1. Introduction
Industrial aqueous wastes are significantly heterogeneous, even within the same factory, their characteristics changing by time, depending on the ongoing production activities. This poses a challenge for their treatment, there being three alternatives: (a) advanced biological processes, which may be able to treat high organic loads with a typical operational stability [
1,
2,
3] and a very low sludge production [
1,
4]; (b) advanced chemical processes [
5,
6,
7,
8,
9]; (c) or a combination of both [
4,
10,
11]. In the past, chemical oxidation has been used for reducing the concentrations of residual organics, removing ammonia, controlling odors, and for disinfection purposes. Nowadays, chemical oxidation processes are recommended for improving the biological treatability of refractory organic compounds and reducing the inhibitory effects of specific substances towards the microbial growth [
12,
13].
The present research was focused on the application of some advanced oxidation processes (AOPs) for the treatment of high strength industrial aqueous wastes. These processes combine several oxidants and/or physical treatments, such as ultraviolet light and ultrasonic irradiation with or without the use of chemical catalysts. AOPs are based on the formation of hydroxyl radicals (
•OH), which promote radical chain reactions leading to the destruction of aromatic compounds, adsorbable organic halogen (AOX), detergents, pesticides, azo dyes, and phenols [
5,
6,
12,
14,
15,
16,
17,
18,
19,
20].
UV radiation is often used in combination with O
3, H
2O
2, Fenton’s (H
2O
2/Fe
2+) reagent and TiO
2 catalyst to accelerate the radical formation and, thus, cause an indirect photolysis [
21]. UV-based processes are negatively affected by high turbidity and intensive colour of wastewater [
14].
In the UV/H
2O
2 combined process, the UV radiation activates H
2O
2, finally leading to the formation of the
•OH radical formation [
22,
23]. The effectiveness of the UV/H
2O
2 process depends on various conditions that affect its ability to degrade organic molecules. These conditions include the type and the concentration of the organic contaminants or dissolved inorganics (such as carbonates and metallic cations), light transmittance of the solutions, pH, temperature, and hydrogen peroxide dosage. An excessive concentration of H
2O
2 would act as a radical scavenger reducing the rate of oxidation, while a too low H
2O
2 dosage brings to an insufficiently hydroxyl radicals formation, thus decreasing the oxidation rate. The UV/H
2O
2 process is sensitive to the scavenging effects of carbonate ions for pH values in the range 8–9 [
7,
18].
The Fenton process has been the most widely used AOP [
24] for wastewater treatment due to its simplicity in terms of equipment and management operation. However, it presents some disadvantages such as the production of chemical sludge, the high acid consumption for decreasing the pH (especially in case of high alkalinity wastewaters), the high concentrations of chloride and sulphate ions (depending on the kind of ferrous salt used) in the treated waste and the significant operating costs, due to sludge disposal and hydrogen peroxide consumption.
Fenton and H2O2/UV processes involve a significant ferrous salt dosage and a high energy consumption, respectively.
The photo-Fenton process (i.e., the Fenton process with additional exposure to UV radiation) overcomes these drawbacks. The reactions involved in this process are the following [
7]:
The two oxidation-reduction reactions occur repeatedly until complete mineralization of the organic pollutants to CO
2 and H
2O is achieved [
7]. The main advantage of the photo-Fenton process compared to the Fenton one lies in the important reduction of reagent consumption and sludge production.
Notwithstanding the huge mole of literature findings, which underline the combined influence of several parameters on process efficiency (temperature, pH, reagent dosage, inorganic salts concentration, etc.) and suggest possible reaction mechanisms [
7,
11,
25,
26], the applicability of AOPs to real wastewater is still an open issue. Therefore, any hypothesis has to be fully validated by means of experimental tests that must be carried out under conditions which must be as close as possible to the real ones. In particular, the composition of the wastewater represents a crucial factor: hence, the real wastes to be treated should be used at this scope, instead of synthetic solutions (which use is more appropriate for theoretical investigations).
The present work was aimed at testing three AOPs (namely H2O2/UV, Fenton and photo-Fenton) for the pre-treatment, upstream a biological process, of six high strength aqueous wastes, four of them also being characterised by a high content of anionic and non-ionic surfactants. Case by case, the preferable treatment process and the optimal operating conditions were defined. Results are thought to be of general interest for practitioners facing the problem of treating such kinds of real wastewaters.
2. Materials and Methods
2.1. Aqueous Wastes
Six aqueous wastes were submitted to chemical oxidation tests (
Table 1). Two of them (aqueous wastes 1, A.W.#1, and 2, A.W.#2) were treated in the phase I of the experimentation. These wastes derived from a pharmaceutical and a chemical factory, respectively. Both show high concentration of organic matter (COD up to >200,000 mg·L
−1). The main components of A.W.#1 were methanol (70,000 mg·L
−1), acetone (50,000 mg·L
−1), and aromatic solvents (110,000–120,000 mg·L
−1). The main organic substances in A.W.#2 were: acetone (75% vol.), dimethylformamide (23.5% vol.) and acetic acid (1.5% vol.).
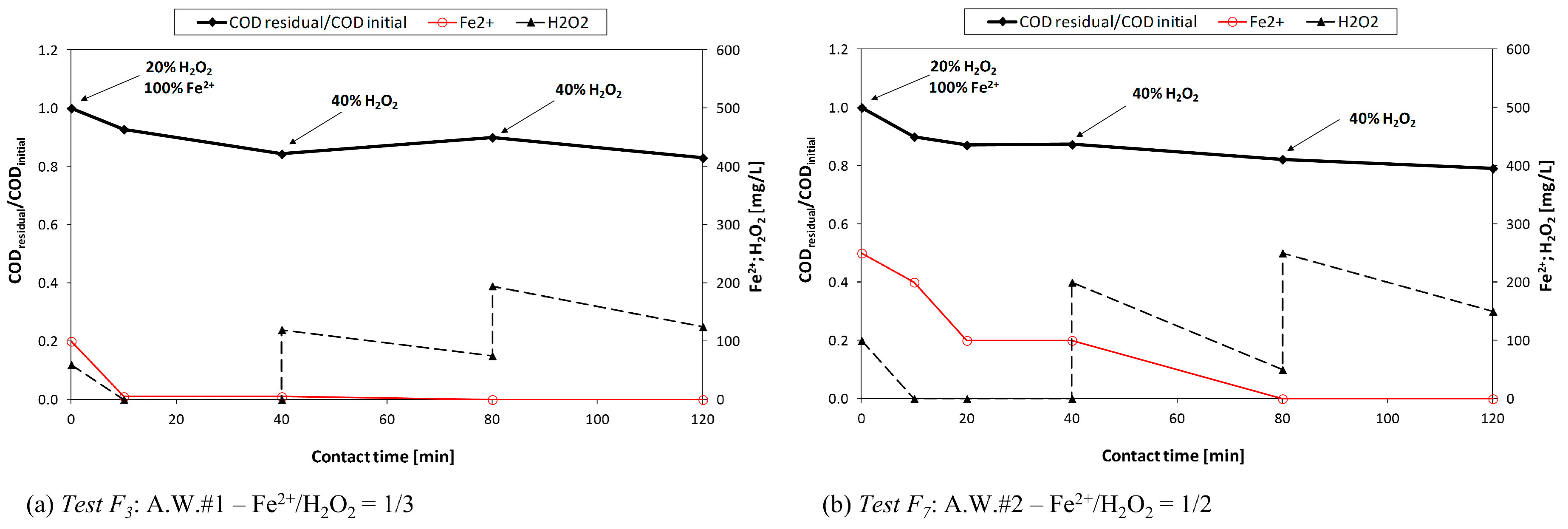
During the second experimental period (phase II), mainly focused on surfactant removal, four aqueous wastes deriving from the detergents production were treated. In this case, together with very high COD concentrations, a significant presence of anionic (MBAS) and non-ionic (TAS) surfactants (up to 13,000 and 17,000 mg·L−1, respectively) was measured. In order to reduce the acid dosage during the Fenton treatment, A.W.#3 was mixed with an acidic aqueous waste (COD = 30,000 mg·L−1, pH < 1.5), thus obtaining two mixtures: mix#1 (83% A.W.#3 + 17% acidic waste) and mix#2 (17% A.W.#3 + 83% acidic waste).
For some of the studied wastewaters, the biodegradability (as appears from the BOD
5/COD ratio) was relatively high, thus suggesting the biological treatability also without the need of a chemical pre-treatment. Nevertheless, BOD measurements are obtained after the dilution of the samples. Indeed, in real applications, this is not the case: the so high level of contamination, poses serious problems to the biomass and microfauna of the activated sludge plant, in terms of metabolic inhibition (namely the nitrification process) and sludge settle-ability [
27,
28,
29]. The results of OUR (Oxygen Uptake Rate) tests carried out on the aqueous wastes (the values obtained vary from 3.7 to 4.2 mgO
2 g
VSS−1·h
−1, with respect to the exogenous value of 4.5 ± 0.7 mgO
2 g
VSS−1·h
−1) clearly demonstrated this. These troubles may be even emphasized by surfactants. Therefore, a chemical pre-treatment might be a proper choice.
2.2. Pilot Scale Plants
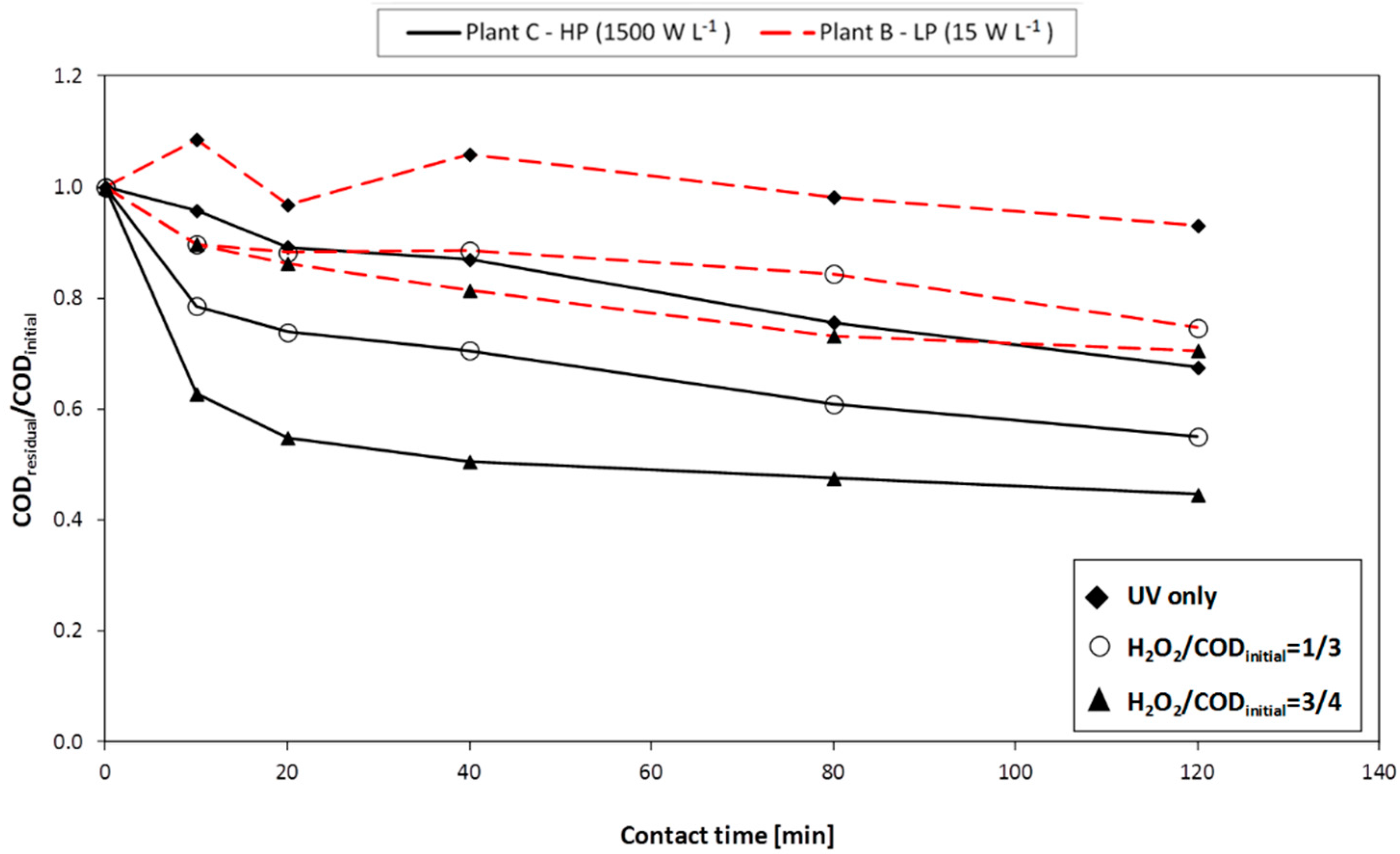
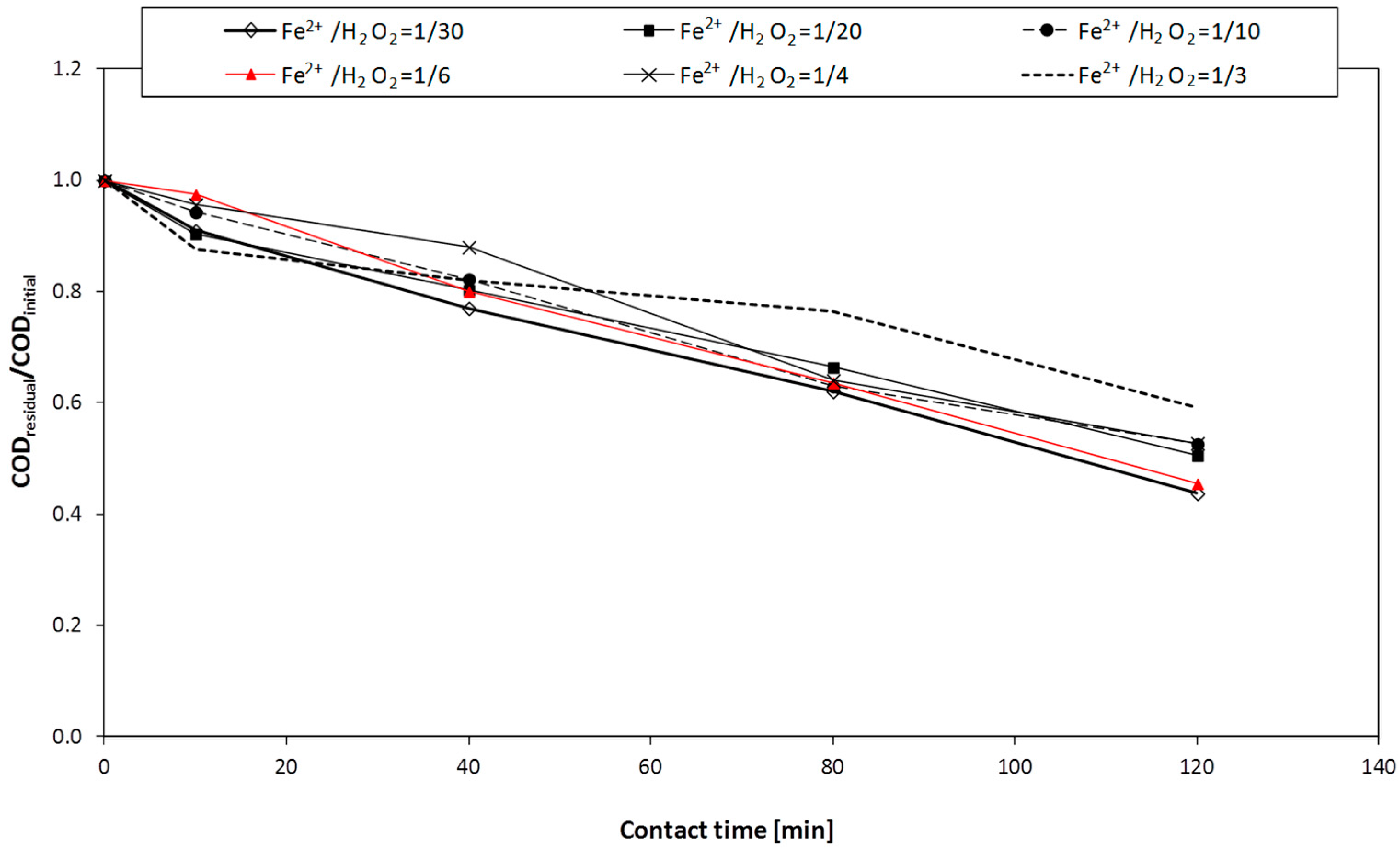
Photo-Fenton and H2O2/UV tests were carried out by means of three different plants (A, B, and C, respectively) with the aim of studying the influence of the UV lamp type (having different energy consumption and emission spectrum) and the reactor shape/geometry, both on the atmospheric oxygen transfer and on process performance. H2O2/UV tests were conducting using all the three plants; photo-Fenton tests were performed on plants A and B.
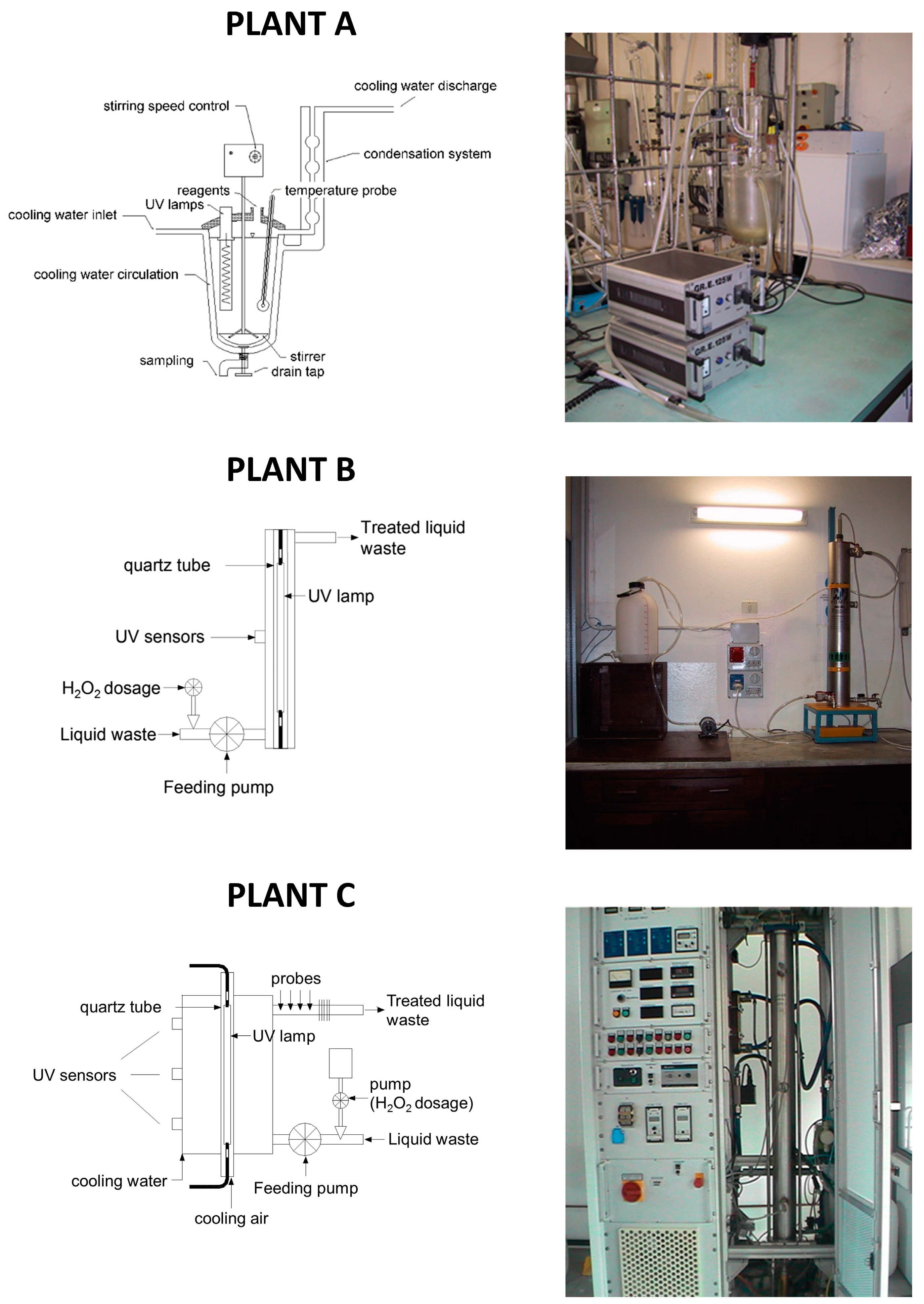
The main characteristics of the pilot scale plants (
Figure 1) are reported below.
Plant A consists of a 2 L glass reactor; the cover is welded and a discharge valve is placed on the bottom. An external jacket connected to a cryostat is used for cooling the system. On the central cone a stirring device is applied. In the lateral cone two medium-high-pressure UV lamps are placed: each lamp has a power of 125 W (emission spectrum: 280–400 nm).
Plant B consists of AISI 316L stainless steel photo-reactor (8 L volume), containing one UV lamp. During the experimental work, two different kinds of lamp were used: the first is a low-pressure lamp with a power of 36 W (emission spectrum: 254 nm); the second is similar but with a power of 120 W. The following advantages may be ascribed to the use of low-pressure lamps: low surface temperature (40–50 °C), high power conversion efficiency (35%–40% of electric energy is converted into useful UV energy) and long duration (8000–10,000 h).
Plant C consists of: an AISI 316L stainless steel photo-reactor (10 L volume), a high-pressure UV lamp (power: 10–30 kW; emission spectrum: 200–700 nm), a feeding pump (with flowrate adjustable from 2 to 10 L·min−1), a pump for H2O2 dosage (flowrate adjustable up to 8 mL·min−1), probes for the measurement of flowrate, electrical conductivity, pH, redox potential, and temperature. The UV lamp used in this plant simulates solar radiation.
As regards the Fenton process, each test was carried out with the use of 1 L flask, rapidly mixed (by means of a magnetic stirrer).
2.3. Experimental Tests
During phase I, the high COD aqueous wastes (A.W.#1 and A.W.#2) were submitted to H
2O
2/UV, photo-Fenton and Fenton processes. During phase II, the Fenton process was tested for the treatment of A.W.#3 to #6, which were characterised by high concentrations of surfactants (see
Table 1).
In
Table 2 and
Table 3 (which concerns the phase I and phase II, respectively) the operating conditions (oxidant dosage, plant used, reaction time) of all tests carried out during the experimental work are reported. The dosage of reagents is reported in terms of absolute concentrations and weight ratio between reagents (H
2O
2/COD
initial and Fe
2+/H
2O
2). The aqueous wastes were diluted in order to limit the amount of reagents and simplify the experimental procedures at the laboratory scale: the concentrations of reagents shown in
Table 2 and
Table 3 are those actually employed for treating the diluted wastewaters.
Since the AOPs were supposed to be used as a pre-treatment to a biological stage, the oxidant dosage was generally under the stoichiometric ratio, with respect to the initial COD of the sample.
Three different reagent dosage criteria were adopted: (1) unique initial dosage; (2) consecutive additions (either at 0 and 40 min, or at 0, 40 and 80 min); or (3) continuous dosage.
As regards UV/H
2O
2 and photo-Fenton tests, the specific power of lamp, expressed as power (W) per volume of reactor (L), is shown in
Table 2.
During some tests, air was inflated into the reactors, in order to assess possible effects on the overall efficiency of the process (e.g., in terms of mixing and mass transfer improvement or oxygen supply). Actually, in real facilities, pressurized air pipelines are often present for other purposes (e.g., the biological treatment plant or other industrial needs), so that the possibility to exploit this opportunity may be an interesting option.
Reaction time was varied from 30 to 240 min, based on the author’s experience on full scale facilities.
The tests exhibiting good performances were repeated in order to confirm the results obtained; about 100 oxidation tests were performed overall.
2.4. Analytical Methods
The concentrations of COD, N-NH
4+, N-NO
2−, N-NO
3−, total nitrogen (TN), total phosphorus (TP), anionic surfactants (MBAS), and non-ionic surfactants (TAS) were measured according to standard methods for water and wastewater [
30]. BOD
5 was determined at 20 °C by inoculation of activated sludge from a municipal wastewater treatment plant.
pH was measured by means of a WTW (Ingolstadt, Germany) Sentix 940-3 probe. Residual concentrations of H2O2 and Fe2+ were measured by means colorimetric test strips (Merck–Darmstadt, Germany–MQuant™).
The chemical analyses were carried out three times on the same sample; the average values are reported in the results session.