Quantitative Analysis and Correction of Temperature Effects on Fluorescent Tracer Concentration Measurement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fluorescent Tracers Used
2.2. Fluorescent Tracer Solutions
2.3. Solution Temperatures
2.4. Model Development
3. Results
3.1. Linear Range and Calibration Model
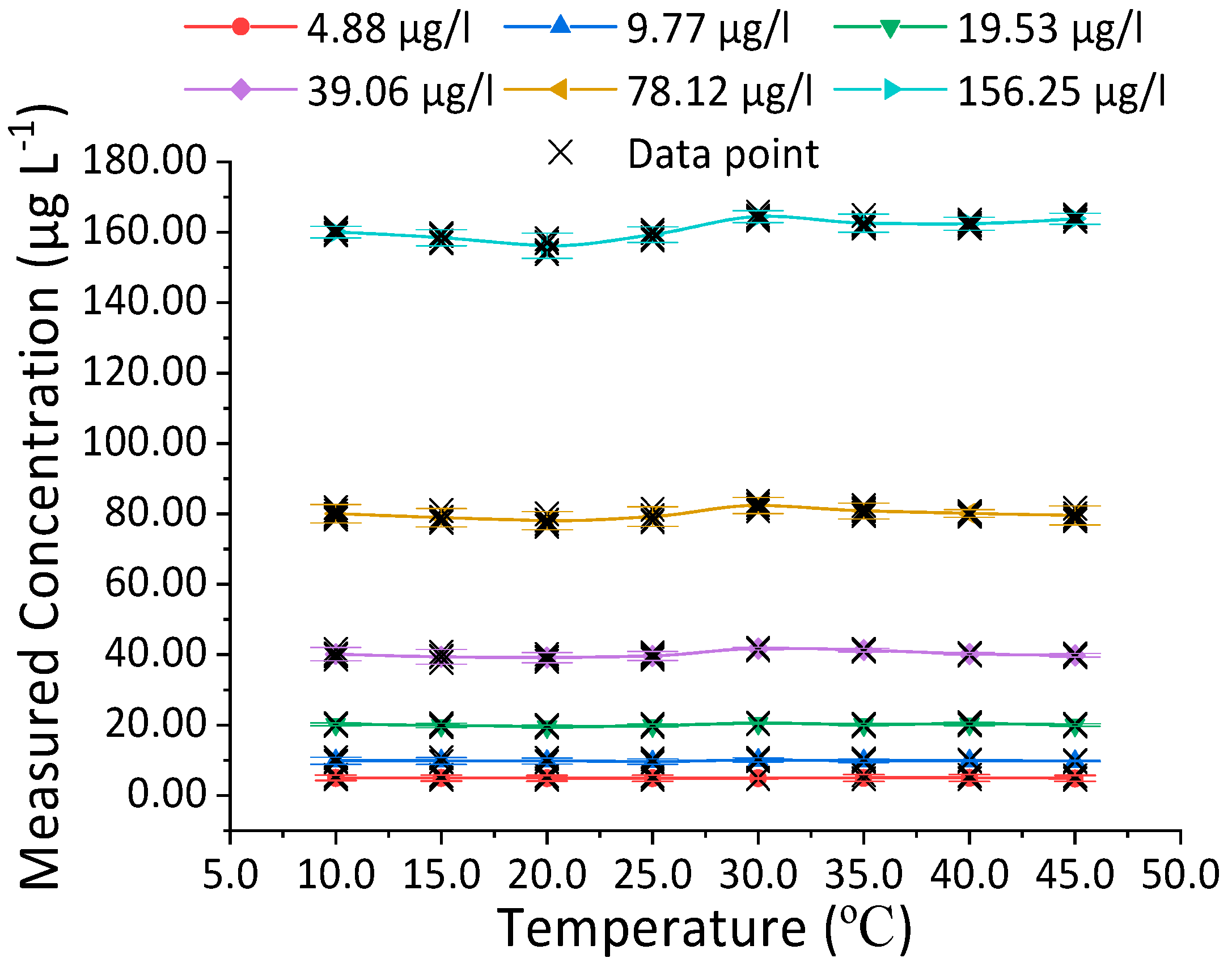
3.2. Effect of Temperature on Measured Concentration of Fluorescent Tracers
3.2.1. Estimation of the Accuracy of the Fluorometer
3.2.2. BSF
3.2.3. Eosin
3.2.4. Fluorescein Sodium Salt
3.3. Correction Models
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Carvalho, F.D.P. Agriculture, pesticides, food security and food safety. Environ. Sci. Policy 2006, 9, 685–692. [Google Scholar] [CrossRef]
- Cooper, J.; Dobson, H. The benefits of pesticides to mankind and the environment. Crop. Prot. 2007, 26, 1337–1348. [Google Scholar] [CrossRef]
- Popp, J.; Pető, K.; Nagy, J. Pesticide productivity and food security. A review. Agron. Sustain. Dev. 2012, 33, 243–255. [Google Scholar] [CrossRef]
- Gil, E.; Arnó, J.; Llorens, J.; Sanz, R.; Llop, J.; Rosell-Polo, J.R.; Gallart, M.; Escola, A. Advanced Technologies for the Improvement of Spray Application Techniques in Spanish Viticulture: An Overview. Sensors 2014, 14, 691–708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pascuzzi, S. Outcomes on the Spray Profiles Produced by the Feasible Adjustments of Commonly Used Sprayers in “Tendone” Vineyards of Apulia (Southern Italy). Sustainability 2016, 8, 1307. [Google Scholar] [CrossRef] [Green Version]
- Bochniak, A.; Kluza, P.A.; Kuna-Broniowska, I.; Koszel, M. Application of Non-Parametric Bootstrap Confidence Intervals for Evaluation of the Expected Value of the Droplet Stain Diameter Following the Spraying Process. Sustainability 2019, 11, 7037. [Google Scholar] [CrossRef] [Green Version]
- Grella, M.; Gallart, M.; Marucco, P.; Balsari, P.; Gil, E. Ground Deposition and Airborne Spray Drift Assessment in Vineyard and Orchard: The Influence of Environmental Variables and Sprayer Settings. Sustainability 2017, 9, 728. [Google Scholar] [CrossRef] [Green Version]
- Qin, W.-C.; Xue, X.; Zhou, Q.; Cai, C.; Wang, B.-K.; Jin, Y.-K. Use of RhB and BSF as fluorescent tracers for determining pesticide spray distribution. Anal. Methods 2018, 10, 4073–4078. [Google Scholar] [CrossRef]
- Vieira, B.C.; Butts, T.R.; Rodrigues, A.O.; Golus, J.A.; Schroeder, K.; Kruger, G.R. Spray particle drift mitigation using field corn (Zea mays L.) as a drift barrier. Pest Manag. Sci. 2018, 74, 2038–2046. [Google Scholar] [CrossRef]
- Bueno, M.R.; da Cunha, J.P.A.R.; de Santana, D.G. Assessment of spray drift from pesticide applications in soybean crops. Biosyst. Eng. 2017, 154, 35–45. [Google Scholar] [CrossRef]
- Wang, G.; Lan, Y.; Yuan, H.; Qi, H.; Chen, P.; Ouyang, F.; Han, Y. Comparison of Spray Deposition, Control Efficacy on Wheat Aphids and Working Efficiency in the Wheat Field of the Unmanned Aerial Vehicle with Boom Sprayer and Two Conventional Knapsack Sprayers. Appl. Sci. 2019, 9, 218. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; He, X.; Wang, X.; Wang, Z.; Wang, S.; Li, L.; Bonds, J.; Herbst, A.; Wang, Z. Testing method and distribution characteristics of spatial pesticide spraying deposition quality balance for unmanned aerial vehicle. Int. J. Agric. Boil. Eng. 2018, 11, 18–26. [Google Scholar] [CrossRef] [Green Version]
- Llop, J.; Gil, E.; Gallart, M.; Contador, F.; Ercilla, M. Spray distribution evaluation of different settings of a hand-held-trolley sprayer used in greenhouse tomato crops. Pest Manag. Sci. 2015, 72, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Nairn, J.J.; Forster, W.A. Due diligence required to quantify and visualise agrichemical spray deposits using dye tracers. Crop. Prot. 2019, 115, 92–98. [Google Scholar] [CrossRef]
- O’Donnell, C.; Hewitt, A.; Dorr, G.; Ferguson, C.; Ledebuhr, M.; Furness, G.; Roten, R. Spraying: Evaluating spray drift and canopy coverage for three types of vineyard sprayers. Wine Viticul. J. 2017, 32, 42. [Google Scholar]
- di Prinzio, A.; Behmer, S.; Magdalena, J.; Chersicla, G. Effect of Pressure on the Quality of Pesticide Application in Orchards. Chil. J. Agric. Res. 2010, 70, 674–678. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Ozkan, H.E.; Zhu, H.; Derksen, R.C.; Krause, C. Spray deposition inside tree canopies from a newly developed variable-rate air-assisted sprayer. Trans. ASABE 2013, 56, 1263–1272. [Google Scholar]
- Felsot, A.S.; Unsworth, J.; Linders, J.; Roberts, G.; Rautman, D.; Harris, C.; Carazo, E. Agrochemical spray drift; assessment and mitigation—A review. J. Environ. Sci. Heal. Part B 2010, 46, 1–23. [Google Scholar] [CrossRef]
- Hoffmann, W.; Farooq, M.; Walker, T.; Fritz, B.; Szumlas, D.; Quinn, B.; Bernier, U.; Hogsette, J.; Lan, Y.; Huang, Y.; et al. Canopy Penetration and Deposition of Barrier Sprays from Electrostatic and Conventional Sprayers1. J. Am. Mosq. Control. Assoc. 2009, 25, 323–331. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, H.; Shen, Y.; Liu, H.; Zondag, R. Spray deposition inside multiple-row nursery trees with a laser-guided sprayer. J. Env. Hortic. 2017, 35, 13–23. [Google Scholar]
- Arvidsson, T.; Bergström, L.; Kreuger, J. Spray drift as influenced by meteorological and technical factors. Pest Manag. Sci. 2011, 67, 586–598. [Google Scholar] [CrossRef] [PubMed]
- Lebeau, F.; Verstraete, A.; Stainier, C.; Destain, M. RTDrift: A real time model for estimating spray drift from ground applications. Comput. Electron. Agric. 2011, 77, 161–174. [Google Scholar] [CrossRef]
- Arvidsson, T.; Bergström, L.; Kreuger, J. Comparison of collectors of airborne spray drift. Experiments in a wind tunnel and field measurements. Pest Manag. Sci. 2011, 67, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Tu, K.; Qin, W.; Lan, Y.; Zhang, H. Drift and deposition of ultra-low altitude and low volume application in paddy field. Int. J. Agric. Biol. Eng. 2014, 7, 23–28. [Google Scholar]
- Zhu, H.; Ozkan, H.E.; Fox, R.D.; Brazee, R.D.; Derksen, R.C. Mixture uniformity in supply lines and spray patterns of a laboratory injection sprayer. Appl. Eng. Agric. 1998, 14, 223–230. [Google Scholar] [CrossRef]
- Lammers, P.S.; Vondricka, J. Direct InjectionDirect Injection Sprayer. In Precision Crop Protection—the Challenge and Use of Heterogeneity; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2010; pp. 295–310. [Google Scholar]
- Zhu, H.; Fox, R.D.; Ozkan, H.E.; Brazee, R.D.; Derksen, R.C. A system to determine lag time and mixture uniformity for inline injection sprayers. Appl. Eng. Agric. 1998, 14, 103–110. [Google Scholar] [CrossRef]
- Shen, Y.; Zhu, H. Embedded computer-controlled premixing inline injection system for air-assisted variable-rate sprayers. Trans. ASABE 2015, 58, 39–46. [Google Scholar]
- Zhang, Z.; Zhu, H.; Guler, H.; Shen, Y. Developing of Premixing Inline Injection System for Agricultural Sprayers Based on Arduino, and Its Performance Evaluation. In Proceedings of the 2018 ASABE Annual International Meeting, Detroit, MI, USA, 29 July–1 August 2018; pp. 1–8. [Google Scholar]
- Bechtold, H.; Rosi, E.; Warren, D.R.; Cole, J. A practical method for measuring integrated solar radiation reaching streambeds using photodegrading dyes. Freshw. Sci. 2012, 31, 1070–1077. [Google Scholar] [CrossRef]
- Khot, L.R.; Salyani, M.; Sweeb, R.D. Solar and Storage Degradations of Oil- and Water-Soluble Fluorescent Dyes. Appl. Eng. Agric. 2011, 27, 211–216. [Google Scholar] [CrossRef]
- You, K.; Zhu, H.; Abbott, J.R. Recovery rates of fluorescent dye on screens and plates as spray deposition collectors. In Proceedings of the 2018 ASABE Annual International Meeting, Detroit, MI, USA, 29 July–1 August 2018; pp. 1–9. [Google Scholar]
- Zhu, H.; Derksen, R.C.; Krause, C.R.; Fox, R.D.; Brazee, R.D.; Ozkan, H.E. Effect of solution pH conditions on fluorescence of spray deposition tracers. Appl. Eng. Agric. 2005, 21, 325–329. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Derksen, R.; Krause, C.; Fox, R.; Brazee, R.; Ozkan, H. Fluorescent Intensity of Dye Solutions under Different pH Conditions. J. ASTM Int. 2005, 2, 12926. [Google Scholar] [CrossRef] [Green Version]
- Turner Designs. Trilogy Laboratory Fluorometer User’s Manual; Turner Designs: San Jose, CA, USA, 2019. [Google Scholar]
- Sutton, J.; Fisher, B.T.; Fleming, J.W. A laser-induced fluorescence measurement for aqueous fluid flows with improved temperature sensitivity. Exp. Fluids 2008, 45, 869–881. [Google Scholar] [CrossRef]
- Deprédurand, V.; Miron, P.; Labergue, A.; Wolff, M.; Castanet, G.; Lemoine, F. A temperature-sensitive tracer suitable for two-colour laser-induced fluorescence thermometry applied to evaporating fuel droplets. Meas. Sci. Technol. 2008, 19, 105403. [Google Scholar] [CrossRef]
- Koch, J.; Hanson, R. Temperature, and excitation wavelength dependencies of 3-pentanone absorption and fluorescence for PLIF applications. Appl. Phys. A 2003, 76, 319–324. [Google Scholar] [CrossRef]
- Estrada-Peérez, C.E.; Hassan, Y.A.; Tan, S. Experimental characterization of temperature sensitive dyes for laser induced fluorescence thermometry. Rev. Sci. Instruments 2011, 82, 74901. [Google Scholar] [CrossRef]
- Ebert, S.; Travis, K.; Lincoln, B.; Guck, J. Fluorescence ratio thermometry in a microfluidic dual-beam laser trap. Opt. Express 2007, 15, 15493–15499. [Google Scholar] [CrossRef]
- Kim, H.J.; Kihm, K.D.; Allen, J.S. Examination of radiometric laser induced fluorescence thermometry for microscale spatial measurement resolution. Int. J. Heat Mass Trans. 2003, 46, 3967–3974. [Google Scholar] [CrossRef]
- Li, Y.; Peng, Y.; Qin, J.; Chao, K. A Correction Method of Mixed Pesticide Content Prediction in Apple by Using Raman Spectra. Appl. Sci. 2019, 9, 1699. [Google Scholar] [CrossRef] [Green Version]
- Gunst, R.F.; Myers, R.H.; Montgomery, D.C. Response Surface Methodology: Process and Product Optimization Using Designed Experiments. Technometrics 1996, 38, 285. [Google Scholar] [CrossRef]
- Myers, R.H.; Khuri, A.I.; Carter, W.H. Response Surface Methodology: 1966–1988. Technometrics 1989, 31, 137. [Google Scholar] [CrossRef]
- Tran, K.H.; Morin, C.; Kühni, M.; Guibert, P. Fluorescence spectroscopy of anisole at elevated temperatures and pressures. Appl. Phys. A 2013, 115, 461–470. [Google Scholar] [CrossRef]

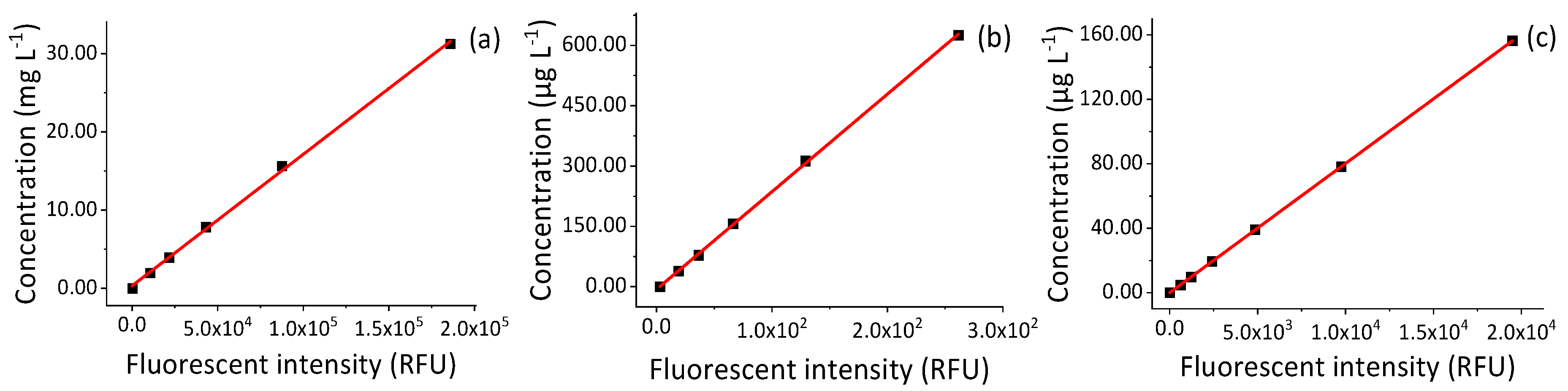





| Fluorescence Tracers | Excitation (nm) | Emission (nm) | CAS Number | Molecular Weight | Formula | Fluorescence Quantum Yield |
|---|---|---|---|---|---|---|
| BSF | 460 | 500 | 2391-30-2 | 404.4 | C19H13N2NaO5S | 0.27 |
| Eosin | 525 | 545 | 15086-94-9 | 647.9 | C20H8Br4O5 | 0.63 |
| Fluorescein sodium salt | 460 | 515 | 518-47-8 | 376.27 | C20H10Na2O5 | 0.97 |
| Fluorescent Tracer | BSF | ||||||
| Nominal concentration (mg L−1) | 5.00 | 10.00 | 15.00 | 20.00 | 25.00 | 30.00 | |
| Measured concentration (mg L−1) (CV (%)) | 4.3 (42.44) | 8.52 (47.58) | 12.83 (52.97) | 17.44 (56.90) | 22.74 (68.87) | 28.19 (78.18) | |
| Fluorescent tracer | Eosin | ||||||
| Nominal concentration (μg L−1) | 37.50 | 75.00 | 150.00 | 300.00 | 600.00 | ||
| Measured concentration (μg L−1) (CV (%)) | 42.64 (10.62) | 84.09 (10.32) | 164.37 (9.90) | 331.64 (10.68) | 655.93 (11.41) | ||
| Fluorescent tracer | Fluorescein sodium salt | ||||||
| Nominal concentration (μg L−1) | 4.88 | 9.77 | 19.53 | 39.06 | 78.12 | 156.25 | |
| Measured concentration (μg L−1) (CV (%)) | 4.90 (1.10) | 9.82 (1.37) | 20.04 (1.54) | 40.16 (2.26) | 79.91 (1.64) | 160.95 (1.79) | |
| Fluorescent Tracer | BSF | |||||
| Nominal concentration (mg L−1) | 5.00 | 10.00 | 15.00 | 20.00 | 25.00 | 30.00 |
| Corrected concentration (mg L−1) (CV (%)) | 4.68 (10.21) | 10.09 (1.76) | 15.22 (2.38) | 20.39 (2.94) | 25.04 (1.62) | 29.52 (2.62) |
| Fluorescent tracer | Eosin | |||||
| Nominal concentration (μg L−1) | 37.50 | 75.00 | 150.00 | 300.00 | 600.00 | |
| Measured concentration (μg L−1) (CV (%)) | 37.53 (3.51) | 75.59 (2.87) | 148.96 (1.19) | 300.74 (0.94) | 599.89 (0.88) | |
| Fluorescent tracer | Fluorescein sodium salt | |||||
| Nominal concentration (μg L−1) | 4.88 | 9.77 | 19.53 | 39.06 | 78.12 | 156.25 |
| Measured concentration (μg L−1) (CV (%)) | 4.90 (1.10) | 9.82 (1.39) | 20.04 (1.53) | 40.16 (2.26) | 79.91 (1.64) | 160.95 (1.79) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Zhu, H.; Guler, H. Quantitative Analysis and Correction of Temperature Effects on Fluorescent Tracer Concentration Measurement. Sustainability 2020, 12, 4501. https://doi.org/10.3390/su12114501
Zhang Z, Zhu H, Guler H. Quantitative Analysis and Correction of Temperature Effects on Fluorescent Tracer Concentration Measurement. Sustainability. 2020; 12(11):4501. https://doi.org/10.3390/su12114501
Chicago/Turabian StyleZhang, Zhihong, Heping Zhu, and Huseyin Guler. 2020. "Quantitative Analysis and Correction of Temperature Effects on Fluorescent Tracer Concentration Measurement" Sustainability 12, no. 11: 4501. https://doi.org/10.3390/su12114501




