Is It Possible to Maintain the Quantity and Quality of Winter Wheat Grain by Replacing Part of the Mineral Nitrogen Dose by Growth Activators and Plant Growth-Promoting Rhizobacteria (PGPR)?
Abstract
1. Introduction
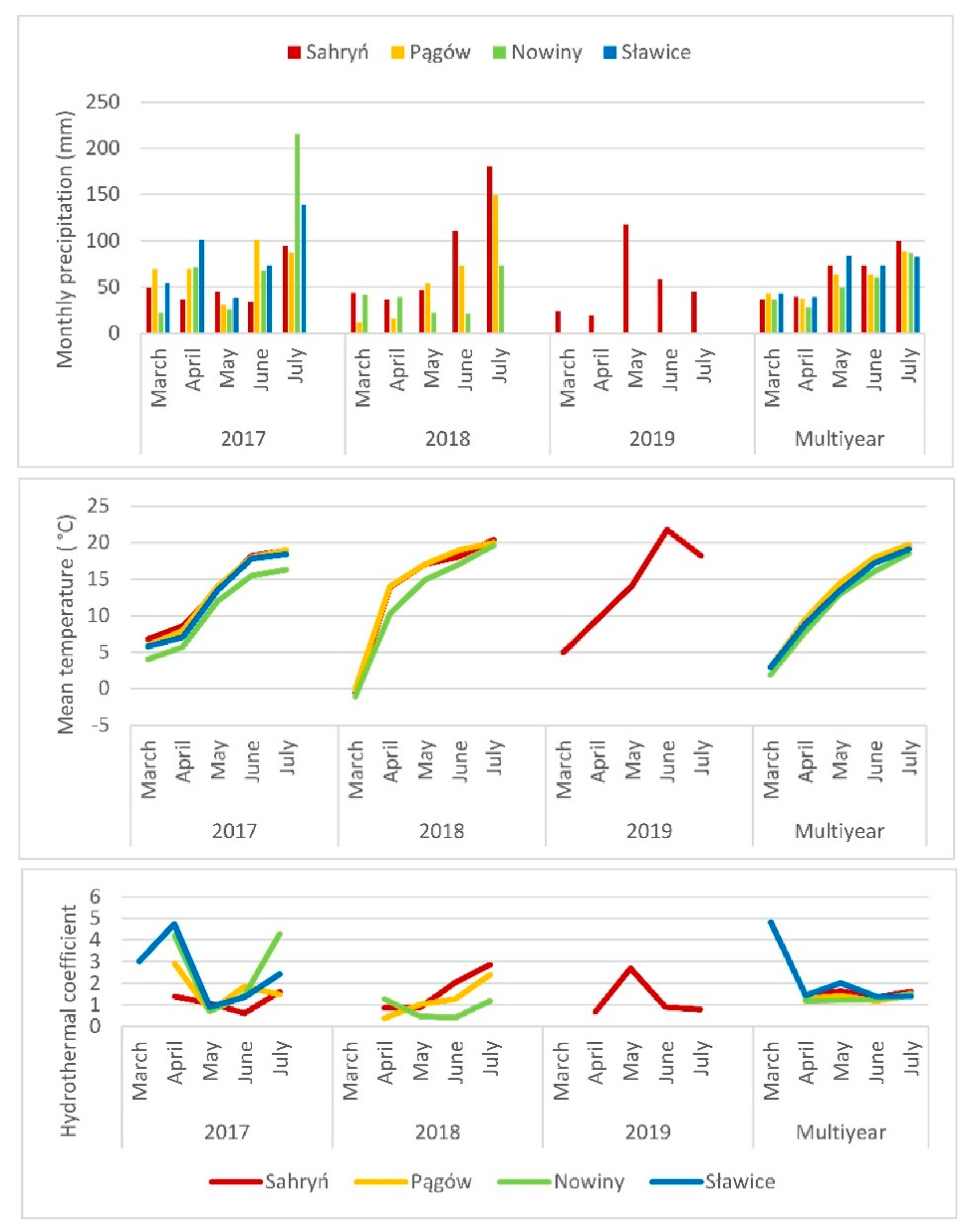
2. Materials and Methods
- Polifoska 6 (N in the form of ammonium—6%, P in the form of mono- and diammonium phosphate—8.7%, K in the form of potassium chloride—24.9%, S in the form of sulphate—2.8%);
- Polifoska 6 Tytan (N in the ammonium form—6%, P in the form of mono- and diammonium—10.9%, K in the form of potassium chloride—20.8%, S in the form of sulphate—2%, Fe—0.5%, Zn—0.05% + Ti);
- Amofoska 3,5-10-24 (N in ammonium form—3.5%, P—4.4%, K—19.9%);
- Potassium salt (K in the form of potassium chloride—49.8%);
- Polidap—ammonium phosphate (N in the ammonium form—18%, P—20%, S—2%);
- Saletrosan 26 (N in the nitrate form—7%, N in the ammonium form 19%, S in the form of sulphate—13%);
- Saletrosan 30 (N in the nitrate form—12%, N in the ammonium form 18%, S in the form of sulphate—7%);
- RSM 32: urea-ammonium nitrate solution (8% N in nitrate form, 8% N in ammonium form, 16% N in amide form);
- Urea (N in the amide form—46%);
- Ammonium nitrate 32% (N in the nitrate form—16%, N in ammonium form—16%);
- Ammonium nitrate 34% (N in the nitrate form—17%, N in ammonium form—17%).
- 0
- control (full nitrogen fertilization dose depending on location—111–238 kg ha−1 N);
- 1
- dose of mineral nitrogen lower by 30% in comparison to full dose before sowing and during vegetation (I the dose of nitrogen in spring is the same as in the control, II and III reduced dose)—97 to 167 kg ha−1 N; Penergetic-K (400 g ha−1)—on the straw (winter rapeseed, maize) of the forecrop or leaves of sugar beet before it is mixed with the soil; Penergetic-K (400 g ha−1) with the first pesticide spray in spring; Penergetic-P (300 g ha−1) with a second pesticide spray in spring; Penergetic-P (300 g ha−1)—3 weeks later;
- 2
- dose of mineral nitrogen lower by 30% in comparison to full dose before sowing and during vegetation (I the nitrogen dose in spring is the same as in the control, II and III reduced dose)—97 to 167 kg ha−1 N; Penergetic-K (400 g ha−1) + Azoter (10 dm3 ha−1)—on the straw (winter rapeseed, maize) of the forecrop or sugar beet leaves before it is mixed with the soil; Penergetic-K (400 g ha−1) + Azoter (10 dm3 ha−1) with the first pesticide spray in spring; Penergetic-P (300 g ha−1) with the second pesticide spray in spring; Penergetic-P (300 g dm3 ha−1)—3 weeks later.
- Grain yield at moisture 14%, t ha−1;
- Grain moisture, %;
- Yield of straw, t ha−1;
- Spike density, pcs. m−2;
- Number of non-productive shoots, pcs. m−2;
- Total number of shoots, pcs. m−2;
- Weight of 1000 grains (14% H2O), g;
- Protein content, % d.m.;
- Content of wet gluten, % d.m.;
- Grain uniformity (fractions separated at sieves 2.5 × 25 mm), %;
- Hagberg falling number, s;
- Zeleny sedimentation value (SDS), ml;
- Height of plants, cm;
- Number of grains per spike, pcs.
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. Food and Agriculture Data. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 7 April 2021).
- Golba, J.; Rozbicki, J.; Gozdowski, D.; Sas, D.; Mądry, W.; Piechociński, M.; Murzyńska, L.; Studnicki, M.; Derejko, A. Adjusting yield components under different levels of N applications in winter wheat. Int. J. Plant Prod. 2013, 7, 139–150. [Google Scholar] [CrossRef]
- Polish Council of Ministers. Regulation of the Polish Council of Ministers on the Adoption of the Action Program to Reduce Water Pollution with Nitrates from Agricultural Sources and to Prevent Further Pollution. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20200000243 (accessed on 21 May 2021).
- Kadziuliene, Z.; Feiziene, D.; Leistrumaite, A.; Semaskiene, R. Peculiarities of some legumes and cereals under organic farming system. NJF Rep. 2005, 1, 103–106. [Google Scholar]
- Pekarskas, J.; Vilkenyte, L.; Sileikiene, D.; Cesoniene, L.; Makarenko, N. Effect of organic nitrogen fertilizers Provita and fermentator Penergetic–K winter wheat and on soil quality. In Proceedings of the 8th International Conference, Environmental Engineering, Vilnius, Lithuania, 19–20 May 2011; pp. 248–254. [Google Scholar]
- Artyszak, A.; Gozdowski, D. Is it possible to replace part of the mineral nitrogen dose in maize for grain by using growth activators and plant growth-promoting rhizobacteria? Agronomy 2020, 10, 1647. [Google Scholar] [CrossRef]
- Franco Junior, K.S.; Terra, A.B.C.; Teruel, T.R.; Mantovani, J.R.; Florentino, L.A. Effect of cover crops and bioactivators in coffee and chemical properties of soil. Coffee Sci. Lavras 2018, 13, 559–567. [Google Scholar]
- De Souza, A.A.; de Almeida, F.Z.; Alberton, O. Growth and yield of soybean with Penergetic application. Sci. Agrar. 2017, 18, 95–98. [Google Scholar]
- Brito, O.R.; Dequech, F.K.; Brito, R.M. Use of Penergetic products P and K in the snap bean production. Annu. Rep. 2012, 55, 279–280. [Google Scholar]
- Jankauskienė, J.; Survilienė, E. Influence of growth regulators on seed germination energy and biometrical parameters of vegetables Scientific works of the Lithuanian Institute of Horticulture and Lithuanian University of Agriculture. Sodininkystė Ir Daržininkystė 2009, 28, 69–77. [Google Scholar]
- Nascente, A.S.; Cobucci, T. Phosphate fertilization in the soil and Penergetic application in the grain yield of common bean. Soils Embrace Life and Universe. In Proceedings of the 20th World Congress of Soil Science, Jeju, Korea, 8–13 June 2014. [Google Scholar]
- Jakiene, E.; Venskutonis, V.; Mickevicius, V. The effect of additional fertilization with liquid complex fertilizers and growth regulators on potato productivity. Scientific works of the Lithuanian Institute of Horticulture and Lithuanian University of Agriculture. Sodininkystė Ir Daržininkystė 2008, 27, 259–267. [Google Scholar]
- Jakiene, E.; Venskutonis, V.; Liakas, V. Fertilization of sugar beet root with ecological fertilizers. Agron. Res. 2009, 7, 269–276. [Google Scholar]
- Artyszak, A.; Gozdowski, D. The effect of growth activators and plant growth-promoting rhizobacteria (PGPR) on the soil properties, root yield, and technological quality of sugar beet. Agronomy 2020, 10, 1262. [Google Scholar] [CrossRef]
- Nunes, P.H.M.P.; Aquino, L.A.; dos Santos, L.P.D.; Xavier, F.O.; Dezordi, L.R.; Assunção, N.S. Yield of the irrigated wheat crop subjected to nitrogen application and to inoculation with Azospirillum brasilense. Rev. Bras. Ciênc. Sol. 2015, 39, 174–182. [Google Scholar] [CrossRef]
- Hungria, M.; Campo, R.J.; Souza, E.M.; Pedrosa, F.O. Inoculation with selected strains of Azospirillum brasilense and A. lipoferum improves yields of maize and wheat in Brazil. Plant Soil 2010, 331, 413–425. [Google Scholar] [CrossRef]
- Piccinin, G.G.; Braccini, A.L.; Dan, L.G.M.; Scapim, C.A.; Ricci, T.T.; Bazo, G.L. Efficiency of seed inoculation with Azospirillum brasilense on agronomic characteristics and yield of wheat. Industr. Crops Prod. 2013, 43, 393–397. [Google Scholar] [CrossRef]
- Pereira, L.C.; Piana, S.C.; Braccini, A.L.; Garcia, M.M.; Ferri, G.C.; Felber, P.H.; Marteli, D.C.V.; Dametto, P.B.I. Wheat (Triticum aestivum) yield response to different inoculation techniques of Azospirillum brasilense. Rev. Ciênc. Agrár. 2017, 40, 105–113. [Google Scholar] [CrossRef]
- Salvo, L.P.; Ferrando, L.; Fernandéz-Scavino, A.; Salamone, I.E.G. Microorganisms reveal what plants do not: Wheat growth and rhizosphere microbial communities after Azospirillum brasilense inoculation and nitrogen fertilization under field conditions. Plant Soil 2018, 424, 405–417. [Google Scholar] [CrossRef]
- Galindo, F.S.; Filho, M.C.M.T.; Buzetti, S.; Rodrigues, W.L.; Santini, J.M.K.; Alves, C.J. Nitrogen fertilisation efficiency and wheat grain yield affected by nitrogen doses and sources associated with Azospirillum brasilense. Acta Agr. Scand. Sec. B Soil Plant Sci. 2019, 69, 606–617. [Google Scholar] [CrossRef]
- Galindo, F.S.; Filho, M.C.M.T.; Buzetti, S.; Santini, J.M.K.; Montanari, R.; Freitas, L.A.; Rodrigues, W.L. Micronutrient accumulation with Azospirillum Brasilense associated with nitrogen fertilization management in wheat. Commun. Soil Sci. Plan. 2019, 2429–2441. [Google Scholar] [CrossRef]
- Munareto, J.D.; Martin, T.N.; Fipke, G.M.; Cunha, V.S.; da Rosa, G.B. Nitrogen management alternatives using Azospirillum brasilense in wheat. Pesq. Agropec. Bras. 2019, 54, e00276. [Google Scholar] [CrossRef]
- Zaheer, M.S.; Raza, M.A.S.; Saleem, M.F.; Khan, I.H.; Ahmad, S.; Iqbal, R.; Manevski, K. Investigating the effect of Azospirillum brasilense and Rhizobium pisi on agronomic traits of wheat (Triticum aestivum L.). Arch. Agron. Soil Sci. 2019, 65, 1554–1564. [Google Scholar] [CrossRef]
- Zaheer, M.S.; Raza, M.A.S.; Saleem, M.F.; Erinle, K.O.; Iqbal, R.; Ahmad, S. Effect of rhizobacteria and cytokinins application on wheat growth and yield under normal vs drought conditions. Commun. Soil Sci. Plan. 2019, 50, 2521–2533. [Google Scholar] [CrossRef]
- Galindo, F.S.; Buzetti, S.; Rodrigues, W.L.; Boleta, E.H.M.; Silva, V.M.; Tavanti, R.F.R.; Fernandes, G.C.; Biagini, A.L.C.; Rosa, P.A.L.; Filho, M.C.M.T. Inoculation of Azospirillum brasilense associated with silicon as a liming source to improve nitrogen fertilization in wheat crops. Sci. Rep. 2020, 10, 6160. [Google Scholar] [CrossRef] [PubMed]
- Galindo, F.S.; Filho, M.C.M.T.; Buzetti, S.; Santini, J.M.K.; Boleta, E.H.M.; Rodrigues, W.L. Macronutrient accumulation in wheat crop (Triticum aestivum L.) with Azospirillum brasilense associated with nitrogen doses and sources. J. Plant Nutr. 2020, 43, 1057–1069. [Google Scholar] [CrossRef]
- Galindo, F.S.; Filho, M.C.M.T.; Buzetti, S.; Pagliari, P.H.; Santini, J.M.K. Can NBPT urease inhibitor in combination with Azospirillum brasilense inoculation improve wheat development? Nutr. Cycl. Agroecosys. 2020, 117, 131–143. [Google Scholar] [CrossRef]
- Juknevičius, D.; Kriaučiūnienė, A.; Jasinskas, A.; Šarauskis, E. Analysis of changes in soil organic carbon, energy consumption and environmental impact using bio-products in the production of winter wheat and oilseed Rape. Sustainability 2020, 12, 8246. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; FAO: Rome, Italy, 2015. [Google Scholar]
- PKN. PN-ISO 10390:1997. Soil Quality—pH Determination; Polish Committee for Standardization: Warsaw, Poland, 1997. [Google Scholar]
- Regional Agrochemical Station. Research Procedure of the Regional Agrochemical Station in Warsaw, 2009; Regional Agrochemical Station: Warsaw, Poland, 2009. [Google Scholar]
- Regional Agrochemical Station. Research Procedure of the Regional Agrochemical Station in Warsaw, 2017; Regional Agrochemical Station: Warsaw, Poland, 2017. [Google Scholar]
- PKN. Polish Standard PN-R-04023:1996. Agro-Chemical Analysis of Soil—Determination of Available Phosphorus. Content in Mineral Soils; Polish Committee for Standardization: Warsaw, Poland, 1996. [Google Scholar]
- PKN. Polish Standard PN-R-04022:1996/Az1:2002. Agro-Chemical Analysis of Soil—Determination of Available Potassium Content in Mineral Soils; Polish Committee for Standardization: Warsaw, Poland, 1996. [Google Scholar]
- PKN. Polish Standard PN-R-04020:1994/Az1:2004. Agro-Chemical Analysis of Soil—Determination of Available Magnesium Content in Mineral Soils; Polish Committee for Standardization: Warsaw, Poland, 1994. [Google Scholar]
- PKN. Polish Standard PN-93/R-04018. Agro-Chemical Analysis of Soil. Determination of Available Boron; Polish Committee for Standardization: Warsaw, Poland, 1993. [Google Scholar]
- PKN. Polish Standard PN-92/R-04017. Agro-Chemical Analysis of Soil. Determination of Available Copper; Polish Committee for Standardization: Warsaw, Poland, 1992. [Google Scholar]
- PKN. Polish Standard PN-R-04021: 1994. Agro-Chemical Analysis of Soil. Determination of Available Iron; Polish Committee for Standardization: Warsaw, Poland, 1994. [Google Scholar]
- PKN. Polish Standard PN-93/R-04019. Agro-Chemical Analysis of Soil. Determination of Available Manganese; Polish Committee for Standardization: Warsaw, Poland, 1993. [Google Scholar]
- PKN. Polish Standard PN-92/R-04016. Agro-Chemical Analysis of Soil. Determination of Available Zinc; Polish Committee for Standardization: Warsaw, Poland, 1992. [Google Scholar]
- AACC International. Approved Methods of the American Association of Cereal Chemists, 11th ed.; The Association AACC International: St. Paul, MN, USA, 2015. [Google Scholar]
- ISO 3093:2009. Wheat, Rye and Their Flours, Durum Wheat and Durum Wheat Semolina—Determination of the Falling Number According to Hagberg-Perten; International Organization for Standardization: Geneva, Switzerland, 2009. [Google Scholar]
- PKN. Polish Industry Standard BN-69 9131-02. Cereals Grain. Determination of Grain Uniformity; Polish Committee for Standardization: Warsaw, Poland, 1969. [Google Scholar]
- PKN. Polish Standard PN-EN ISO 520. Cereals and Pulses—Determination of the Mass 1000 Grains; Polish Committee for Standardization: Warsaw, Poland, 2010. [Google Scholar]
- Salantur, A.; Ozturk, A.; Akten, S. Growth and yield response of spring wheat (Triticum aestivum L.) to inoculation with rhizobacteria. Plant Soil Environ. 2011, 52, 111–118. [Google Scholar] [CrossRef]
- Kumar, V.; Behl, R.K.; Narula, N. Establishment of phosphate-solubilizing strains of Azotobacter Chroococcum in the rhizosphere and their effect on wheat cultivars under green house conditions. Microbiol. Res. 2001, 156, 87–93. [Google Scholar] [CrossRef]
- Dal Cortivo, C.; Ferrari, M.; Visioli, G.; Lauro, M.; Fornasier, F.; Barion, G.; Panozzo, A.; Vamerali, T. Effects of seed-applied biofertilizers on rhizosphere biodiversity and growth of common wheat (Triticum aestivum L.) in the field. Front. Plant Sci. 2020, 11, 72. [Google Scholar] [CrossRef]
- Alves, C.J.; Arf, O.; Ramos, A.F.; Galindo, F.S.; Nogueira, L.M.; Rodrigues, R.A.F. Irrigated wheat subjected to inoculation with Azospirillum brasilense and nitrogen doses as top-dressing. R. Bras. Eng. Agr. Amb. 2017, 21, 537–542. [Google Scholar] [CrossRef][Green Version]
- Galindo, F.S.; Filho, M.C.M.T.; Buzetti, S.; Santini, J.M.K.; Alves, C.J.; Ludkiewicz, M.G.Z. Wheat yield in the Cerrado as affected by nitrogen fertilization and inoculation with Azospirillum brasilense. Pesqui. Agropecu. Bras. 2017, 52, 794–805. [Google Scholar] [CrossRef]
- Fahsi, N.; Mahdi, I.; Mesfioui, A.; Biskri, L.; Allaoui, A. Plant Growth-Promoting Rhizobacteria isolated from the Jujube (Ziziphus lotus) plant enhance wheat growth, Zn uptake, and heavy metal tolerance. Agriculture 2021, 11, 316. [Google Scholar] [CrossRef]
- Nagaraju, Y.; Triveni, S.; Vijaya Gopal, A.; Thirumal, G.; Prasanna Kumar, B.; Jhansi, P. In vitro Screening of Zn solubilizing and Potassium releasing isolates for Plant Growth Promoting (PGP) characters. Bull. Environ. Pharmacol. Life Sci. 2017, 6, 590–597. [Google Scholar]
- Rahman, C.H.; Ahcene, B.; Miloud, B.; Rachid, D. Screening and characterization of plant growth promoting traits of phosphate solubilizing bacteria isolated from wheat rhizosphere of Algerian saline soil. Malays. J. Microbiol. 2017, 13, 124–131. [Google Scholar]
- Mukhtar, S.; Shahid, I.; Mehnaz, S.; Malik, K.A. Assessment of two carrier materials for phosphate solubilizing biofertilizers and their effect on growth of wheat (Triticum aestivum L.). Microbiol. Res. 2017, 205, 107–117. [Google Scholar] [CrossRef]
- Boubekri, K.; Soumare, A.; Mardad, I.; Lyamlouli, K.; Hafidi, M.; Ouhdouch, Y.; Kouisni, L. The Screening of potassium- and phosphate-solubilizing actinobacteria and the assessment of their ability to promote wheat growth parameters. Microorganisms 2021, 9, 470. [Google Scholar] [CrossRef] [PubMed]
- Abaid-Ullah, M.; Nadeem, M.; Hassan, M.; Ganter, J.; Muhammad, B.; Nawaz, K.; Shah, A.S.; Hafeez, F.Y. Plant Growth Promoting Rhizobacteria: An alternate way to improve yield and quality of wheat (Triticum aestivum). Int. J. Agric. Biol. 2015, 17, 51–60. [Google Scholar]
- Timmusk, S.; Abd El-Daim, I.A.; Copolovici, L.; Tanilas, T.; Kännaste, A.; Behers, L.; Nevo, E.; Seisenbaeva, G.; Stenström, E.; Niinemets, Ü. Drought-tolerance of wheat improved by rhizosphere bacteria from harsh environments: Enhanced biomass production and reduced emissions of stress volatiles. PLoS ONE 2014, 9, e96086. [Google Scholar] [CrossRef]
- Ilyas, N.; Mumtaz, K.; Akhtar, N.; Yasmin, H.; Sayyed, R.Z.; Khan, W.; El Enshasy, H.A.; Dailin, D.J.; Elsayed, E.A.; Ali, Z. Exopolysaccharides producing bacteria for the amelioration of drought stress in wheat. Sustainability 2020, 12, 8876. [Google Scholar] [CrossRef]
- Barnawal, D.; Bharti, N.; Pandey, S.S.; Pandey, A.; Chanotiya, C.S.; Kalra, A. Plant growth-promoting rhizobacteria enhance wheat salt and drought stress tolerance by altering endogenous phytohormone levels and TaCTR1/TaDREB2 expression. Physiol. Plant. 2017, 161, 502–514. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Yadav, A.N.; Khannam, K.S.; Kumar, S.; Saxena, A.K.; Suman, A. Molecular diversity and multifarious plant growth promoting attributes of Bacilli associated with wheat (Triticum aestivum L.) rhizosphere from six diverse agro-ecological zones of India. J. Basic Microbiol. 2016, 56, 44–58. [Google Scholar] [CrossRef]
- Chrouqi, L.; Lahcen, O.; Jadrane, I.; Koussa, T.; Alfeddy, M.N. Screening of soil rhizobacteria isolated from wheat plants grown in the Marrakech Region (Morocco, North Africa) for plant growth promoting activities. JMES 2017, 8, 3382–3390. [Google Scholar]
- Bharti, N.; Pandey, S.S.; Barnawal, D.; Patel, V.K.; Kalra, A. Plant growth promoting rhizobacteria Dietzia natronolimnaea modulates the expression of stress responsive genes providing protection of wheat from salinity stress. Sci. Rep. 2016, 6, 34768. [Google Scholar] [CrossRef]
- Gontia-Mishra, I.; Sapre, S.; Sharma, A.; Tiwari, S. Alleviation of mercury toxicity in wheat by the interaction of mercury-tolerant Plant Growth-Promoting Rhizobacteria. J. Plant Growth Regul. 2016, 35, 1000–1012. [Google Scholar] [CrossRef]
- Rana, A.; Saharan, B.; Joshi, M.; Prasanna, R.; Kumar, K.; Nain, L. Identification of multi-trait PGPR isolates and evaluating their potential as inoculants for wheat. Ann. Microbiol. 2011, 61, 893–900. [Google Scholar] [CrossRef]
| Treatment | p-Value Based on ANOVA | |||||
|---|---|---|---|---|---|---|
| Trait | 0 | 1 | 2 | Treat-Ment (T) | Environ-Ment (E: Year × Location) | Inter-Action: T × E |
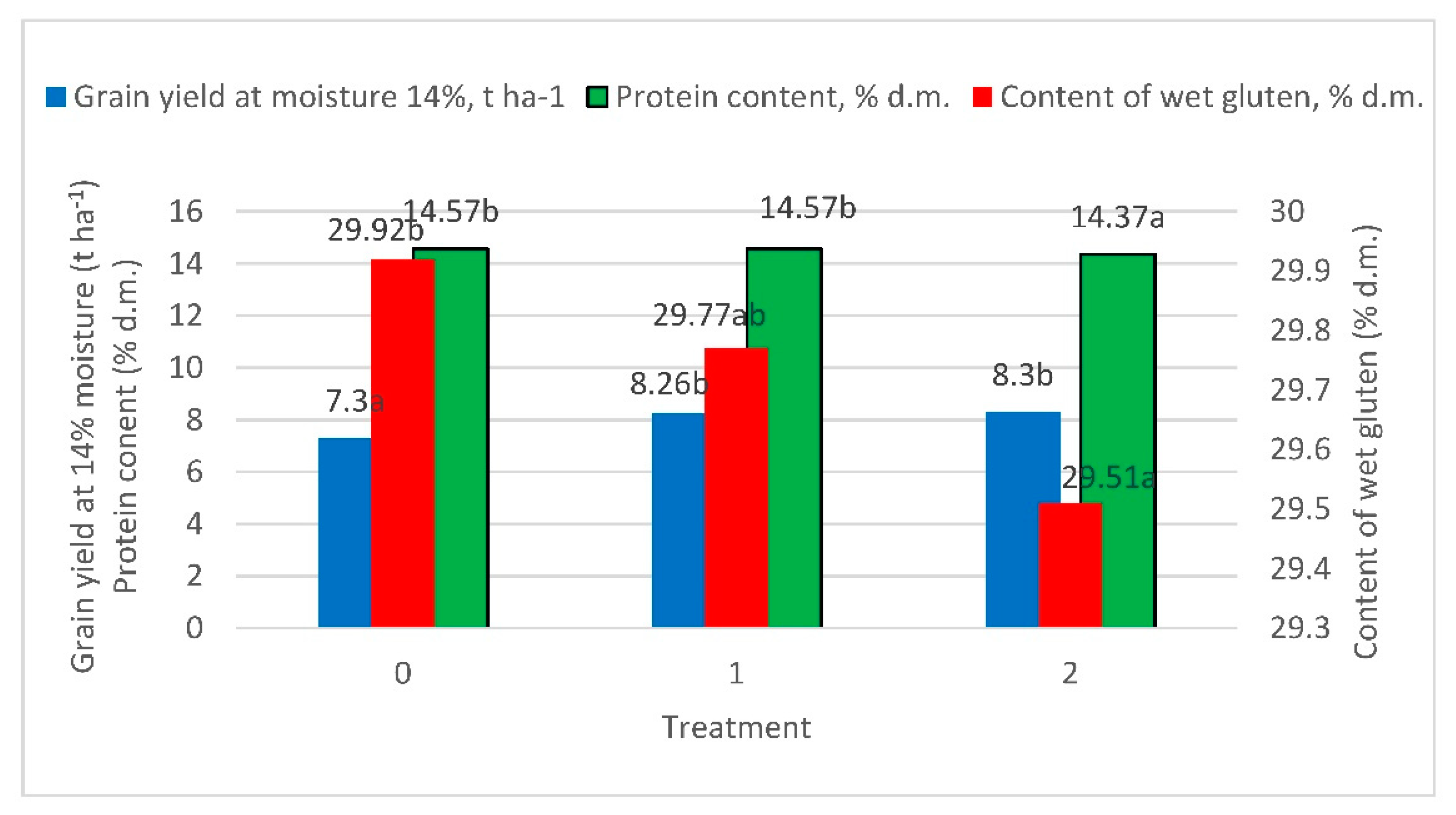
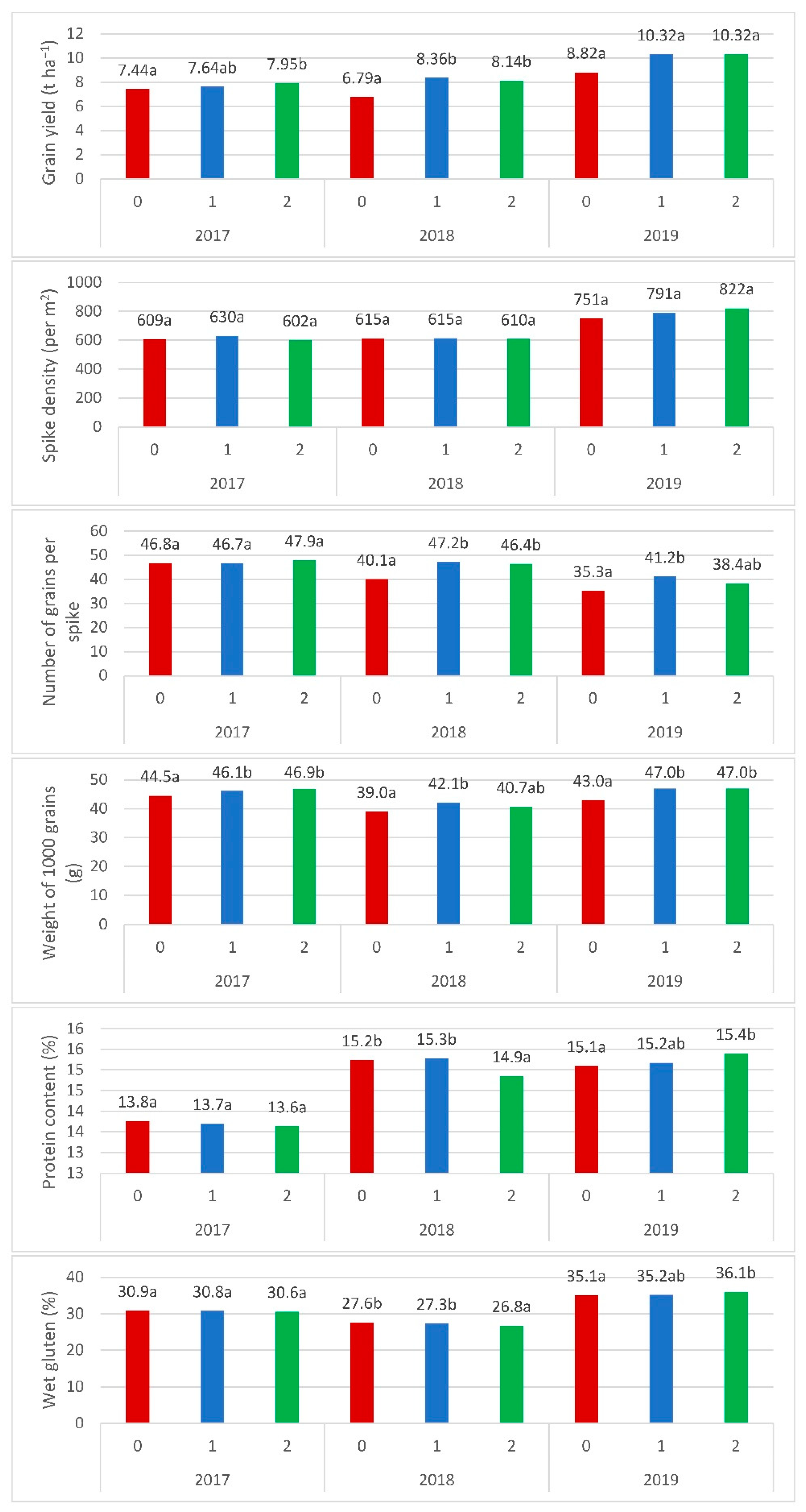
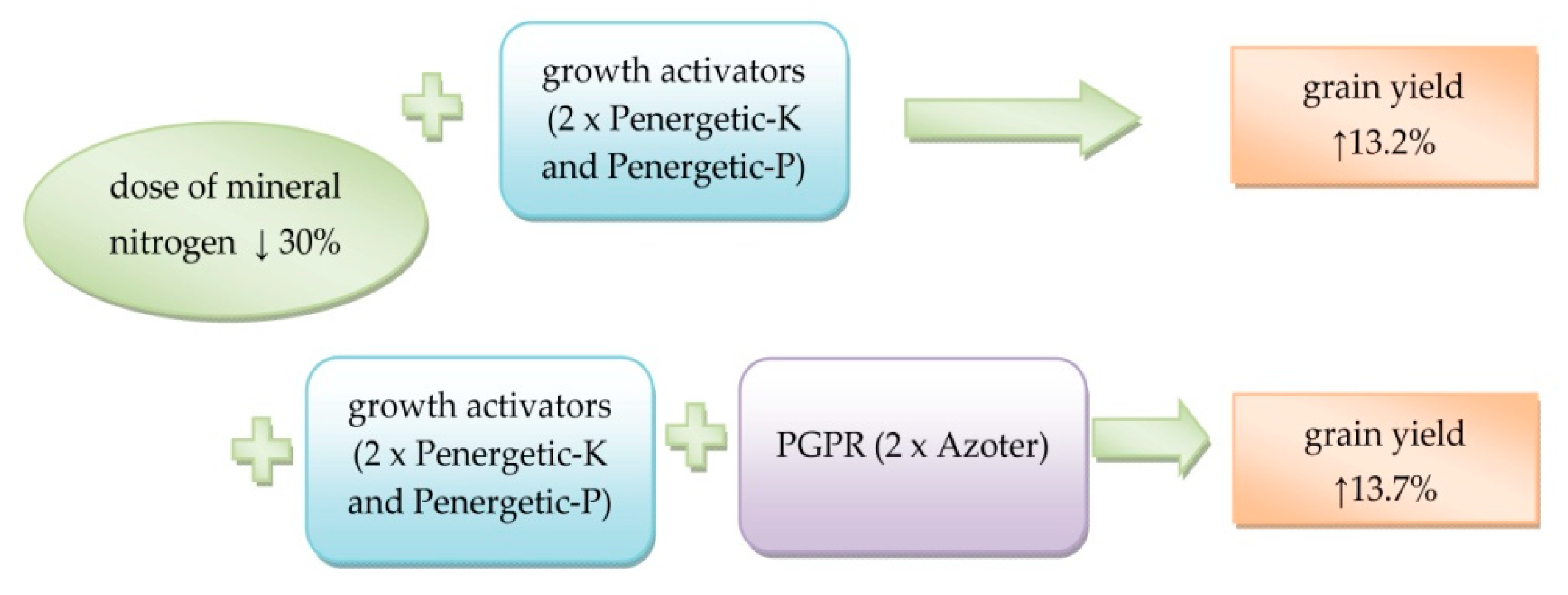
| Grain yield at moisture 14%, t ha−1 | 7.30 a * | 8.26 b | 8.30 b | <0.001 | <0.001 | 0.128 |
| Grain moisture, % | 12.21 a | 12.23 a | 12.27 a | <0.001 | 0.630 | 0.001 |
| Yield of straw, t ha−1 | 5.57 a | 6.01 ab | 6.37 b | <0.001 | 0.012 | 0.184 |
| Spike density, pcs. m−2 | 627.25 a | 641.44 a | 630.06 a | <0.001 | 0.384 | 0.035 |
| Number of non-productive shoots, pcs. m−2 | 55.50 b | 42.72 a | 46.11 a | <0.001 | 0.008 | 0.160 |
| Total number of shoots, pcs. m−2 | 682.75 a | 683.89 a | 676.17 a | <0.001 | 0.772 | 0.144 |
| Weight of 1000 grains (14% H2O), g | 41.87 a | 44.42 b | 44.14 b | <0.001 | <0.001 | 0.043 |
| Protein content, % d.m. | 14.57 b | 14.57 b | 14.37 a | <0.001 | 0.004 | <0.001 |
| Content of wet gluten, % d.m. | 29.92 b | 29.77 ab | 29.51 a | <0.001 | 0.026 | <0.001 |
| Grain uniformity (fractions separated at sieves 2.5 × 25 mm), % | 79.83 a | 81.29 b | 82.21 c | <0.001 | <0.001 | <0.001 |
| Hagberg falling number, s | 308.72 a | 320.67 b | 322.22 b | <0.001 | 0.005 | 0.002 |
| Zeleny sedimentation value (SDS), ml | 48.60 b | 47.71 a | 48.92 b | <0.001 | 0.020 | <0.001 |
| Height of plants, cm | 67.14 a | 69.67 b | 71.93 c | <0.001 | <0.001 | <0.001 |
| Number of grains per spike, pcs. | 42.52 a | 46.30 b | 46.18 b | <0.001 | <0.001 | 0.009 |
| Trait | Mean | Minimum | Maximum | Standard Deviation (SD) | Coefficient of Variation (CV), % |
|---|---|---|---|---|---|
| Grain yield at moisture 14%, t ha−1 | 7.95 | 2.62 | 11.48 | 1.76 | 22.19 |
| Grain moisture, % | 12.24 | 9.80 | 15.00 | 1.55 | 12.65 |
| Yield of straw, t ha−1 | 5.99 | 1.84 | 14.72 | 2.58 | 43.17 |
| Spike density, pcs. m−2 | 632.92 | 393.00 | 885.00 | 130.19 | 20.57 |
| Number of non-productive shoots, pcs. m−2 | 48.11 | 3.00 | 180.00 | 37.47 | 77.88 |
| Total number of shoots, pcs. m−2 | 680.94 | 431.00 | 977.00 | 150.23 | 22.06 |
| Weight of 1000 grains (14% H2O), g | 43.48 | 24.40 | 54.30 | 6.64 | 15.26 |
| Protein content, % d.m. | 14.50 | 12.20 | 16.80 | 1.14 | 7.83 |
| Content of wet gluten, % d.m. | 29.73 | 20.80 | 36.90 | 4.03 | 13.55 |
| Grain uniformity (fractions separated at sieves 2.5 × 25 mm), % | 81.11 | 66.00 | 91.80 | 6.54 | 8.07 |
| Hagberg falling number, s | 317.20 | 241.00 | 390.00 | 35.15 | 11.08 |
| Zeleny sedimentation value (SDS), ml | 48.41 | 21.00 | 71.00 | 16.08 | 33.22 |
| Height of plants, cm | 69.58 | 48.50 | 94.50 | 11.85 | 17.04 |
| Number of grains per spike, pcs. | 45.00 | 31.00 | 64.80 | 7.67 | 17.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Artyszak, A.; Gozdowski, D. Is It Possible to Maintain the Quantity and Quality of Winter Wheat Grain by Replacing Part of the Mineral Nitrogen Dose by Growth Activators and Plant Growth-Promoting Rhizobacteria (PGPR)? Sustainability 2021, 13, 5834. https://doi.org/10.3390/su13115834
Artyszak A, Gozdowski D. Is It Possible to Maintain the Quantity and Quality of Winter Wheat Grain by Replacing Part of the Mineral Nitrogen Dose by Growth Activators and Plant Growth-Promoting Rhizobacteria (PGPR)? Sustainability. 2021; 13(11):5834. https://doi.org/10.3390/su13115834
Chicago/Turabian StyleArtyszak, Arkadiusz, and Dariusz Gozdowski. 2021. "Is It Possible to Maintain the Quantity and Quality of Winter Wheat Grain by Replacing Part of the Mineral Nitrogen Dose by Growth Activators and Plant Growth-Promoting Rhizobacteria (PGPR)?" Sustainability 13, no. 11: 5834. https://doi.org/10.3390/su13115834
APA StyleArtyszak, A., & Gozdowski, D. (2021). Is It Possible to Maintain the Quantity and Quality of Winter Wheat Grain by Replacing Part of the Mineral Nitrogen Dose by Growth Activators and Plant Growth-Promoting Rhizobacteria (PGPR)? Sustainability, 13(11), 5834. https://doi.org/10.3390/su13115834







