Biogenic Amines as Quality Marker in Organic and Fair-Trade Cocoa-Based Products
Abstract
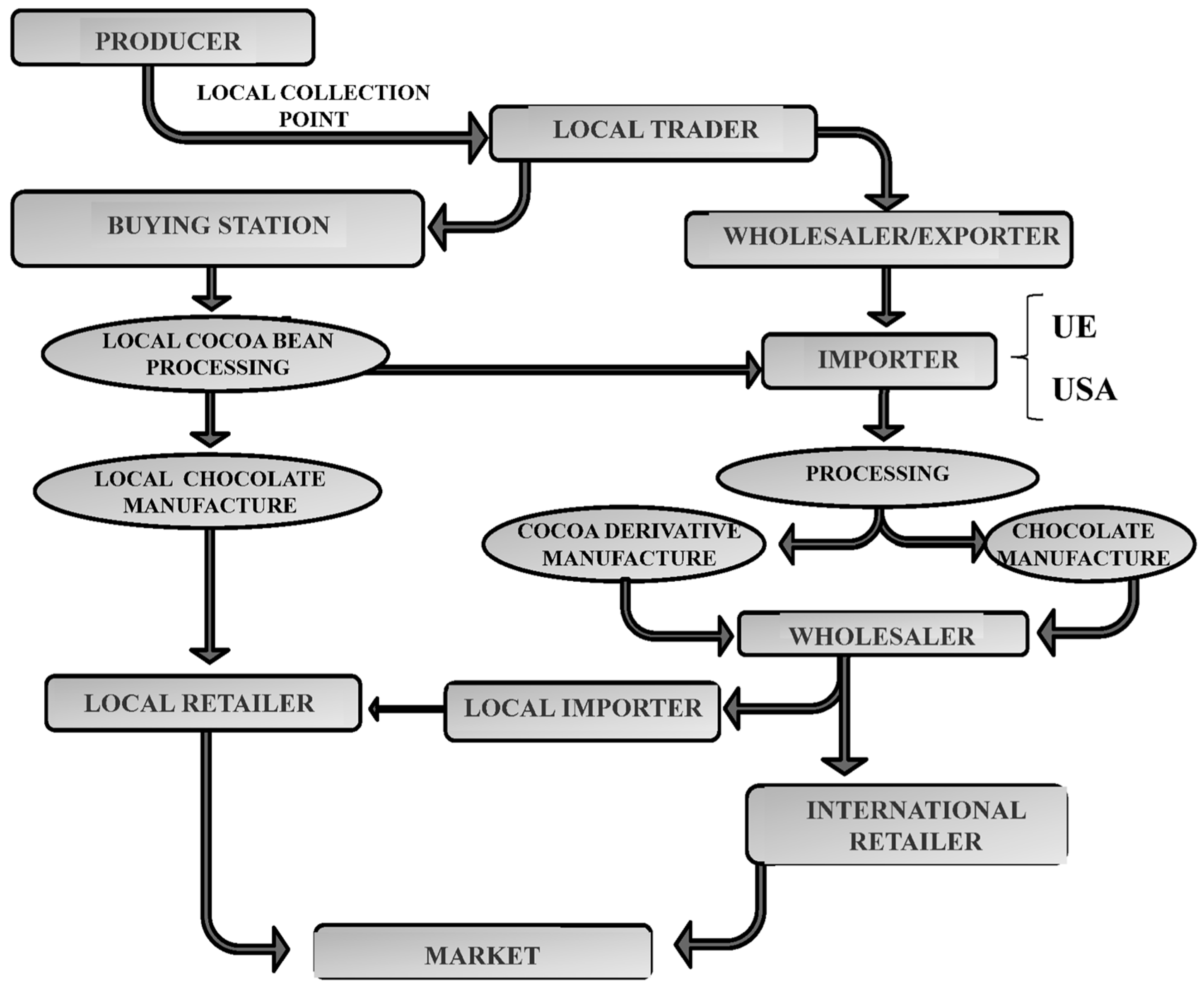
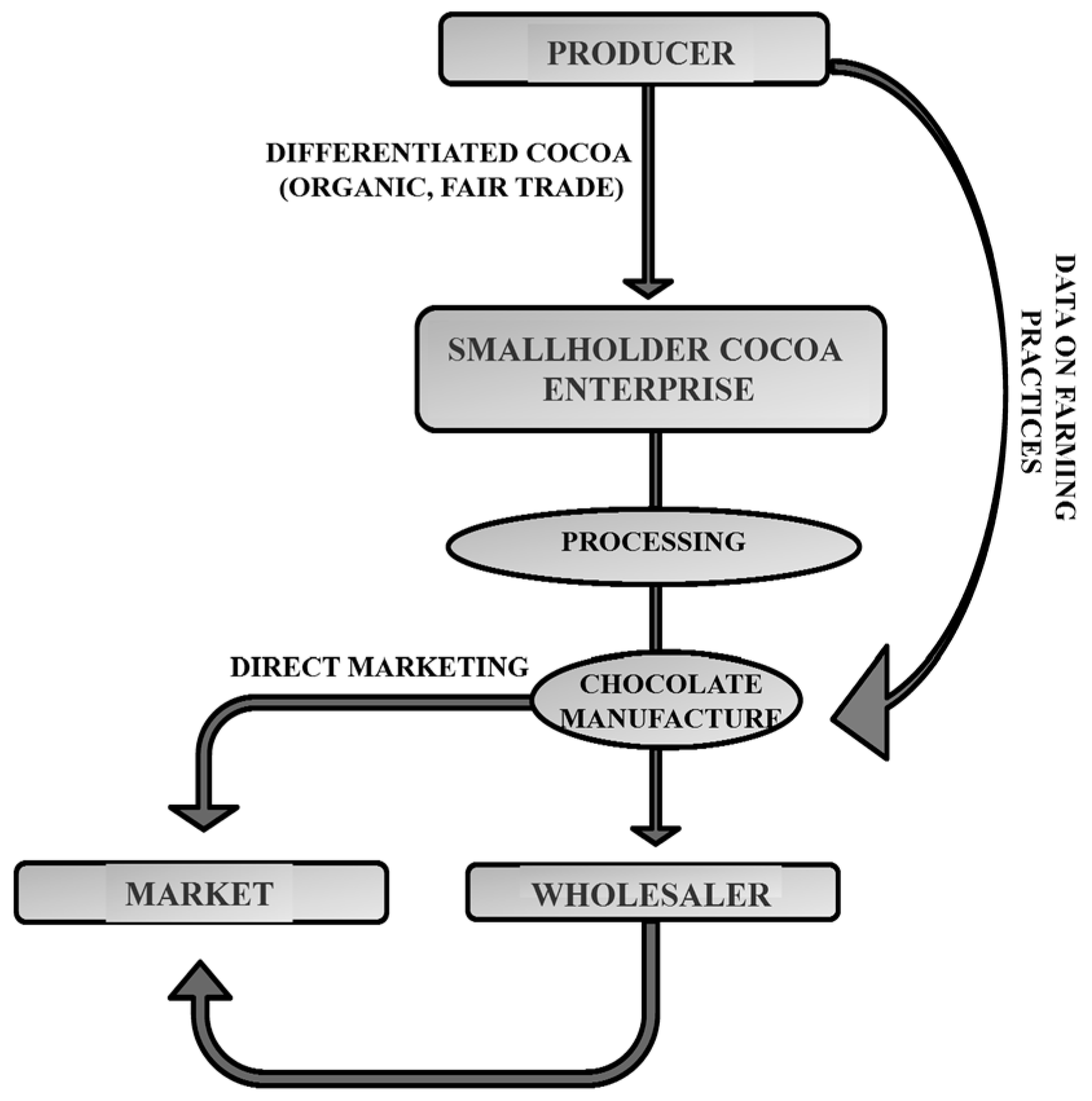
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Chemicals
2.3. Amine Standard Solutions and Calibration
2.4. BA Extraction and Purification
2.5. Equipment
2.6. Data Handling
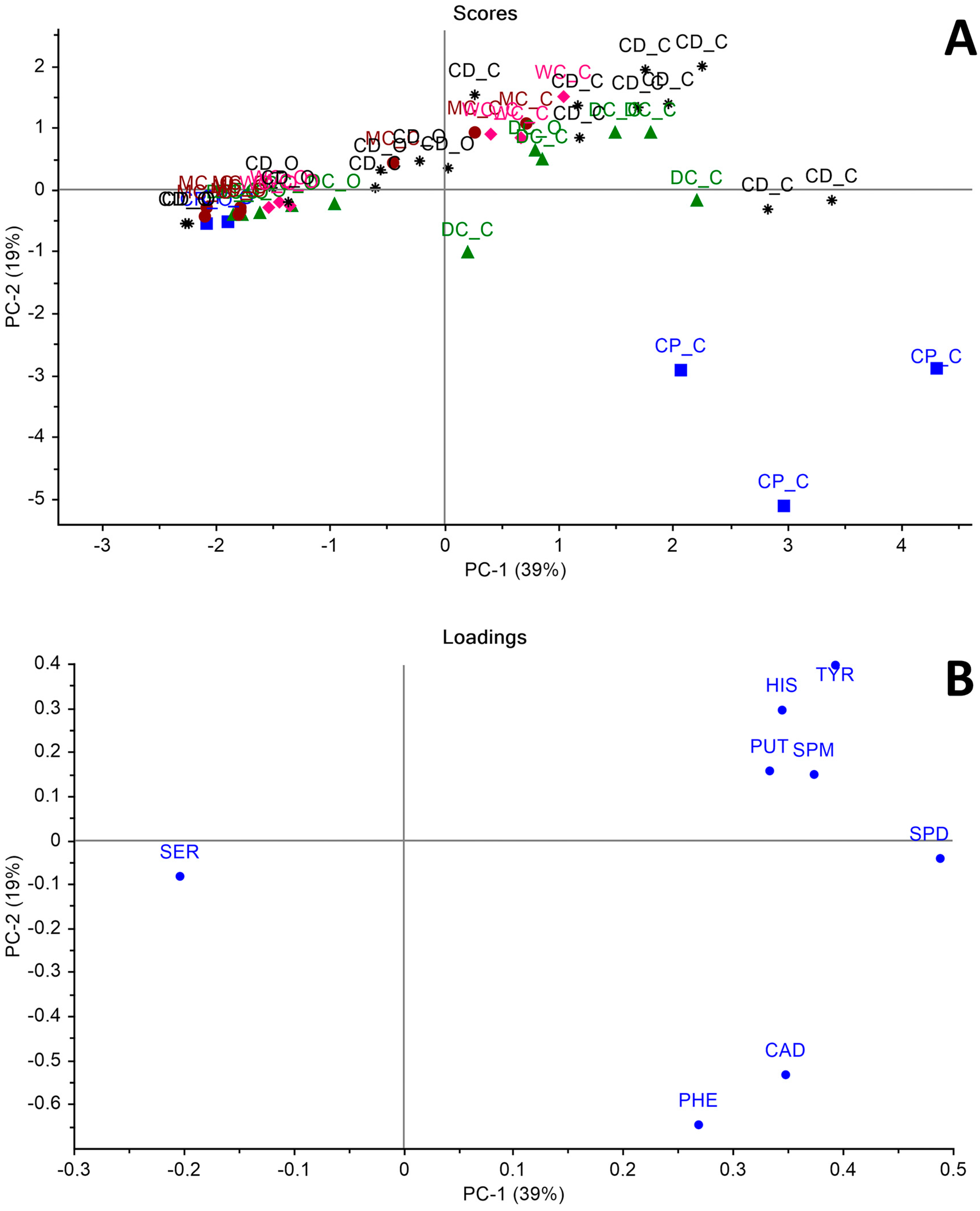
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- ICCO. Quarterly Bulletin of Cocoa Statistics. Available online: http://www.icco.org/statistics/quarterly-bulletin-cocoa-statistics.html (accessed on 31 May 2016).
- Sukha, D.; Butler, D.; Umaharan, P.; Boult, E. The use of an optimized organoleptic assessment protocol to describe and quantify different flavor attributes of cocoa liquors made from Ghana and Trinitario beans. Eur. Food Res. Technol. 2008, 226, 405–413. [Google Scholar] [CrossRef]
- Saltini, R.; Akkerman, R.; Frosch, S. Optimizing chocolate production through traceability: A review of the influence of farming practices on cocoa bean quality. Food Control 2013, 29, 167–187. [Google Scholar] [CrossRef] [Green Version]
- Caligiani, A.; Cirlini, M.; Palla, G.; Ravaglia, R.; Arlorio, M. GCeMS detection of chiral markers in cocoa beans of different quality and geographic origin. Chirality 2007, 19, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Rohsius, C.; Matissek, R.; Lieberei, R. Free amino acid amounts in raw cocoas from different origins. Eur. Food Res. Technol. 2006, 222, 432–438. [Google Scholar] [CrossRef]
- Haynes, J.; Cubbage, F.; Mercer, E.; Sills, E. The search for value and meaning in the cocoa supply chain in Costa Rica. Sustainability 2012, 4, 1466–1487. [Google Scholar] [CrossRef]
- Méndez, V.E.; Bacon, C.M.; Olson, M.; Petchers, S.; Herrador, D.; Carranza, C.; Trujillo, L.; Guadarrama-Zugasti, C.; Cordón, A.; Mendoza, A. Effects of fair trade and organic certifications on small-scale coffee farmer households in Central America and Mexico. Renew. Agric. Food Syst. 2010, 25, 236–251. [Google Scholar] [CrossRef]
- Jacobi, J.; Andres, C.; Schneider, M.; Calizaya, M.P.P.; Rist, S. Carbon stocks, tree diversity, and the role of organic certification in different cocoa production systems in Alto Beni Bolivia. Agrofor. Syst. 2014, 88, 1117–1132. [Google Scholar] [CrossRef]
- Araujo, Q.R.; Fernandes, C.A.F.; Ribeiro, D.O.; Efraim, P.; Steinmacher, D.; Lieberei, R.; Bastide, P.; Araujo, T.G. Cocoa quality index—A proposal. Food Control 2014, 46, 49–54. [Google Scholar] [CrossRef]
- Badrie, N.; Bekele, F.; Sikora, E.; Sikora, M. Cocoa agronomy, quality, nutritional, and health aspects. Crit. Rev. Food Sci. Nutr. 2015, 55, 620–659. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.D.; Noerrung, B.; Budka, H. Scientific opinion on risk based control of biogenic amine formation in fermented foods. EFSA J. 2011, 9, 1–92. [Google Scholar]
- Granvogl, M.; Bugan, S.; Schieberle, P. Formation of amines and aldehydes from parent amino acids during thermal processing of cocoa and model systems: New insights into pathways of the Strecker reaction. J. Agric. Food Chem. 2006, 54, 1730–1739. [Google Scholar] [CrossRef] [PubMed]
- Granvogl, M.; Schieberle, P. Quantification of 3-aminopropionamide in cocoa, coffee and cereal products. Eur. Food Res. Technol. 2007, 225, 857–863. [Google Scholar] [CrossRef]
- Oracz, J.; Nebesny, E. Influence of roasting conditions on the biogenic amine content in cocoa beans of different Theobroma cacao cultivars. Food Res. Int. 2014, 55, 1–10. [Google Scholar] [CrossRef]
- Pastore, P.; Favaro, G.; Badocco, D.; Tapparo, A.; Cavalli, S.; Saccani, G. Determination of biogenic amines in chocolate by ion chromatographic separation and pulsed integrated amperometric detection with implemented wave-form at Au disposable electrode. J. Chromatogr. A 2005, 1098, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, F.J.; Navarro, J.L.; Delgado, R.M.; Zamora, R. Histamine formation by lipid oxidation products. Food Res. Int. 2013, 52, 206–213. [Google Scholar] [CrossRef]
- Zamora, R.; Delgado, R.M.; Hidalgo, F.J. Formation of β-phenylethylamine as a consequence of lipid oxidation. Food Res. Int. 2012, 46, 321–325. [Google Scholar] [CrossRef]
- Restuccia, D.; Spizzirri, U.G.; Puoci, F.; Picci, N. Determination of biogenic amine profiles in conventional and organic cocoa-based products. Food Addit. Contam. A 2015, 32, 1156–1163. [Google Scholar] [CrossRef] [PubMed]
- Spizzirri, U.G.; Parisi, O.I.; Picci, N.; Restuccia, D. Application of LC with Evaporative Light Scattering detector for biogenic amines determination in fair trade cocoa-based products. Food Anal. Methods 2016, 9, 2200–2209. [Google Scholar] [CrossRef]
- Lavizzari, T.; Veciana-Nogués, M.T.; Bover-Cid, S.; Mariné-Font, A.; Vidal-Carou, M.C. Improved method for the determination of biogenic amines and polyamines in vegetable products by ion-pair high-performance liquid chromatography. J. Chromatogr. A 2006, 1129, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Mayr, C.M.; Schieberle, P. Development of stable isotope dilution assays for the simultaneous quantitation of biogenic amines and polyamines in foods by LC-MS/MS. J. Agr. Food Chem. 2012, 60, 3026–3032. [Google Scholar] [CrossRef] [PubMed]
- Baranowska, I.; Płonka, J. Simultaneous determination of biogenic amines and methylxanthines in foodstuff-sample preparation with HPLC-DAD-FL analysis. Food Anal. Methods 2015, 8, 963–972. [Google Scholar] [CrossRef]
- Cirilo, M.P.G.; Coelho, A.F.S.; Araújo, C.M.; Gonçalves, F.R.B.; Nogueira, F.D.; Glória, M.B.A. Profile and levels of bioactive amines in green and roasted coffee. Food Chem. 2003, 82, 397–402. [Google Scholar] [CrossRef]
- Gomiero, T.; Pimentel, D.; Paoletti, M.G. Environmental impact of different agricultural management practices: Conventional vs. organic agriculture. Crit. Rev. Plant Sci. 2011, 30, 95–124. [Google Scholar] [CrossRef]
- Santos, W.C.; Souza, M.R.; Cerqueira, M.M.O.P.; Glória, M.B.A. Bioactive amines formation in milk by Lactococcus in the presence or not of rennet and NaCl at 20 and 32 °C. Food Chem. 2003, 81, 595–606. [Google Scholar] [CrossRef]
- Izquierdo-Pulido, M.; Mariné-Font, A.; Vidal-Carou, M.C. Biogenic Amines formation during malting and brewing. J. Food Sci. 1994, 59, 1104–1107. [Google Scholar] [CrossRef]
- Restuccia, D.; Spizzirri, U.G.; Parisi, O.I.; Cirillo, G.; Picci, N. Brewing effect on levels of biogenic amines in different coffee samples as determined by LC-UV. Food Chem. 2015, 175, 143–150. [Google Scholar] [CrossRef] [PubMed]
- De Luca, M.; Restuccia, D.; Clodoveo, M.L.; Puoci, F.; Ragno, G. Chemometric analysis for discrimination of extra virgin olive oils from whole and stoned olive pastes. Food Chem. 2016, 202, 432–437. [Google Scholar] [CrossRef] [PubMed]
- De Luca, M.; Hiri, A.; Ioele, G.; Balouki, A.; Basbassi, E.; Kzaiber, F.; Oussama, A.; Ragno, G. Chemometric classification of citrus juices of Moroccan cultivars by infrared spectroscopy. Czech J. Food Sci. 2015, 33, 137–142. [Google Scholar]
- Parente, E.; Martuscelli, M.; Gardini, F.; Grieco, S.; Crudele, M.A.; Suzzi, G. Evolution of microbial populations and biogenic amine production in dry sausages produced in Southern Italy. J. Appl. Microbiol. 2001, 90, 882–891. [Google Scholar] [CrossRef] [PubMed]
| Sample # | Sample Type | % Cocoa | Cocoa Origin | Cultivation | Biogenic Amines (µg∙g−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PHE | PUT | CAD | HIS | TYR | SPD | SER | SPM | Total | |||||
| 1 | Cocoa powder | 80 | NR | Conventional | 2.0 ± 0.1 | 9.1 ± 0.3 | 5.3 ± 0.3 | 23.8 ± 0.6 | 13.1 ± 0.4 | 9.7 ± 0.3 | - | 9.3 ± 0.3 | 72.3 ± 0.5 |
| 2 | Cocoa powder | 80 | NR | Conventional | 1.8 ± 0.1 | 7.1 ± 0.4 | 3.3 ± 0.2 | 20.1 ± 0.4 | 6.4 ± 0.3 | 10.2 ± 0.2 | - | - | 48.9 ± 0.6 |
| 3 | Cocoa powder | 80 | NR | Conventional | 3.2 ± 0.2 | 10.1 ± 0.2 | 6.1 ± 0.3 | 15.8 ± 0.4 | 9.7 ± 0.2 | 5.9 ± 0.3 | - | 1.3 ± 0.1 | 50.8 ± 0.5 |
| 4 | Cocoa powder | 80 | Dominican Republic | Organic-Fair Trade | - | 1.0 ± 0.1 | - | 2.4 ± 0.2 | 1.3 ± 0.1 | 1.0 ± 0.1 | - | - | 5.7 ± 0.2 |
| 5 | Cocoa powder | 80 | Cameroon | Organic-Fair trade | - | - | - | 2.9 ± 0.2 | 1.7 ± 0.1 | 1.8 ± 0.1 | - | 0.6 ± 0.1 | 7.0 ± 0.2 |
| 6 | Cocoa powder | 80 | Panama | Organic-Fair trade | - | 0.8 ± 0.1 | - | 2.2 ± 0.1 | 1.6 ± 0.1- | 1.4 ± 0.1 | - | 0.9 ± 0.1 | 6.9 ± 0.2 |
| 7 | Dark Chocolate | 60 | NR | Conventional | - | 4.7 ± 0.2 | - | 20.0 ± 0.5 | 8.1 ± 0.2 | 8.2 ± 0.3 | - | 5.3 ± 0.2 | 46.3 ± 0.4 |
| 8 | Dark Chocolate | 70 | NR | Conventional | 1.0 ± 0.1 | 5.4 ± 0.3 | - | 35.0 ± 0.4 | 6.7 ± 0.3 | 9.8 ± 0.2 | - | 7.1 ± 0.3 | 65.0 ± 0.3 |
| 9 | Dark Chocolate | 70 | NR | Conventional | - | 6.1 ± 0.4 | - | 30.3 ± 0.5 | 9.4 ± 0.4 | 8.2 ± 0.3 | - | 6.8 ± 0.3 | 60.8 ± 0.4 |
| 10 | Dark Chocolate | 70 | Ecuador | Organic-Fair Trade | - | 4.0 ± 0.2 | - | 20.0 ± 0.4 | 12.0 ± 0.3 | 7.3 ± 0.2 | - | 4.9 ± 0.1 | 48.2 ± 0.3 |
| 11 | Dark Chocolate | 70 | Dominican Republic | Organic-Fair Trade | - | 1.2 ± 0.1 | - | 3.4 ± 0.2 | 2.1 ± 0.2 | 1.8 ± 0.2 | - | 1.9 ± 0.1 | 10.4 ± 0.3 |
| 12 | Dark Chocolate | 70 | Ecuador | Organic-Fair trade | - | 1.9 ± 0.2 | - | 5.8 ± 0.3 | 2.1 ± 0.1 | 2.1 ± 0.1 | - | 2.8 ± 0.2 | 14.7 ± 0.2 |
| 13 | Dark Chocolate orange flavour | 49 | NR | Conventional | 1.1 ± 0.1 | 2.3 ± 0.1 | - | 10.5 ± 0.3 | 7.0 ± 0.2 | 5.0 ± 0.2 | - | 4.3 ± 0.2 | 30.2 ± 0.2 |
| 14 | Dark Chocolate orange flavour | 55 | Cameroon | Organic | - | 0.9 ± 0.1 | - | 3.8 ± 0.1 | 2.2 ± 0.3 | 3.2 ± 0.2 | - | 5.0 ± 0.1 | 15.1 ± 0.2 |
| 15 | Dark Chocolate with cocoa beans | 73 | Dominican Republic | Organic-Fair Trade | - | 0.9 ± 0.1 | - | 1.9 ± 0.1 | 2.3 ± 0.2 | 1.0 ± 0.1 | - | 1.6 ± 0.1 | 7.7 ± 0.2 |
| 16 | Extra Dark Chocolate | 75 | NR | Conventional | - | 7.9 ± 0.1 | - | 19.5 ± 0.3 | 12.4 ± 0.3 | 9.0 ± 0.2 | - | 8.8 ± 0.1 | 57.6 ± 0.3 |
| 17 | Extra Dark Chocolate | 75 | Panama | Organic-Fair trade | - | 1.2 ± 0.1 | - | 2.9 ± 0.2 | 1.6 ± 0.1 | 1.1 ± 0.1 | - | 1.9 ± 0.1 | 8.6 ± 0.1 |
| 18 | Milk Chocolate | 32 | NR | Conventional | - | 2.1 ± 0.1 | - | 37.1 ± 0.5 | 4.7 ± 0.1 | 3.5 ± 0.2 | - | - | 47.4 ± 0.3 |
| 19 | Milk Chocolate | 32 | NR | Conventional | - | 3.1 ± 0.1 | - | 45.4 ± 0.4 | 14.1 ± 0.2 | 6.1 ± 0.3 | - | - | 68.7 ± 0.6 |
| 20 | Milk Chocolate | 33 | NR | Conventional | - | 3.9 ± 0.2 | - | 27.9 ± 0.6 | 19.6 ± 0.3 | 4.7 ± 0.3 | - | - | 56.1 ± 0.4 |
| 21 | Milk Chocolate | 35 | Ghana | Organic | - | 1.6 ± 0.1 | - | 7.3 ± 0.2 | 2.1 ± 0.1 | 1.4 ± 0.2 | - | - | 12.4 ± 0.3 |
| 22 | Milk Chocolate | 34 | Dominican Republic | Organic-Fair Trade | - | 0.9 ± 0.1 | - | 12.0 ± 0.2 | 1.8 ± 0.1 | 1.0 ± 0.1 | - | - | 15.7 ± 0.2 |
| 23 | Milk Chocolate | 39 | Ecuador | Organic-Fair trade | - | 0.8 ± 0.1 | - | 10.0 ± 0.2 | 2.0 ± 0.1 | 1.3 ± 0.1 | - | - | 14.1 ± 0.2 |
| 24 | Milk Chocolate with coffee cream | 15 | NR | Conventional | - | 2.3 ± 0.1 | - | 11.9 ± 0.2 | 6.9 ± 0.1 | 1.1 ± 0.1 | 5.2 ± 0.1 | 3.1 ± 0.2 | 30.5 ± 0.2 |
| 25 | Milk Chocolate with coffee cream | 15 | Bolivia | Organic | - | 1.0 ± 0.1 | - | 7.9 ± 0.1 | 4.1 ± 0.2 | 1.2 ± 0.1 | 3.0 ± 0.2 | 2.0 ± 0.1 | 19.2 ± 0.3 |
| 26 | White Chocolate | NR | NR | Conventional | - | - | - | 38.1 ± 0.5 | 10.6 ± 0.2 | 6.2 ± 0.1 | - | 3.5 ± 0.1 | 58.4 ± 0.4 |
| 27 | White Chocolate | 39 | NR | Conventional | - | - | - | 35.8 ± 0.5 | 17.1 ± 0.3 | 6.1 ± 0.2 | - | - | 59.0 ± 0.6 |
| 28 | White Chocolate | 39 | NR | Conventional | - | - | - | 47.2 ± 0.5 | 20.6 ± 0.4 | 4.7 ± 0.3 | - | 2.8 ± 0.2 | 75.3 ± 0.4 |
| 29 | White Chocolate | 39 | Cameroon | Organic | - | - | - | 9.5 ± 0.3 | 4.1 ± 0.2 | 2.8 ± 0.2 | - | 1.1 ± 0.1 | 16.4 ± 0.3 |
| 30 | White Chocolate | 39 | Dominican Republic | Organic-Fair trade | - | - | 12.0 ± 0.3 | 3.3 ± 0.1 | 2.4 ± 0.1 | - | - | 17.7 ± 0.2 | |
| 31 | White Chocolate | 39 | Ecuador | Organic-Fair trade | - | - | 12.4 ± 0.3 | 3.0 ± 0.1 | 2.0 ± 0.1 | - | 1.2 ± 0.1 | 18.6 ± 0.2 | |
| 32 | Milk chocolate egg | 15 | NR | Conventional | - | 13.1 ± 0.2 | - | 23.1 ± 0.3 | 28.9 ± 0.4 | - | - | - | 65.1 ± 0.3 |
| 33 | Cocoa and hazelnut spread | NR | NR | Conventional | - | 15.2 ± 0.2 | - | 21.9 ± 0.4 | 31.2 ± 0.5 | 2.9 ± 0.1 | - | 6.1 ± 0.2 | 77.3 ± 0.4 |
| 34 | Dark cocoa spread | NR | NR | Conventional | - | 10.9 ± 0.2 | - | 16.3 ± 0.3 | 24.6 ± 0.3 | 4.0 ± 0.1 | - | 5.6 ± 0.2 | 61.4 ± 0.3 |
| 35 | Dark cocoa spread | 25 | Ghana | Organic | - | 4.6 ± 0.2 | - | 8.1 ± 0.2 | 11.2 ± 0.2 | 2.2 ± 0.1 | - | 3.8 ± 0.2 | 29.9 ± 0.2 |
| 36 | Soluble chocolate powder | NR | NR | Conventional | - | 9.6 ± 0.2 | - | 8.7 ± 0.2 | 7.1 ± 0.2 | 4.4 ± 0.2 | - | - | 29.8 ± 0.2 |
| 37 | Soluble chocolate powder | 20 | Cameroon | Organic | - | 6.9 ± 0.2 | - | 3.5 ± 0.1 | 5.4 ± 0.2 | 2.1 ± 0.1 | - | - | 17.9 ± 0.3 |
| 38 | Gianduja Chocolate | 32 | NR | Conventional | - | 18.1 ± 0.3 | - | 28.4 ± 0.2 | 16.2 ± 0.2 | 6.2 ± 0.2 | - | 4.1 ± 0.1 | 73.0 ± 0.4 |
| 39 | Coffee confectionary | 32 | Bolivia | Organic-Fair Trade | - | 0.9 ± 0.1 | - | 4.9 ± 0.1 | 4.3 ± 0.2 | 1.3 ± 0.1 | 2.8 ± 0.2 | 1.1 ± 0.1 | 15.3 ± 0.2 |
| 40 | Coffee confectionary | 30 | Dominican Republic | Organic-Fair trade | 0.9 ± 0.1 | 5.0 ± 0.1 | 4.3 ± 0.2 | 1.3 ± 0.1 | 2.9 ± 0.1 | 1.0 ± 0.1 | 15.4 ± 0.2 | ||
| 41 | Giandujotto confectionary | 21 | NR | Conventional | - | 32.7 ± 0.4 | - | 10.5 ± 0.2 | 11.3 ± 0.2 | 6.1 ± 0.2 | - | - | 60.6 ± 0.3 |
| 42 | Cocoa glaze | 15 | NR | Conventional | - | 7.6 ± 0.2 | 4.3 ± 0.1 | 19.7 ± 0.2 | 21.1 ± 0.4 | 8.6 ± 0.2 | - | 6.7 ± 0.3 | 68.0 ± 0.3 |
| 43 | Chocolate syrup | 20 | NR | Conventional | - | 11.2 ± 0.2 | 5.1 ± 0.2 | 17.3 ± 0.2 | 29.9 ± 0.5 | 7.9 ± 0.2 | - | 6.4 ± 0.2 | 77.8 ± 0.4 |
| 44 | Chocolate syrup | 20 | Ecuador | Organic | - | 5.6 ± 0.2 | - | 7.9 ± 0.2 | 12.1 ± 0.2 | 5.0 ± 0.1 | - | 4.1 ± 0.2 | 34.7 ± 0.3 |
| 45 | Powder to prepare cocoa drink | 25 | Dominican Republic | Organic-Fair trade | - | 0.9 ± 0.1 | - | 9.8 ± 0.2 | 7.9 ± 0.2 | - | - | 1.7 ± 0.1 | 20.3 ± 0.2 |
| 46 | Powder to prepare cocoa mousse | NR | NR | Conventional | - | 13.3 ± 0.2 | - | 25.1 ± 0.4 | 19.7 ± 0.2 | 7.4 ± 0.2 | - | 6.0 ± 0.2 | 71.5 ± 0.3 |
| 47 | Powder to prepare cocoa pudding | NR | NR | Conventional | - | 10.1 ± 0.2 | - | 23.4 ± 0.4 | 31.7 ± 0.5 | 4.9 ± 0.2 | - | 8.9 ± 0.1 | 79.0 ± 0.4 |
| 48 | Powder to prepare cocoa pudding | 19 | Dominican Republic | Organic-Fair trade | - | 3.7 ± 0.2 | - | 15.0 ± 0.3 | 12.9 ± 0.3 | 3.6 ± 0.3 | - | 2.9 ± 0.2 | 38.1 ± 0.3 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Restuccia, D.; Spizzirri, U.G.; Luca, M.D.; Parisi, O.I.; Picci, N. Biogenic Amines as Quality Marker in Organic and Fair-Trade Cocoa-Based Products. Sustainability 2016, 8, 856. https://doi.org/10.3390/su8090856
Restuccia D, Spizzirri UG, Luca MD, Parisi OI, Picci N. Biogenic Amines as Quality Marker in Organic and Fair-Trade Cocoa-Based Products. Sustainability. 2016; 8(9):856. https://doi.org/10.3390/su8090856
Chicago/Turabian StyleRestuccia, Donatella, Umile Gianfranco Spizzirri, Michele De Luca, Ortensia Ilaria Parisi, and Nevio Picci. 2016. "Biogenic Amines as Quality Marker in Organic and Fair-Trade Cocoa-Based Products" Sustainability 8, no. 9: 856. https://doi.org/10.3390/su8090856






