Oxidative Photodegradation of Pyrene and Fluoranthene by Fe-Based and Zn-Based Fenton Reagents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Preparation
2.2. Photolysis Experiment
2.3. Sample Preparation and Analysis
2.4. Kinetic Modeling
3. Results and Discussions
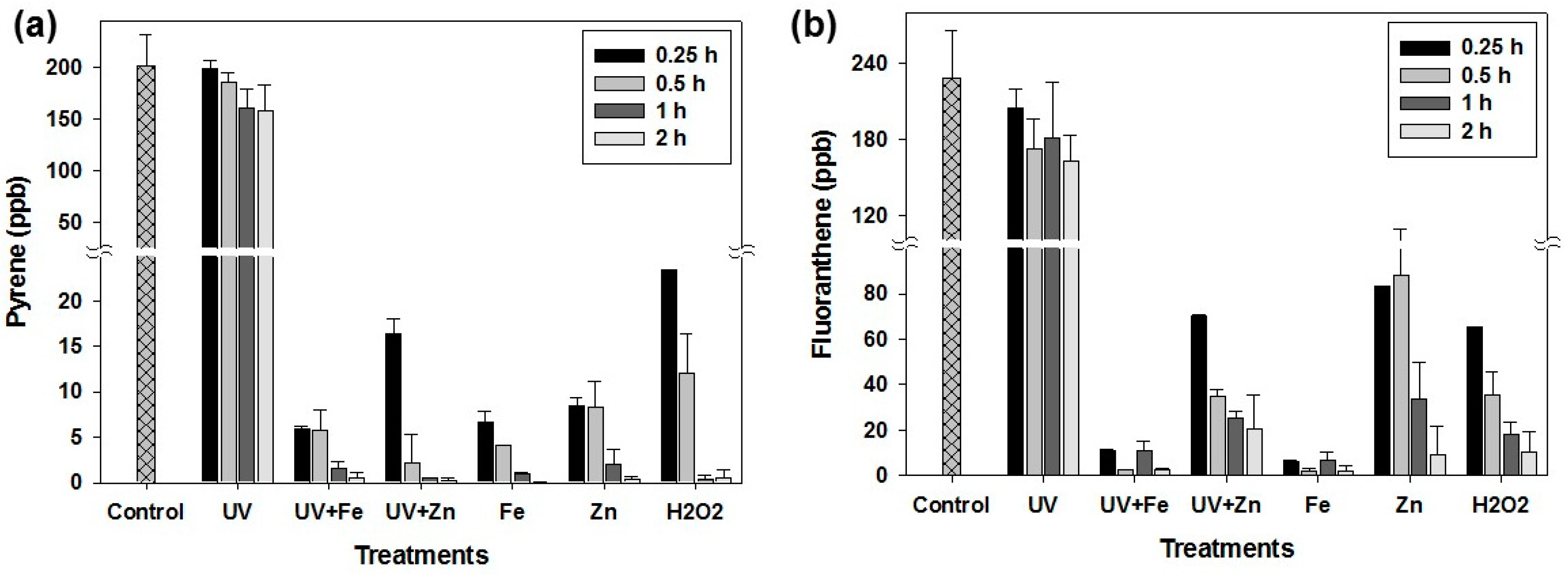
3.1. PAH Degradation
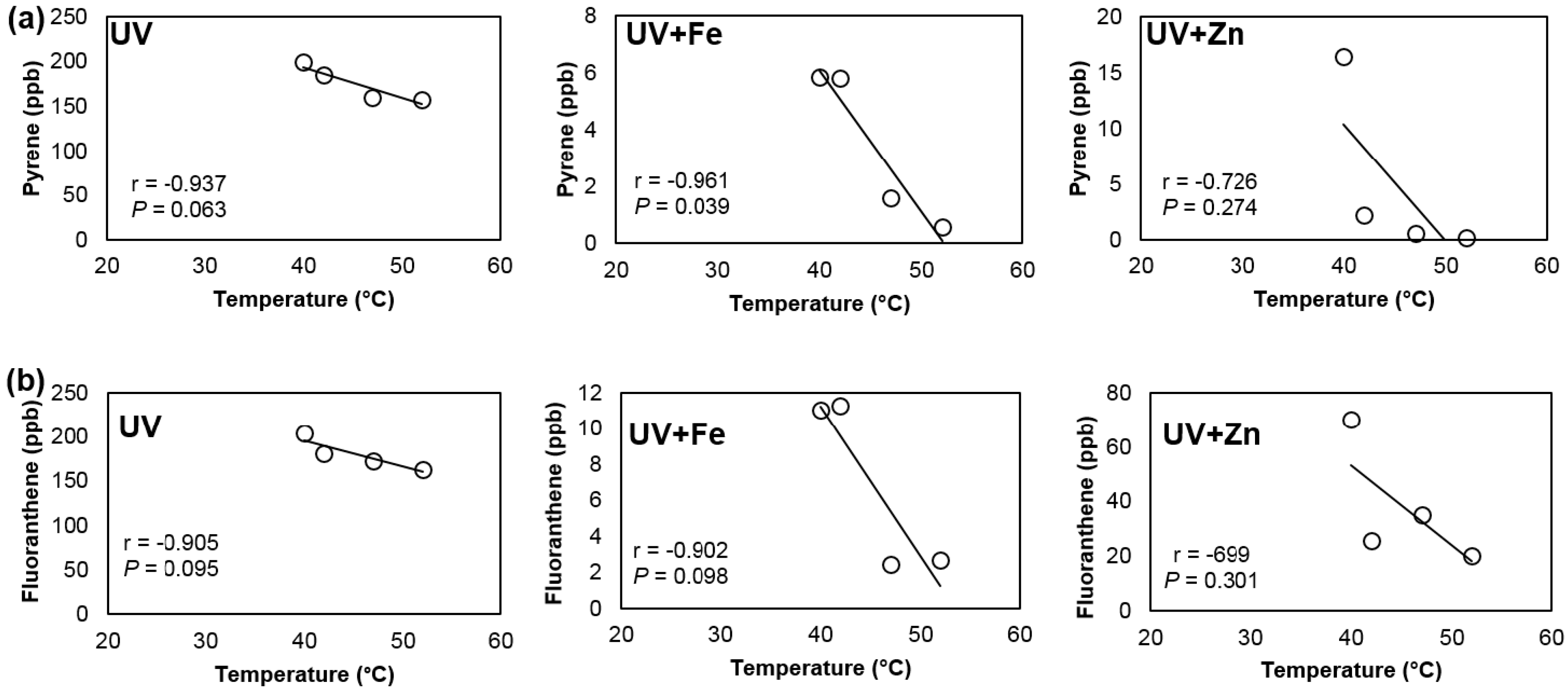
3.2. Effect of UV Chamber Temperature on Degradation
3.3 Dynamics of PAH Degradation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Freije, A.M. Heavy metal, trace element and petroleum hydrocarbon pollution in the Arabian Gulf: Review. J. Assoc. Arab Univ. Basic Appl. Sci. 2015, 17, 90–100. [Google Scholar] [CrossRef]
- Primbs, T.; Piekarz, A.; Wilson, G.; Schmedding, D.; Higginbotham, C.; Field, J.; Simonich, S.M. Influence of Asian and Western United States Urban Areas and Fires on the Atmospheric Transport of Polycyclic Aromatic Hydrocarbons, Polychlorinated Biphenyls, and Fluorotelomer Alcohols in the Western United States. Environ. Sci. Technol. 2008, 42, 6385–6391. [Google Scholar] [CrossRef] [PubMed]
- Genualdi, S.A.; Killin, R.K.; Woods, J.; Wilson, G.; Schmedding, D.; Simonich, S.L.M. Trans-Pacific and regional atmospheric transport of polycyclic aromatic hydrocarbons and pesticides in biomass burning emissions to western North America. Environ. Sci. Technol. 2009, 43, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- EL-Saeid, M.H.; Al-Turki, A.M.; Nadeem, M.E.A.; Hassanin, A.S.; Al-Wabel, M.I. Photolysis degradation of polyaromatic hydrocarbons (PAHs) on surface sandy soil. Environ. Sci. Pollut. Res. 2015, 22, 9603–9616. [Google Scholar] [CrossRef] [PubMed]
- El-Mubarak, A.H.; Rushdi, A.I.; Al-Mutlaq, K.F.; Bazeyad, A.Y.; Simonich, S.L.M.; Simoneit, B.R.T. Identification and source apportionment of polycyclic aromatic hydrocarbons in ambient air particulate matter of Riyadh, Saudi Arabia. Environ. Sci. Pollut. Res. 2013, 21, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, G.; Traina, S.J.; Swanston, C.W. Black Carbon’s Properties and Role in the Environment: A Comprehensive Review. Sustainability 2010, 2, 294–320. [Google Scholar] [CrossRef]
- Wilcke, W. Global patterns of polycyclic aromatic hydrocarbons (PAHs) in soil. Geoderma 2007, 141, 157–166. [Google Scholar] [CrossRef]
- Ravindra, K.; Sokhi, R.; Van Grieken, R. Atmospheric polycyclic aromatic hydrocarbons: Source attribution, emission factors and regulation. Atmos. Environ. 2008, 42, 2895–2921. [Google Scholar] [CrossRef]
- Vu, B.; Alves, C.A.; Gonçalves, C.; Pio, C.; Gonçalves, F.; Pereira, R. Mutagenicity assessment of aerosols in emissions from wood combustion in Portugal. Environ. Pollut. 2012, 166, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Gan, S.; Lau, E.V.; Ng, H.K. Remediation of soils contaminated with polycyclic aromatic hydrocarbons (PAHs). J. Hazard. Mater. 2009, 172, 532–549. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.; Rodriguez, W.; Kukor, J.J. Enhanced degradation of polycyclic aromatic hydrocarbons by biodegradation combined with a modified Fenton reaction. Chemosphere 2001, 45, 11–20. [Google Scholar] [CrossRef]
- Yap, C.L.; Gan, S.; Ng, H.K. Fenton based remediation of polycyclic aromatic hydrocarbons-contaminated soils. Chemosphere 2011, 83, 1414–1430. [Google Scholar] [CrossRef] [PubMed]
- Canzano, S.; Capasso, S.; Natale, M.D.; Erto, A.; Iovino, P.; Musmarra, D. Remediation of Groundwater Polluted by Aromatic Compounds by Means of Adsorption. Sustainability 2014, 6, 4807–4822. [Google Scholar] [CrossRef]
- Andreozzi, R.; Caprio, V.; Insola, A.; Marotta, R. Advanced oxidation processes (AOP) for water purification and recovery. Catal. Today 1999, 53, 51–59. [Google Scholar] [CrossRef]
- Munter, R. Advanced oxidation processes—Current status and prospects. Proc. Estonian Acad. Sci. Chem. 2001, 50, 59–80. [Google Scholar]
- Cheng, M.; Zeng, G.; Huang, D.; Lai, C.; Xu, P.; Zhang, C.; Liu, Y. Hydroxyl radicals based advanced oxidation processes (AOPs) for remediation of soils contaminated with organic compounds: A review. Chem. Eng. J. 2016, 284, 582–598. [Google Scholar] [CrossRef]
- Watts, R.J.; Stanton, P.C.; Howsawkeng, J.; Teel, A.L. Mineralization of a sorbed polycyclic aromatic hydrocarbon in two soils using catalyzed hydrogen peroxide. Water Res. 2002, 36, 4283–4292. [Google Scholar] [CrossRef]
- Mirzaee, E.; Gitipour, S.; Mousavi, M.; Amini, S. Optimization of total petroleum hydrocarbons removal from Mahshahr contaminated soil using magnetite nanoparticle catalyzed Fenton-like oxidation. Environ. Earth Sci. 2017, 76, 165. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Pedrazzani, R.; Sorlini, S.; Abbà, A.; Bertanza, G. H2O2 Based Oxidation Processes for the Treatment of Real High Strength Aqueous Wastes. Sustainability 2017, 9, 244. [Google Scholar] [CrossRef]
- Jung, Y.S.; Lim, W.T.; Park, J.-Y.; Kim, Y.-H. Effect of pH on Fenton and Fenton-like oxidation. Environ. Technol. 2009, 30, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.I.; Ahmad, K.; Ashraf, M.; Parveen, R.; Mustafa, I.; Khan, A.; Bibi, Z.; Akram, N.A. Bioaccumulation of heavy metals and metalloids in luffa (Luffa cylinderica L.) irrigated with domestic wastewater in Jhang, Pakistan: A prospect for human nutrition. Pak. J. Bot. 2015, 47, 217–224. [Google Scholar]
- Woo, O.T.; Chung, W.K.; Wong, K.H.; Chow, A.T.; Wong, P.K. Photocatalytic oxidation of polycyclic aromatic hydrocarbons: Intermediates identification and toxicity testing. J. Hazard. Mater. 2009, 168, 1192–1199. [Google Scholar] [CrossRef] [PubMed]
- Karaca, G.; Baskaya, H.S.; Tasdemir, Y. Removal of polycyclic aromatic hydrocarbons (PAHs) from inorganic clay mineral: Bentonite. Environ. Sci. Pollut. Res. Int. 2016, 23, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Tsuzuki, T. Commercial scale production of inorganic nanoparticles. Int. J. Nanotechnol. 2009, 6, 567–578. [Google Scholar] [CrossRef]
- Tang, L.; Tang, X.Y.; Zhu, Y.G.; Zheng, M.H.; Miao, Q.L. Contamination of polycyclic aromatic hydrocarbons (PAHs) in urban soils in Beijing, China. Environ. Int. 2005, 31, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-F.; Cullen, W.R.; Reimer, K.J.; Le, X.C. Microbial degradation of pyrene and characterization of a metabolite. Sci. Total Environ. 1996, 177, 17–29. [Google Scholar] [CrossRef]
- Rababah, A.; Matsuzawa, S. Treatment system for solid matrix contaminated with fluoranthene. I—Modified extraction technique. Chemosphere 2002, 46, 39–47. [Google Scholar] [CrossRef]
- Šepič, E.; Bricelj, M.; Leskovšek, H. Toxicity of fluoranthene and its biodegradation metabolites to aquatic organisms. Chemosphere 2003, 52, 1125–1133. [Google Scholar] [CrossRef]
- Lei, A.P.; Hu, Z.L.; Wong, Y.S.; Tam, N.F.-Y. Removal of fluoranthene and pyrene by different microalgal species. Bioresour. Technol. 2007, 98, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Vela, N.; Martínez-Menchón, M.; Navarro, G.; Pérez-Lucas, G.; Navarro, S. Removal of polycyclic aromatic hydrocarbons (PAHs) from groundwater by heterogeneous photocatalysis under natural sunlight. J. Photochem. Photobiol. Chem. 2012, 232, 32–40. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, C.; Chen, Z.; Li, X.; Li, P. Photodegradation of pyrene on soil surfaces under UV light irradiation. J. Hazard. Mater. 2010, 173, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.D.; Hua, R.M.; Tang, F.; Chen, X. Effects of soil particle size on distribution and photodegradation of selected pesticides in soil. J. Anhui Agric. Univ. China 1993, 4, 4. [Google Scholar]
- Cavoski, I.; Caboni, P.; Sarais, G.; Cabras, P.; Miano, T. Photodegradation of Rotenone in Soils under Environmental Conditions. J. Agric. Food Chem. 2007, 55, 7069–7074. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, S.; Persson, Y.; Frankki, S.; van Bavel, B.; Lundstedt, S.; Haglund, P.; Tysklind, M. Degradation of polycyclic aromatic hydrocarbons (PAHs) in contaminated soils by Fenton’s reagent: A multivariate evaluation of the importance of soil characteristics and PAH properties. J. Hazard. Mater. 2007, 149, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Kitti, A.; Harju, M.; Tysklind, M.; van Bavel, B. Multivariate characterization of polycyclic aromatic hydrocarbons using semi-empirical molecule orbital calculations and physical data. Chemosphere 2003, 50, 627–637. [Google Scholar] [CrossRef]
- Pauporté, T.; Lincot, D. Hydrogen peroxide oxygen precursor for zinc oxide electrodeposition II—Mechanistic aspects. J. Electroanal. Chem. 2001, 517, 54–62. [Google Scholar] [CrossRef]
- Machulek, A.; Oliveira, S.C.; Osugi, M.E.; Ferreira, V.S.; Quina, F.H.; Dantas, R.F.; Oliveira, S.L.; Casagrande, G.A.; Anaissi, F.J.; Silva, V.O.; et al. Application of Different Advanced Oxidation Processes for the Degradation of Organic Pollutants. In Organic Pollutants—Monitoring, Risk and Treatment; Rashed, M.N., Ed.; InTech: Rijeka, Croatia, 2013. [Google Scholar]
- Mondal, K.; Sharma, A. Photocatalytic oxidation of pollutant dyes in wastewater by TiO2 and ZnO nano-materials—A mini-review. In Nanoscience & Technology for Mankind; The Academy of Sciences India (NASI): Allahabad, India, 2014; pp. 36–72. [Google Scholar]
- Plazinski, W.; Rudzinski, W.; Plazinska, A. Theoretical models of sorption kinetics including a surface reaction mechanism: A review. Adv. Colloid Interface Sci. 2009, 152, 2–13. [Google Scholar] [CrossRef] [PubMed]

| Compound | RT (Min) | Ion Polarity | Mass | Product Mass | CE | Recovery % ± SD |
|---|---|---|---|---|---|---|
| Fluoranthene-Q | 19.94 | Positive | 202.08 | 176.08 | 35 | 97.58 ± 2.66 |
| Fluoranthene | 19.94 | Positive | 202.08 | 200.08 | 30 | |
| Pyrene | 20.5 | Positive | 202.08 | 176.08 | 35 | 99.65 ± 2.68 |
| Pyrene-Q | 20.5 | Positive | 202.08 | 200.08 | 30 |
| Treatment | Pseudo-First-Order Parameters | ||
|---|---|---|---|
| R2 | k (min−1) | t1/2 (min) | |
| Pyrene | |||
| UV | 0.928 | 0.002 | 346.574 |
| UV + Fe | 0.953 | 0.024 | 28.881 |
| UV + Zn | 0.968 | 0.038 | 18.241 |
| Fe | 0.942 | 0.060 | 11.552 |
| Zn | 0.982 | 0.033 | 21.004 |
| H2O2 | 0.988 | 0.036 | 19.254 |
| Fluoranthene | |||
| UV | 0.979 | 0.002 | 346.574 |
| UV + Fe | 0.986 | 0.015 | 46.210 |
| UV + Zn | 0.909 | 0.009 | 77.016 |
| Fe | 0.933 | 0.011 | 63.013 |
| Zn | 0.975 | 0.018 | 38.508 |
| H2O2 | 0.909 | 0.013 | 53.319 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, A.; Al-Barakah, F.N.; Al-Sewailem, M.; El-Saeid, M.H.; Waqar, M.; Ahmad, M. Oxidative Photodegradation of Pyrene and Fluoranthene by Fe-Based and Zn-Based Fenton Reagents. Sustainability 2017, 9, 870. https://doi.org/10.3390/su9050870
Hussain A, Al-Barakah FN, Al-Sewailem M, El-Saeid MH, Waqar M, Ahmad M. Oxidative Photodegradation of Pyrene and Fluoranthene by Fe-Based and Zn-Based Fenton Reagents. Sustainability. 2017; 9(5):870. https://doi.org/10.3390/su9050870
Chicago/Turabian StyleHussain, Abid, Fahad N. Al-Barakah, Mohamed Al-Sewailem, Mohamed H. El-Saeid, Muhammad Waqar, and Mahtab Ahmad. 2017. "Oxidative Photodegradation of Pyrene and Fluoranthene by Fe-Based and Zn-Based Fenton Reagents" Sustainability 9, no. 5: 870. https://doi.org/10.3390/su9050870






