; et al. Biomarkers of Nutrition for Development-Folate Review. J. Nutr. 2015, 145, 1636S–1680S. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.; Sequeira, J.M.; Quadros, E.V. The metabolic basis for developmental disorders due to defective folate transport. Biochimie 2016, 126, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Wahl, S.E.; Kennedy, A.E.; Wyatt, B.H.; Moore, A.D.; Pridgen, D.E.; Cherry, A.M.; Mavila, C.B.; Dickinson, A.J. The role of folate metabolism in orofacial development and clefting. Dev. Biol. 2015, 405, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.; O’Dowd, T.; Reulbach, U. Use of folic acid supplements and risk of cleft lip and palate in infants: A population-based cohort study. Br. J. Gen. Pract. 2012, 62, e466–e472. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, E.; Murillo, J.; Barrio, C.; del Río, A.; Pérez-Miguelsanz, J.; López-Gordillo, Y.; Partearroyo, T.; Paradas, I.; Maestro, C.; Martínez-Sanz, E.; et al. Occurrence of cleft-palate and alteration of Tgf-β(3) expression and the mechanisms leading to palatal fusion in mice following dietary folic-acid deficiency. Cells Tissues Organs 2011, 194, 406–420. [Google Scholar] [CrossRef] [PubMed]
- Joubert, B.R.; den Dekker, H.T.; Felix, J.F.; Bohlin, J.; Ligthart, S.; Beckett, E.; Tiemeier, H.; van Meurs, J.B.; Uitterlinden, A.G.; Hofman, A.; et al. Maternal plasma folate impacts differential DNA methylation in an epigenome-wide meta-analysis of newborns. Nat. Commun. 2016, 7, 10577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parr, C.L.; Magnus, M.C.; Karlstad, Ø.; Haugen, M.; Refsum, H.; Ueland, P.M.; McCann, A.; Nafstad, P.; Håberg, S.E.; Nystad, W.; et al. Maternal Folate Intake during Pregnancy and Childhood Asthma in a Population-based Cohort. Am. J. Respir. Crit. Care Med. 2017, 195, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ge, X.; Zhu, B.; Xuan, Y.; Huang, K.; Rutayisire, E.; Mao, L.; Huang, S.; Yan, S.; Tao, F. Maternal Continuing Folic Acid Supplementation after the First Trimester of Pregnancy Increased the Risk of Large-for-Gestational-Age Birth: A Population-Based Birth Cohort Study. Nutrients 2016, 8, 493. [Google Scholar] [CrossRef] [PubMed]
- Maestro-de-las-Casas, C.; Pérez-Miguelsanz, J.; López-Gordillo, Y.; Maldonado, E.; Partearroyo, T.; Varela-Moreiras, G.; Martínez-Álvarez, C. Maternal folic acid-deficient diet causes congenital malformations in the mouse eye. Birth Defects Res. A Clin. Mol. Teratol. 2013, 97, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Hansen, D.K.; Horsley, E.T.; Tang, Y.S.; Khan, R.A.; Stabler, S.P.; Jayaram, H.N.; Antony, A.C. Maternal folate deficiency results in selective upregulation of folate receptors and heterogeneous nuclear ribonucleoprotein-E1 associated with multiple subtle aberrations in fetal tissues. Birth Defects Res. A Clin. Mol. Teratol. 2005, 73, 6–28. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Rozen, R. Maternal folate deficiency affects proliferation, but not apoptosis, in embryonic mouse heart. J. Nutr. 2006, 136, 1774–1778. [Google Scholar] [PubMed]
- Zhang, D.; Ighaniyan, S.; Stathopoulos, L.; Rollo, B.; Landman, K.; Hutson, J.; Newgreen, D. The neural crest: A versatile organ system. Birth Defects Res. C Embryo Today 2014, 102, 275–298. [Google Scholar] [CrossRef] [PubMed]
- Gonseth, S.; Roy, R.; Houseman, E.A.; de Smith, A.J.; Zhou, M.; Lee, S.; Nusslé, S.; Singer, A.W.; Wrensch, M.R.; Metayer, C.; et al. Periconceptional folate consumption is associated with neonatal DNA methylation modifications in neural crest regulatory and cancer development genes. Epigenetics 2015, 10, 1166–1176. [Google Scholar] [CrossRef] [PubMed]
- Karunamuni, G.H.; Ma, P.; Gu, S.; Rollins, A.M.; Jenkins, M.W.; Watanabe, M. Connecting teratogen-induced congenital heart defects to neural crest cells and their effect on cardiac function. Birth Defects Res. C Embryo Today 2014, 102, 227–250. [Google Scholar] [CrossRef] [PubMed]
- Rosenquist, T.H. Folate, homocysteine and the cardiac neural crest. Dev. Dyn. 2013, 242, 201–218. [Google Scholar] [CrossRef] [PubMed]
- Trainor, P.A. Craniofacial birth defects: The role of neural crest cells in the etiology and pathogenesis of Treacher Collins syndrome and the potential for prevention. Am. J. Med. Genet. A 2010, 152A, 2984–2994. [Google Scholar] [CrossRef] [PubMed]
- Bernal, A.J.; Jirtle, R.L. Epigenomic disruption: The effects of early developmental exposures. Birth Defects Res. A Clin. Mol. Teratol. 2010, 88, 938–944. [Google Scholar] [CrossRef] [PubMed]
- Geraghty, A.A.; Lindsay, K.L.; Alberdi, G.; McAuliffe, F.M.; Gibney, E.R. Nutrition During Pregnancy Impacts Offspring’s Epigenetic Status-Evidence from Human and Animal Studies. Nutr. Metab. Insights 2016, 8, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Heijmans, B.; Tobi, E.; Stein, A.; Putter, H.; Blauw, G.J.; Susser, E.S.; Slagboom, P.E.; Lumey, L.H. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc. Natl. Acad. Sci. USA 2008, 105, 17046–17049. [Google Scholar] [CrossRef] [PubMed]
- Lehnen, H.; Zechner, U.; Haaf, T. Epigenetics of gestational diabetes mellitus and offspring health: The time for action is in early stages of life. Mol. Hum. Reprod. 2013, 19, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Marsit, C.J. Influence of environmental exposure on human epigenetic regulation. J. Exp. Biol. 2015, 3, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Tobi, E.; Slagboom, P.; van Dongen, J.; Kremer, D.; Stein, A.D.; Putter, H.; Heijmans, B.T.; Lumey, L.H. Prenatal famine and genetic variation are independently and additively associated with DNA methylation at regulatory loci within IGF2/H19. PLoS ONE 2012, 7, e37933. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.; Maxson, R.E., Jr. Recent advances in craniofacial morphogenesis. Dev. Dyn. 2006, 235, 2353–2375. [Google Scholar] [CrossRef] [PubMed]
- Noden, D.M.; Trainor, P.A. Relations and interactions between cranial mesoderm and neural crest populations. J. Anat. 2005, 207, 575–601. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.B.; Trainor, P.A. Craniofacial malformations: Intrinsic vs extrinsic neural crest cell defects in Treacher Collins and 22q11 deletion syndromes. Clin. Genet. 2006, 69, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Emmanouil-Nikoloussi, E.N.; Kerameos-Foroglou, C. Developmental malformations of human tongue and associated syndromes (review). Bull. Group Int. Rech. Sci. Stomatol. Odontol. 1992, 35, 5–12. [Google Scholar] [PubMed]
- Gekas, J.; Li, B.; Kamnasaran, D. Current perspectives on the etiology of agnathia-otocephaly. Eur. J. Med. Genet. 2010, 53, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Hogeveen, M.; van Beynum, I.; van Rooij, A.; Kluijtmans, L.; den Heijer, M.; Blom, H. Methylmalonic acid values in healthy Dutch children. Eur. J. Nutr. 2008, 47, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Kerr, M.A.; Livingstone, B.; Bates, C.J.; Bradbury, I.; Scott, J.M.; Ward, M.; Pentieva, K.; Mansoor, M.A.; McNulty, H. Folate, related B vitamins, and homocysteine in childhood and adolescence: Potential implications for disease risk in later life. Pediatrics 2009, 123, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Pinhas-Hamiel, O.; Doron-Panush, N.; Reichman, B.; Nitzan-Kaluski, D.; Shalitin, S.; Geva-Lerner, L. Obese children and adolescents: A risk group for low vitamin B12 concentration. Arch. Pediatr. Adolesc. Med. 2006, 160, 933–936. [Google Scholar] [CrossRef] [PubMed]
- González-Gross, M.; Benser, J.; Breidenassel, C.; Albers, U.; Huybrechts, I.; Valtueña, J.; Spinneker, A.; Segoviano, M.; Widhalm, K.; Molnar, D.; et al. Gender and age influence blood folate, vitamin B 12, vitamin B 6, and homocysteine levels in European adolescents: The Helena Study. Nutr. Res. 2012, 32, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Roman Viñas, B.; Ribas Barba, L.; Ngo, J.; Gurinovic, M.; Novakovic, R.; Cavelaars, A.; de Groot, L.C.; van’t Veer, P.; Matthys, C.; Serra Majem, L. Projected Prevalence of Inadequate Nutrient Intakes in Europe. Ann. Nutr. Metab. 2011, 59, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Fabian, E.; Elmadfa, I. Nutritional Situation of the Elderly in the European Union: Data of the European Nutrition and Health Report (2004). Ann. Nutr. Metab. 2008, 52, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Planells, E.; Sánchez, C.; Montellano, M.A.; Mataix, J.; Llopis, J. Vitamins B6 and B12 and folate status in an adult Mediterranean population. Eur. J. Clin. Nutr. 2003, 57, 777–785. [Google Scholar] [CrossRef] [PubMed]


| Weeks on Maternal Fad Diet | Mothers (n) | Fetuses (n) | Tongue Malformation Fetuses n (% Total) | Microglossia Fetuses n (% Group; % Total) | Aglossia Fetuses n (% Group; % Total) |
|---|---|---|---|---|---|
| Control | 5 | 25 | 0 (0) | 0 (0.0; 0.0) | 0 (0.0; 0.0) |
| 2 | 4 | 29 | 0 (0) | 0 (0.0; 0.0) | 0 (0.0; 0.0) |
| 4 | 7 | 46 | 0 (0) | 0 (0.0; 0.0) | 0 (0.0; 0.0) |
| 6 | 4 | 13 | 2 (0.9) | 0 (0.0; 0.0) | 2 (100.0; 0.9) |
| 8 | 5 | 25 | 4 (1.7) | 1 (25.0; 0.4) | 3 (75.0; 1.3) |
| 10 | 9 | 54 | 12 (5.1) | 4 (33.3; 1.7) | 8 (66.7; 3.4) |
| 12 | 5 | 21 | 2 (0.9) | 2 (100.0; 0.9) | 0 (0.0; 0.0) |
| 14 | 2 | 11 | 2 (0.9) | 0 (0.0; 0.0) | 2 (100.0; 0.9) |
| 16 | 2 | 11 | 1 (0.4) | 0 (0.0; 0.0) | 1 (100.0; 0.4) |
| Total | 43 | 235 | 23 (9.8) | 7 (30.4; 3.0) | 16 (69.6; 6.8) |
| Weeks on Maternal FAD Diet | |||||
|---|---|---|---|---|---|
| 0 | 2–6 | 8–16 | Total | ||
| Mothers without tongue malformation fetuses | n | 5 a | 14 a | 10 b | 29 |
| % group | 100.0 | 93.3 | 43.5 | 67.4 | |
| Mothers with tongue malformation fetuses | n | 0 a | 1 a | 13 b | 14 |
| % group | 0.0 | 6.7 | 56.5 | 32.6 | |
| Total | n | 5 | 15 | 23 | 43 |
| % group | 100.0 | 100.0 | 100.0 | 100.0 | |
| Weeks on Maternal FAD Diet (n) | Tongue Width | Base of the Tongue Width | Tongue Height | Tongue Area | ||||
|---|---|---|---|---|---|---|---|---|
| (μm) | VvC (%) | (μm) | VvC (%) | (μm) | VvC (%) | (mm2) | VvC (%) | |
| Control | 1629.7 | 1392.2 | 767.8 | 1.297 | ||||
| (n = 15) | (1534.8–1717.6) | (1305.5–1453.4) | (702.0–816.0) | (1.243–1.500) | ||||
| 2 | 1504.0 | −7.7 | 1296.9 *** | −6.8 | 726.1 | −5.4 | 1.042 | −19.6 |
| (n = 29) | (1602.3–1641.4) | (1296.9–1348.0) | (687.5–776.8) | (1.047–1.203) | ||||
| 4 | 1688.0 | 3.6 | 1430.0 | 2.7 | 820.00 | 6.8 | 1.389 | 7.1 |
| (n = 46) | (1505.6–1734.4) | (1225.0–1515.0) | (716.9–868.7) | (1.125–1.583) | ||||
| 6 | 1412.9 | −13.3 | 1239.0 | −11.0 | 668.6 | −12.9 | 1.013 ** | −21.9 |
| (n = 13) | (1266.2–1624.3) | (1195.2–1355.0) | (593.9–699.9) | (0.871–1.193) | ||||
| 8 | 1504.9 * | −7.7 | 1321.3 | −5.1 | 680.3 | −11.4 | 1.051 ** | −19.0 |
| (n = 25) | (1287.0–1565.5) | (1203.0–1371.7) | (609.0–748.8) | (0.977–1.179) | ||||
| 10 | 1499.5 * | −8.0 | 1274.9 | −8.4 | 744.1 | −3.1 | 1.147 | −11.6 |
| (n = 54) | (1393.5–1593.2) | (1174.2–1444.8) | (635.0–833.5) | (0.922–1.417) | ||||
| 12 | 1386.0 * | −14.9 | 1252.5 | −10.0 | 710.6 | −7.5 | 1.029 ** | −20.7 |
| (n = 21) | (1298.2–1430.4) | (1127.3–1310.6) | (650.9–791.8) | (0.933–1.119) | ||||
| 14 | 1392.9 ** | −14.5 | 1166.6 * | −16.2 | 716.7 | −6.7 | 0.969 ** | −46.3 |
| (n = 11) | (1171.0–1455.7) | (1077.8–1217.0) | (663.6–782.2) | (0.825–1.080) | ||||
| 16 | 1314.3 *** | −19.4 | 1032.9 *** | −25.8 | 604.5 | −21.3 | 0.888 *** | −31.5 |
| (n = 11) | (1130.1–1360.1) | (856.9–1188.2) | (543.3–723.3) | (0.612–0.949) | ||||
| Weeks of Maternal FAD Diet (n) | Head Width | Head Height | Head Area | |||
|---|---|---|---|---|---|---|
| (mm) | VvC (%) | (mm) | VvC (%) | (mm2) | VvC (%) | |
| Control | 5.0 | 3.9 | 17.9 | |||
| (n = 15) | (4.7–5.3) | (3.7–4.8) | (15.7–19.0) | |||
| 2 | 5.0 | 0.0 | 4.0 | 2.6 | 15.3 | −14.5 |
| (n = 29) | (4.7–5.2) | (3.7–4.1) | (14.2–16.1) | |||
| 4 | 5.0 | 0.0 | 4.1 | 5.1 | 17.2 | −3.9 |
| (n = 46) | (4.4–5.2) | (3.9–4.4) | (14.7–18.5) | |||
| 6 | 4.6 | −8.0 | 4.0 | 2.6 | 14.7 | −17.8 |
| (n = 13) | (4.3–5.1) | (3.8–4.1) | (12.7–16.4) | |||
| 8 | 4.5 | −10.0 | 3.8 | −2.6 | 14.4 | −19.5 |
| (n = 25) | (4.3–4.8) | (3.7–4.1) | (13.6–15.8) | |||
| 10 | 4.7 | −6.0 | 4.0 | 2.6 | 12.6 ** | −29.6 |
| (n = 54) | (3.8–5.1) | (3.7–4.5) | (15.3–18.9) | |||
| 12 | 4.3 ** | −14.0 | 3.8 | −2.6 | 13.3 ** | −25.7 |
| (n = 21) | (4.1–4.5) | (3.7–4.0) | (12.6–14.6) | |||
| 14 | 4.4 | −12.0 | 4.0 | 2.6 | 13.7 | −23.5 |
| (n = 11) | (3.8–4.7) | (3.5–4.2) | (10.7–16.0) | |||
| 16 | 3.7 *** | −26.0 | 3.5 | −10.2 | 11.1 *** | −40.0 |
| (n = 11) | (3.6–4.1) | (3.2–4.1) | (9.2–13.4) | |||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maldonado, E.; López-Gordillo, Y.; Partearroyo, T.; Varela-Moreiras, G.; Martínez-Álvarez, C.; Pérez-Miguelsanz, J. Tongue Abnormalities Are Associated to a Maternal Folic Acid Deficient Diet in Mice. Nutrients 2018, 10, 26. https://doi.org/10.3390/nu10010026
Maldonado E, López-Gordillo Y, Partearroyo T, Varela-Moreiras G, Martínez-Álvarez C, Pérez-Miguelsanz J. Tongue Abnormalities Are Associated to a Maternal Folic Acid Deficient Diet in Mice. Nutrients. 2018; 10(1):26. https://doi.org/10.3390/nu10010026
Chicago/Turabian StyleMaldonado, Estela, Yamila López-Gordillo, Teresa Partearroyo, Gregorio Varela-Moreiras, Concepción Martínez-Álvarez, and Juliana Pérez-Miguelsanz. 2018. "Tongue Abnormalities Are Associated to a Maternal Folic Acid Deficient Diet in Mice" Nutrients 10, no. 1: 26. https://doi.org/10.3390/nu10010026





