Adsorptive Removal of Reactive Black 5 from Wastewater Using Bentonite Clay: Isotherms, Kinetics and Thermodynamics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Adsorbent Preparation
2.2. Surface Characterization
2.3. Adsorbate Solution Preparation
2.4. Batch Experimentation
2.5. Equilibrium Adsorption Isotherm Models
2.6. Adsorption Kinetics
2.7. Statistical Analysis
3. Results and discussions
3.1. Surface Characterization of Bentonite Clay
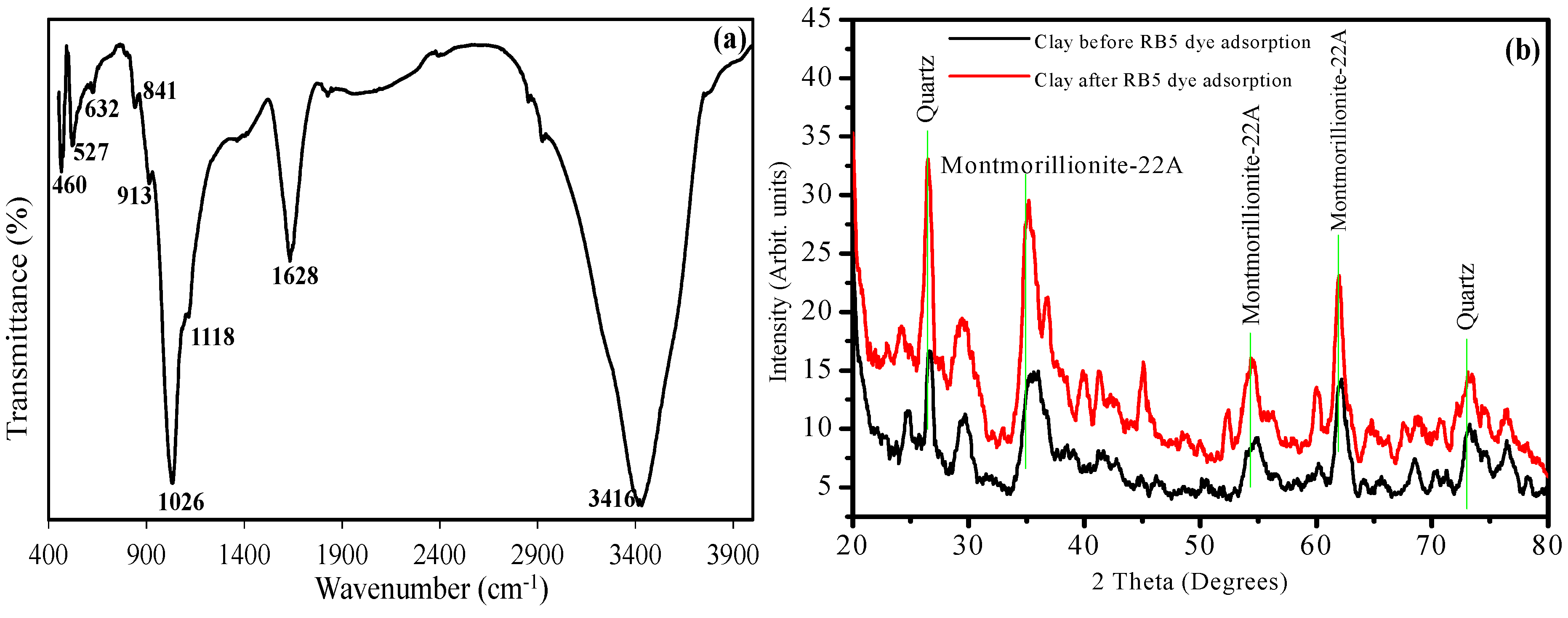
3.2. Effects of Different Reaction Parameters
3.2.1. Effects of the Shaking Speed on the Bentonite Clay Capacity for RB5
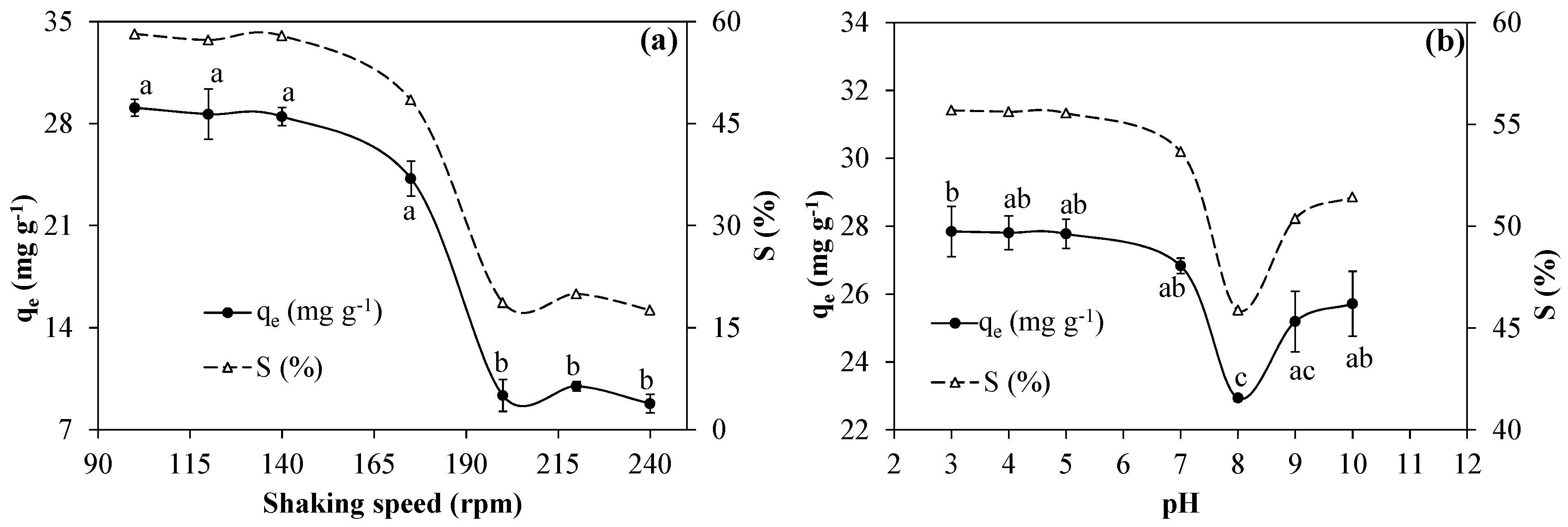
3.2.2. Effects of pH
3.2.3. Effects of Temperature
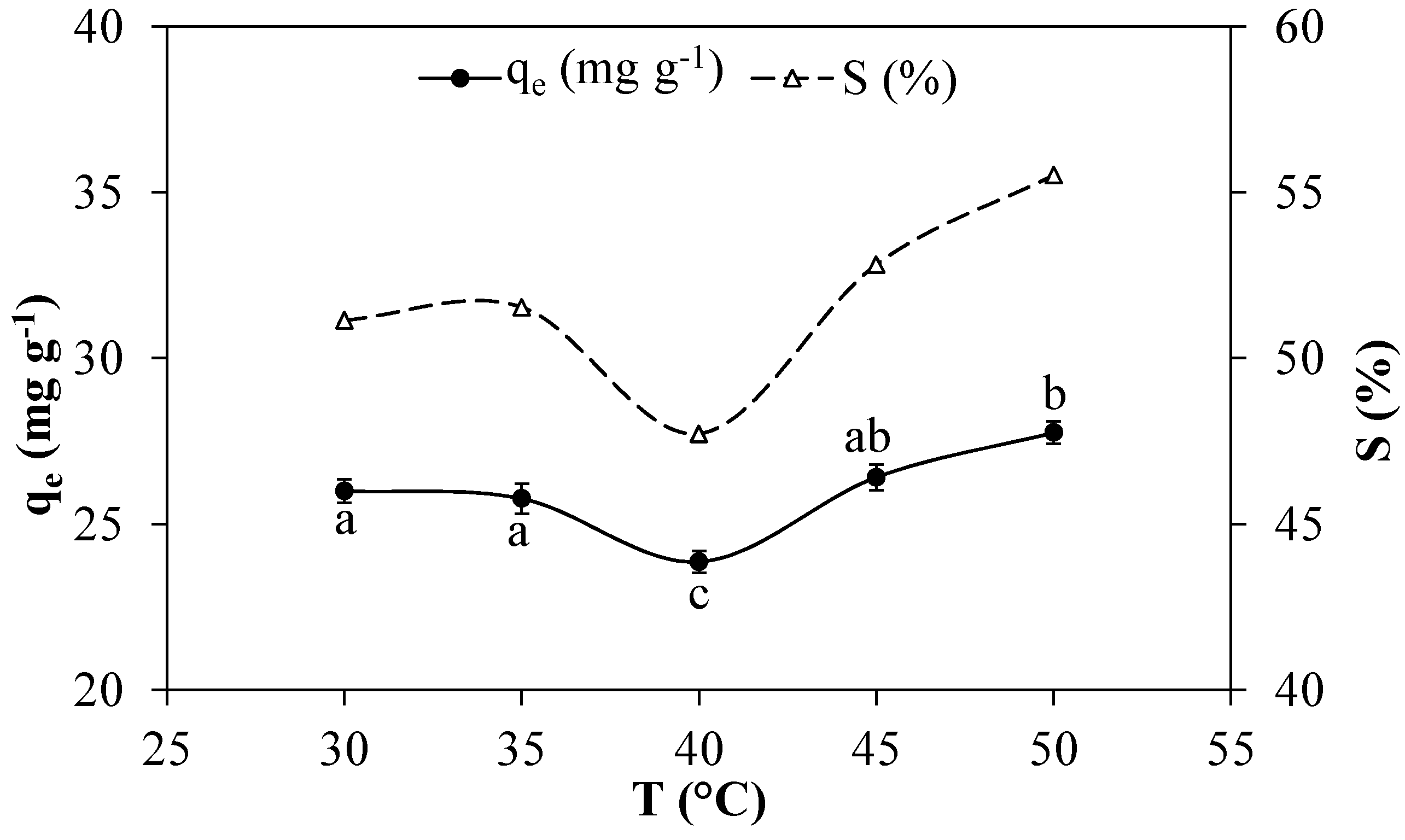
3.2.4. Effects of the Contact Time
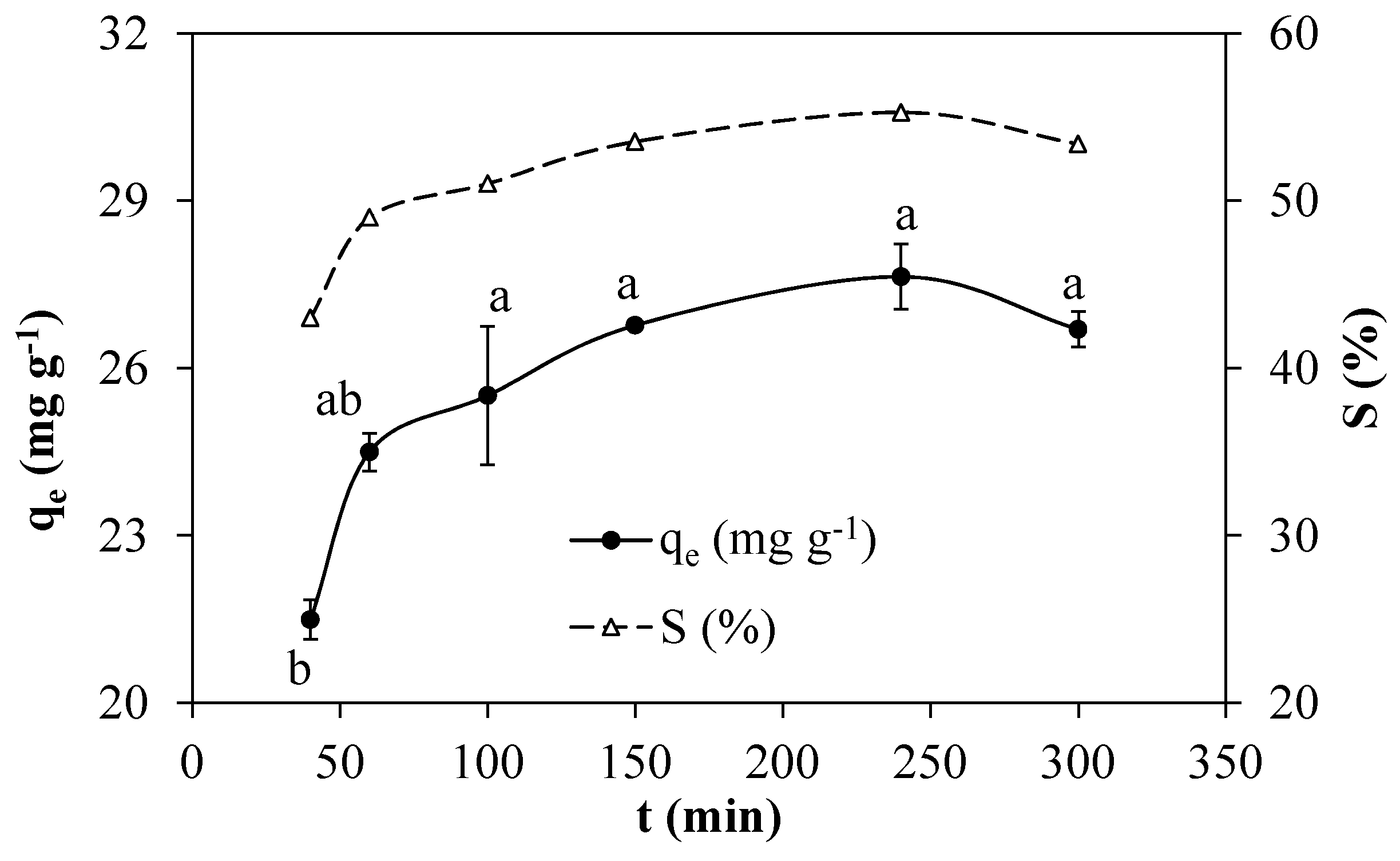
3.2.5. Effects of the Initial Adsorbate Concentration
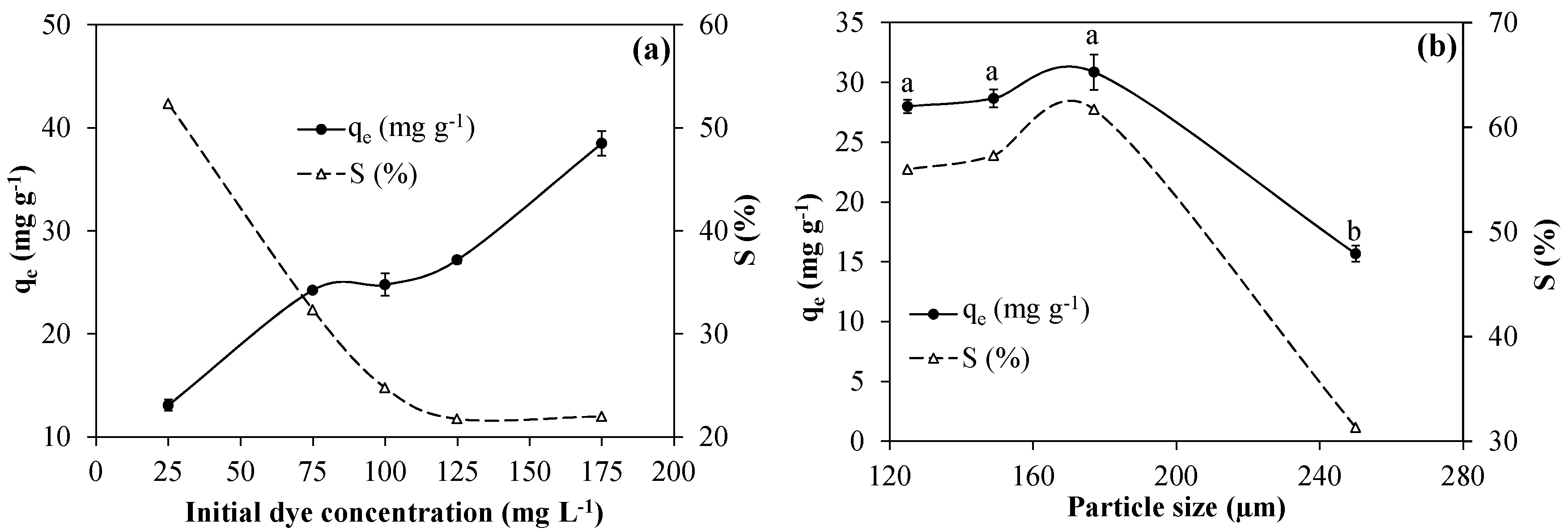
3.2.6. Effects of the Particle Size
3.3. Adsorption Equilibrium Isotherms
3.3.1. Langmuir Isotherm
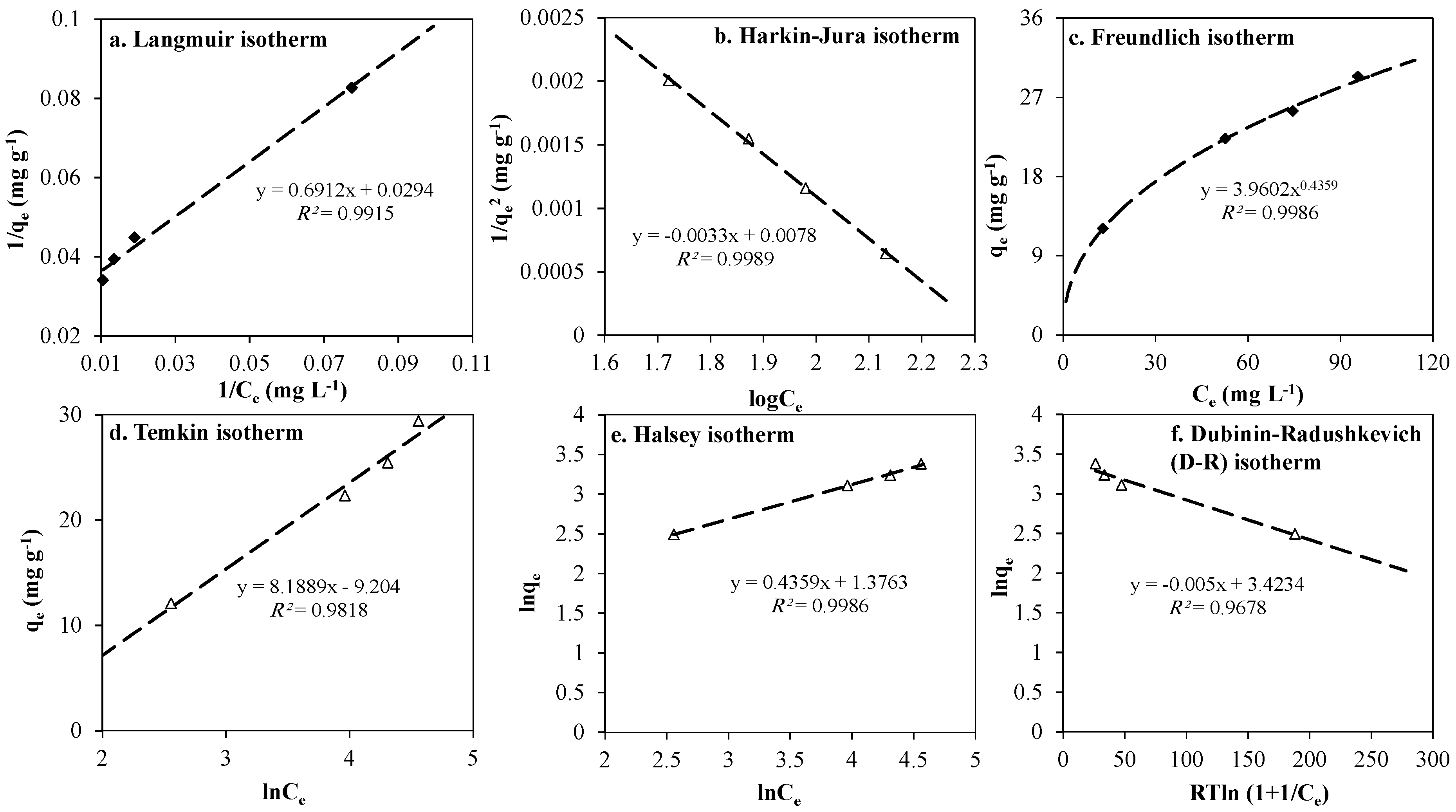
3.3.2. Harkin-Jura (H-J) Isotherm
| Isotherm | Parameter | Unit | Value |
|---|---|---|---|
| Langmuir | qexp | mg·g−1 | 29.38 |
| qmax | mg·g−1 | 34.01 | |
| Kads | L·mg−1 | 0.043 | |
| R2 | 0.9915 | ||
| Harkin-Jura | A | mg·g−1 | 303 |
| B | 2.36 | ||
| R2 | 0.9989 | ||
| Freundlich | 1/n | 0.4359 | |
| n | 2.294 | ||
| KF | (L·g−1) | 28.91 | |
| R2 | 0.9986 | ||
| Temkin | AT | L·mg−1 | 3.08 |
| bT | kJ·mol−1 | 312.85 | |
| R2 | 0.98182 | ||
| Halsey | qcal | 28.91 | |
| n | −2.29 | ||
| k | 23.51 | ||
| R2 | 0.9986 | ||
| Dubinin-Radushkevich | qDR | mol·g−1 | 30.67 |
| β | (mol·g−1)2 | 0.003 | |
| E | kJ·mol−1 | 14.14 | |
| R2 | 0.9678 | ||
3.3.3. Freundlich Isotherm
3.3.4. Temkin Isotherm
3.3.5. Halsey Isotherm
3.3.6. Dubinin–Radushkevich (D-R) isotherm
3.4. Adsorption Kinetics
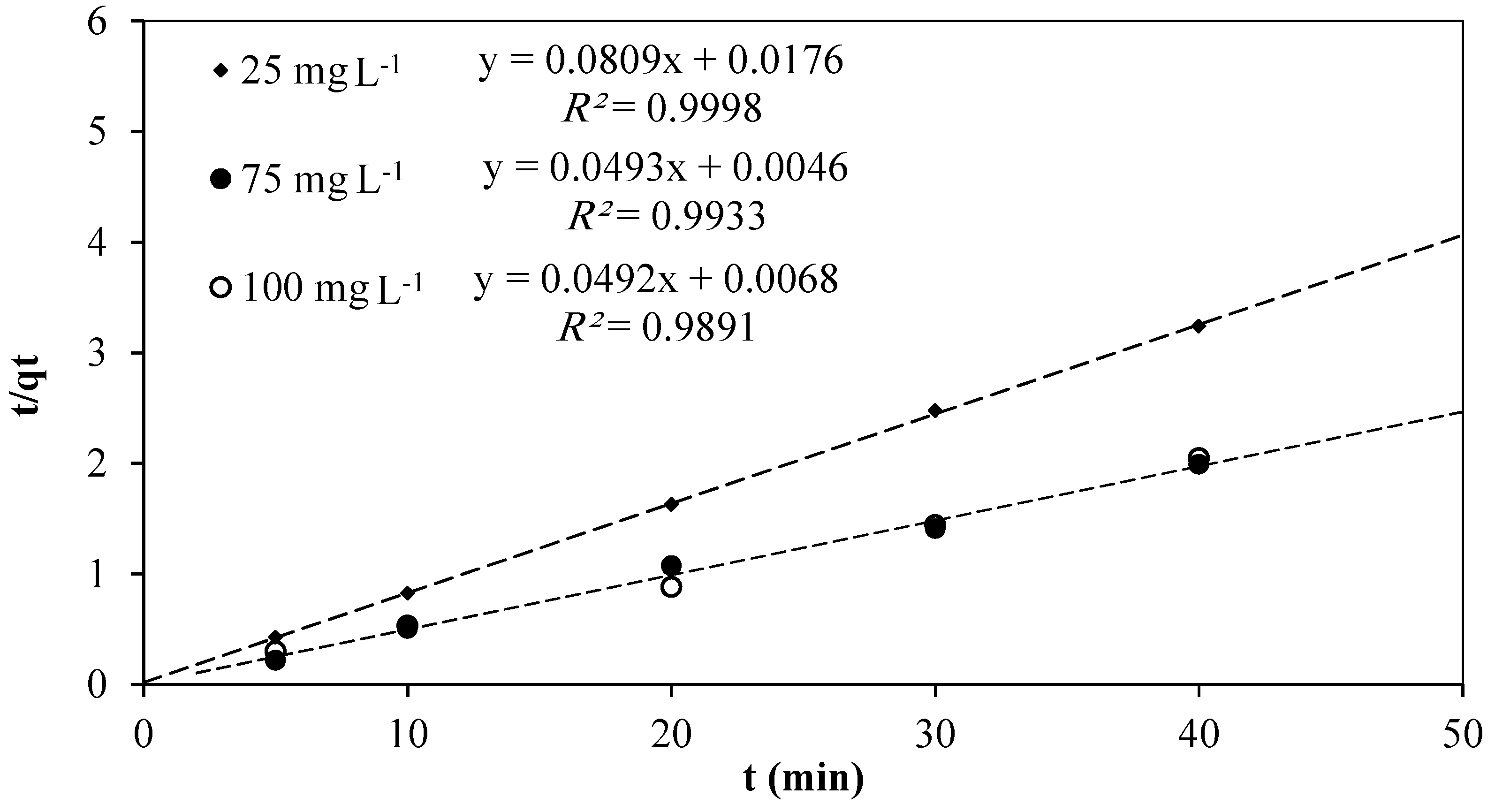
| Initial RB5 Dye Conc. (mg·L−1) | Pseudo-Second Order | ||||
|---|---|---|---|---|---|
| qe exp. (mg·g−1) | K2 (g·mg−1·min−1) | qe cal (mg·g−1) | H (mg·g−1·min−1) | R2 | |
| 25 | 13.07 | 0.3719 | 12.36 | 56.81 | 0.9998 |
| 75 | 27.18 | 0.5284 | 20.28 | 147.06 | 0.9933 |
| 100 | 22.71 | 0.3560 | 20.33 | 147.06 | 0.9891 |
3.5. Thermodynamic Study
| ΔH/(kJ·mol−1) | ΔS/(kJ·mol−1) | Δ G/(kJ·mol−1) | |||||
|---|---|---|---|---|---|---|---|
| 303 K | 308 K | 313 K | 318 K | 323 K | |||
| T1 | −0.0006 | 0.0011 | −0.335 | −0.341 | −0.346 | ||
| T2 | 0.0014 | −0.002 | 0.64 | 0.65 | 0.66 | ||
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mughal, M.J.; Saeed, R.; Naeem, M.; Ahmed, M.A.; Yasmien, A.; Siddiqui, Q.; Iqbal, M. Dye fixation and decolourization of vinyl sulphone reactive dyes by using dicyanidiamide fixer in the presence of ferric chloride. J. Saudi Chem. Soc. 2013, 17, 23–28. [Google Scholar] [CrossRef]
- Almeida, C.A.P.; Debacher, N.A.; Downs, A.J.; Cottet, L.; Mello, C.A.D. Removal of methylene blue from colored effluents by adsorption on montmorillonite clay. J. Colloid Interface Sci. 2009, 332, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Babu, B.R.; Parande, A.K.; Kumar, T.P. Cotton Textile Processing: Waste Generation and Effluent Treatment. J. Cotton Sci. 2007, 11, 141–153. [Google Scholar]
- Rai, H.S.; Bhattacharyya, M.S.; Singh, J.; Bansal, T.K.; Vats, P.; Banerjee, U.C. Removal of Dyes from the Effluent of Textile and Dyestuff Manufacturing Industry: A Review of Emerging Techniques With Reference to Biological Treatment. Crit. Rev. Environ. Sci. Technol. 2005, 35, 219–238. [Google Scholar] [CrossRef]
- Reddy, M.C.S.; Sivaramakrishna, L.; Reddy, A.V. The use of an agricultural waste material, Jujuba seeds for the removal of anionic dye (Congo red) from aqueous medium. J. Hazard. Mater. 2012, 203–204, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, K. Adsorption characteristics of the dye, Brilliant Green, on Neem leaf powder. Dyes Pigment. 2003, 57, 211–222. [Google Scholar] [CrossRef]
- Crini, G. Non-conventional low-cost adsorbents for dye removal: A review. Bioresour. Technol. 2006, 97, 1061–1085. [Google Scholar] [CrossRef] [PubMed]
- Robinson, T.; McMullan, G.; Marchant, R.; Nigam, P. Remediation of dyes in textile effluent: A critical review on current treatment technologies with a proposed alternative. Bioresour. Technol. 2001, 77, 247–255. [Google Scholar] [CrossRef]
- Suteu, D.; Biliuta, G.; Rusu, L.; Coseri, S.; Nacu, G. Cellulose Cellets as new type of Adsorbent for the removal of Dyes from aqueous media. Environ. Eng. Manag. J. 2015, 14, 525–532. [Google Scholar]
- Gupta, V.K.; Suhas. Application of low-cost adsorbents for dye removal—A review. J. Environ. Manag. 2009, 90, 2313–2342. [Google Scholar] [CrossRef] [PubMed]
- Etim, U.J.; Umoren, S.A.; Eduok, U.M. Coconut coir dust as a low cost adsorbent for the removal of cationic dye from aqueous solution. J. Saudi Chem. Soc. 2012. [Google Scholar] [CrossRef]
- Kanawade, S.M.; Gaikwad, R.W. Removal of Methylene Blue from Effluent by Using Activated Carbon and Water Hyacinth as Adsorbent. Int. J. Chem. Eng. Appl. 2011, 2, 317–319. [Google Scholar] [CrossRef]
- Khan, N.A.; Ibrahim, S.; Subramaniam, P. Elimination of heavy metals from wastewater using agricultural wastes as adsorbents. Malays. J. Sci. 2004, 23, 43. [Google Scholar]
- Parmar, M.; Thakur, L.S. Heavy metal Cu, Ni and Zn: Toxicity, health hazards and their removal techniques by low cost adsorbents: A short overview. Int. J. Plant Anim. Environ. Sci. 2013, 3, 143–157. [Google Scholar]
- Tahir, H.; Hammed, U.; Sultan, M.; Jahanzeb, Q. Batch adsorption technique for the removal of malachite green and fast green dyes by using montmorillonite clay as adsorbent. Afr. J. Biotechnol. 2010, 9, 8206–8214. [Google Scholar]
- Tahir, S.S.; Rauf, N. Removal of a cationic dye from aqueous solutions by adsorption onto bentonite clay. Chemosphere 2006, 63, 1842–1848. [Google Scholar] [CrossRef] [PubMed]
- Hameed, B.H.; Chin, L.H.; Rengaraj, S. Adsorption of 4-chlorophenol onto activated carbon prepared from rattan sawdust. Desalination 2008, 225, 185–198. [Google Scholar] [CrossRef]
- Rehman, M.S.; Kim, I.; Han, J.-I. Adsorption of methylene blue dye from aqueous solution by sugar extracted spent rice biomass. Carbohydr. Polym. 2012, 90, 1314–1322. [Google Scholar] [CrossRef] [PubMed]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 385–471. [Google Scholar]
- Nguyen, C.; Do, D.D. The Dubinin-Radushkevich equation and the underlying microscopic adsorption description. Carbon 2001, 39, 1327–1336. [Google Scholar] [CrossRef]
- Temkin, M.J.; Pyzhev, V. Kinetics of the Synthesis of Ammonia on Promoted Iron Catalysts. Acta Physicochim. 1940, 12, 217–222. [Google Scholar]
- Garg, U.K.; Kaur, M.P.; Garg, V.K.; Sud, D. Removal of hexavalent chromium from aqueous solution by agricultural waste biomass. J. Hazard. Mater. 2007, 140, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Al-Duri, B. Kinetic modeling of liquid-phase adsorption of reactive dyes on activated carbon. J. Colloid Interface Sci. 2005, 287, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Hall, K.R.; Eagleton, L.C.; Acrivos, A.; Vermeulen, T. Pore- and Solid-Diffusion Kinetics in Fixed-Bed Adsorption under Constant-Pattern Conditions. Ind. Eng. Chem. Fundam. 1966, 5, 212–223. [Google Scholar] [CrossRef]
- Boraa, M.; Gangulia, J.N.; Duttaa, D.K. Thermal and spectroscopic studies on the decomposition of [Ni{di(2-aminoethyl)amine}2]- and [Ni(2,20:60,200-terpyridine)2]-Montmorillonite intercalated composites. Thermochim. Acta 2000, 346, 169–175. [Google Scholar] [CrossRef]
- Aharoni, C.; Ungarish, M. Kinetics of activated chemisorption. Part 2.—Theoretical models. J. Chem. Soc. Faraday Trans. 1977, 73, 456–464. [Google Scholar] [CrossRef]
- Kumar, P.S.; Ramakrishnan, K.; Gayathri, R. Removal of Nickel (II) from Aqueous Solutions by Ceralite IR 120 Cationic Exchange Resins. J. Eng. Sci. Technol. 2010, 5, 232–243. [Google Scholar]
- AlSawalha, M.; Novikova, L.; Roessner, F.; Bel’chinskaya, L. Acidity of different Jordanian Clays characterized by TPD-NH3 and MBOH Conversion. World Acad. Sci. Eng. Technol. 2011, 5, 7–29. [Google Scholar]
- Leite, I.F.; Soares, A.P.S.; Carvalho, L.H.; Raposo, C.M.O.; Malta, O.M.L.; Silva, S.M.L. Characterization of pristine and purified organobentonites. J. Therm. Anal. Calorim. 2009, 100, 563–569. [Google Scholar] [CrossRef]
- Madejová, J. FTIR techniques in clay mineral studies. Vib. Spectrosc. 2003, 31, 1–10. [Google Scholar] [CrossRef]
- Tomul, F.; Balci, S. Synthesis and Characterization of Al-Pillared Interlayered Bentonites. GU J. Sci. 2008, 21, 21–31. [Google Scholar]
- Cótica, L.F.; Freitas, V.F.; Santos, I.A.; Barabach, M.; Anaissi, F.J.; Miyahara, R.Y.; Sarvezuk, P.W.C. Cobalt-modified Brazilian bentonites: Preparation, characterisation, and thermal stability. Appl. Clay Sci. 2011, 51, 187–191. [Google Scholar] [CrossRef]
- Shao, D.D.; Xu, D.; Wang, S.W.; Fang, Q.H.; Wang, W.S.; Dong, Y.H.; Wang, X.K. Modeling of radionickel sorption on MX-80 bentonite as a function of pH and ionic strength. Sci. China Ser. B Chem. 2008, 52, 362–371. [Google Scholar] [CrossRef]
- Zeng, G.; Zhang, C.; Huang, G.; Yu, J.; Wang, Q.; Li, J.; Xi, B.; Liu, H. Adsorption behavior of bisphenol A on sediments in Xiangjiang River, Central-south China. Chemosphere 2006, 65, 1490–1499. [Google Scholar] [CrossRef] [PubMed]
- Kalavathy, M.H.; Miranda, L.R. Comparison of copper adsorption from aqueous solution using modified and unmodified Hevea brasiliensis saw dust. Desalination 2010, 255, 165–174. [Google Scholar] [CrossRef]
- Srivastava, V.C.; Mall, I.D.; Mishra, I.M. Characterization of mesoporous rice husk ash (RHA) and adsorption kinetics of metal ions from aqueous solution onto RHA. J. Hazard. Mater. 2006, 134, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Ahmaruzzaman, M.; Gayatri, S.L. Activated Tea Waste as a Potential Low-Cost Adsorbent for the Removal of p-Nitrophenol from Wastewater. J. Chem. Eng. Data 2010, 55, 4614–4623. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amin, M.T.; Alazba, A.A.; Shafiq, M. Adsorptive Removal of Reactive Black 5 from Wastewater Using Bentonite Clay: Isotherms, Kinetics and Thermodynamics. Sustainability 2015, 7, 15302-15318. https://doi.org/10.3390/su71115302
Amin MT, Alazba AA, Shafiq M. Adsorptive Removal of Reactive Black 5 from Wastewater Using Bentonite Clay: Isotherms, Kinetics and Thermodynamics. Sustainability. 2015; 7(11):15302-15318. https://doi.org/10.3390/su71115302
Chicago/Turabian StyleAmin, Muhammad Tahir, Abdulrahman Ali Alazba, and Muhammad Shafiq. 2015. "Adsorptive Removal of Reactive Black 5 from Wastewater Using Bentonite Clay: Isotherms, Kinetics and Thermodynamics" Sustainability 7, no. 11: 15302-15318. https://doi.org/10.3390/su71115302





