Investigation of a Farm-scale Multitrophic Recirculating Aquaculture System with the Addition of Rhodovulum sulfidophilum for Milkfish (Chanos chanos) Coastal Aquaculture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sediment Sampling and Sampling Site
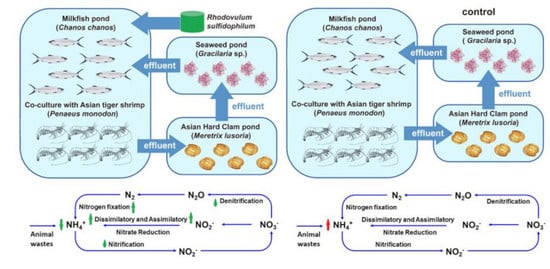
2.2. Experimental Design
2.3. Analysis of the Chemical Compositions of Water from MRAS
2.4. Next Generation Sequencing and Data Analysis
3. Results
3.1. Chemical Compositions in the Water of the MRAS
3.2. Analysis of Photosynthetic Bacteria, Cyanobacteria, and Algae Communities
3.3. Analysis of Potential Pathogenic Bacterial Communities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Islam, M.; Yasmin, R. Impact of aquaculture and contemporary environmental issues in Bangladesh. Int. J. Fish. Aquat. Stud. 2017, 5, 100–107. [Google Scholar]
- Zhang, J.; Kitazawa, D. Assessing the bio-mitigation effect of integrated multi-trophic aquaculture on marine environment by a numerical approach. Mar. Pollut. Bull. 2016, 110, 484–492. [Google Scholar] [CrossRef]
- Hadley, S.; Wild-Allen, K.; Johnson, C.; Macleod, C. Investigation of broad scale implementation of integrated multitrophic aquaculture using a 3D model of an estuary. Mar. Pollut. Bull. 2018, 133, 448–459. [Google Scholar] [CrossRef]
- Qiu, T.; Liu, L.; Gao, M.; Zhang, L.; Tursun, H.; Wang, X. Effects of solid-phase denitrification on the nitrate removal and bacterial community structure in recirculating aquaculture system. Biodegradation 2016, 27, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Shen, J.; Ruan, Y.; Guo, X.; Ye, Z.; Deng, Y.; Shi, M. The effects of different seeding ratios on nitrification performance and biofilm formation in marine recirculating aquaculture system biofilter. Environ. Sci. Pollut. Res. 2016, 23, 14540–14548. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Liu, X.; Ma, Z.; Liu, Q.; Wu, Z.; Zeng, X.; Shi, X.; Gu, Z. Vertical Segregation and Phylogenetic Characterization of Ammonia-Oxidizing Bacteria and Archaea in the Sediment of a Freshwater Aquaculture Pond. Front. Microbiol. 2016, 6, 1539. [Google Scholar] [CrossRef]
- Brown, M.N.; Briones, A.; Diana, J.; Raskin, L. Ammonia-oxidizing archaea and nitrite-oxidizing nitrospiras in the biofilter of a shrimp recirculating aquaculture system. FEMS Microbiol. Ecol. 2013, 83, 17–25. [Google Scholar] [CrossRef]
- Ferreira, M.G.P.; Melo, F.P.; Lima, J.P.V.; Andrade, H.A.; Severi, W.; Correia1, E.S. Bioremediation and biocontrol of commercial probiotic in marine shrimp culture with biofloc. Lat. Am. J. Aquat. Res. 2017, 45, 167–176. [Google Scholar] [CrossRef]
- Lopes, R.B.; Olinda, R.A.; Souza, B.A.; Cyrino, J.E.; Dias, C.T.; Queiroz, J.F.; Tavares, L.H. Efficiency of bioaugmentation in the removal of organic matter in aquaculture systems. Braz. J. Biol. 2011, 71, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.W.; Tsai, L.L.; Chang, B.V. Anaerobic degradation of sulfamethoxazole in mangrove sediments. Sci. Total. Environ. 2018, 643, 1446–1455. [Google Scholar] [CrossRef]
- Akhter, N.; Wu, B.; Memon, A.M.; Mohsin, M. Probiotics and prebiotics associated with aquaculture: A review. Fish Shellfish Immunol. 2015, 45, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Egerton, S.; Culloty, S.; Whooley, J.; Stanton, C.; Ross, R.P. The Gut Microbiota of Marine Fish. Front. Microbiol. 2018, 9, 873. [Google Scholar] [CrossRef] [PubMed]
- Loo, P.L.; Chong, V.C.; Vikineswary, S. Rhodovulum sulfidophilum, a phototrophic bacterium, grown in palm oil mill effluent improves the larval survival of marble goby Oxyeleotris marmorata (Bleeker). Aquacult. Res. 2013, 44, 495–507. [Google Scholar] [CrossRef]
- Loo, P.L.; Chong, V.C.; Ibrahim, S.; Sabaratnam, V. Manipulating Culture Conditions and Feed Quality to Increase the Survival of Larval Marble Goby Oxyeleotris marmorata. North. Am. J. Aquac. 2015, 77, 149–159. [Google Scholar] [CrossRef]
- Yang, C.W.; Hsiao, W.C.; Chang, B.V. Biodegradation of sulfonamide antibiotics in sludge. Chemosphere 2016, 150, 559–565. [Google Scholar] [CrossRef]
- Cole, J.R.; Wang, Q.; Fish, J.A.; Chai, B.; McGarrell, D.M.; Sun, Y.; Brown, C.T.; Porras-Alfaro, A.; Kuske, C.R.; Tiedje, J.M. Ribosomal Database Project: data and tools for high throughput rRNA analysis. Nucleic Acids Res. 2014, 42, D633–D642. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Tanabe, M.; Sato, Y.; Morishima, K. KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017, 45, D353–D361. [Google Scholar] [CrossRef]
- The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 11 March 2019).
- Krausfeldt, L.E.; Tang, X.; van de Kamp, J.; Gao, G.; Bodrossy, L.; Boyer, G.L.; Wilhelm, S.W. Spatial and temporal variability in the nitrogen cyclers of hypereutrophic Lake Taihu. FEMS Microbiol. Ecol. 2017, 93, fix024. [Google Scholar] [CrossRef]
- Ajin, A.M.; Silvester, R.; Alexander, D.; M, N.; Abdulla, M.H. Characterization of blooming algae and bloom-associated changes in the water quality parameters of traditional pokkali cum prawn fields along the South West coast of India. Environ. Monit. Assess. 2016, 188, 145. [Google Scholar]
- Raszl, S.M.; Froelich, B.A.; Vieira, C.R.; Blackwood, A.D.; Noble, R.T. Vibrio parahaemolyticus and Vibrio vulnificus in South America: water, seafood and human infections. J. Appl. Microbiol. 2016, 121, 1201–1222. [Google Scholar] [CrossRef]
- Baker-Austin, C.; Oliver, J.D.; Alam, M.; Ali, A.; Waldor, M.K.; Qadri, F.; Martinez-Urtaza, J. Vibrio spp. infections. Nat. Rev. Dis. Primers 2018, 4, 8. [Google Scholar] [CrossRef] [PubMed]
- Leung, H.M.; Leung, S.K.; Au, C.K.; Cheung, K.C.; Wong, Y.K.; Leung, A.O.; Yung, K.K. Comparative assessment of water quality parameters of mariculture for fish production in Hong Kong Waters. Mar. Pollut. Bull. 2015, 94, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Herbeck, L.S.; Unger, D.; Wu, Y.; Jennerjahn, T.C. Effluent, nutrient and organic matter export from shrimp and fish ponds causing eutrophication in coastal and back-reef waters of NE Hainan, tropical China. Cont. Shelf. Res. 2013, 57, 92–104. [Google Scholar] [CrossRef]
- Hozumi, A.; Hong, P.Y.; Kaartvedt, S.; Røstad, A.; Jones, B.H. Water quality, seasonality, and trajectory of an aquaculture-wastewater plume in the Red Sea. Aquacult. Environ. Interact. 2018, 10, 61–77. [Google Scholar] [CrossRef]
- Turcios, A.E.; Papenbrock, J. Sustainable Treatment of Aquaculture Effluents-What Can We Learn from the Past for the Future? Sustainability 2014, 6, 836–856. [Google Scholar] [CrossRef]
- Chen, L.; Feng, Q.; Li, C.; Wei, Y.; Zhao, Y.; Feng, Y.; Zheng, H.; Li, F.; Li, H. Impacts of aquaculture wastewater irrigation on soil microbial functional diversity and community structure in arid regions. Sci. Rep. 2017, 7, 11193. [Google Scholar] [CrossRef]
- Lenzi, M. The Future of Aquaculture. J. Aquac. Res. Develop. 2013, 4, e106. [Google Scholar] [CrossRef]
- Buck, B.H.; Troell, M.F.; Krause, G.; Angel, D.L.; Grote, B.; Chopin, T. State of the Art and Challenges for Offshore Integrated Multi-Trophic Aquaculture (IMTA). Front. Mar. Sci. 2018, 5, 165. [Google Scholar] [CrossRef]
- Guerra-García, J.M.; Hachero-Cruzado, I.; González-Romero, P.; Jiménez-Prada, P.; Cassell, C.; Ros, M. Towards Integrated Multi-Trophic Aquaculture: Lessons from Caprellids (Crustacea: Amphipoda). PLoS ONE 2016, 11, e0154776. [Google Scholar] [CrossRef] [PubMed]
- Largo, D.B.; Diola, A.G.; Marababol, M.S. Development of an integrated multi-trophic aquaculture (IMTA) system for tropical marine species in southern cebu, Central Philippines. Aquacult. Rep. 2016, 3, 67–76. [Google Scholar] [CrossRef]
- Zhang, X.; Shu, M.; Wang, Y.; Fu, L.; Li, W.; Deng, B.; Liang, Q.; Shen, W. Effect of photosynthetic bacteria on water quality and microbiota in grass carp culture. World J. Microbiol. Biotechnol. 2014, 30, 2523–2531. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.Q.; Xue, M.; Liang, H.F.; Wu, Y.; Li, X. Beneficial effects of Ectothiorhodospira shaposhnikovii WF on larval cultivation of Litopenaeus vannamei. Benef. Microbes 2015, 6, 525–533. [Google Scholar]









© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, B.-V.; Liao, C.-S.; Chang, Y.-T.; Chao, W.-L.; Yeh, S.-L.; Kuo, D.-L.; Yang, C.-W. Investigation of a Farm-scale Multitrophic Recirculating Aquaculture System with the Addition of Rhodovulum sulfidophilum for Milkfish (Chanos chanos) Coastal Aquaculture. Sustainability 2019, 11, 1880. https://doi.org/10.3390/su11071880
Chang B-V, Liao C-S, Chang Y-T, Chao W-L, Yeh S-L, Kuo D-L, Yang C-W. Investigation of a Farm-scale Multitrophic Recirculating Aquaculture System with the Addition of Rhodovulum sulfidophilum for Milkfish (Chanos chanos) Coastal Aquaculture. Sustainability. 2019; 11(7):1880. https://doi.org/10.3390/su11071880
Chicago/Turabian StyleChang, Bea-Ven, Chien-Sen Liao, Yi-Tang Chang, Wei-Liang Chao, Shinn-Lih Yeh, Dong-Lin Kuo, and Chu-Wen Yang. 2019. "Investigation of a Farm-scale Multitrophic Recirculating Aquaculture System with the Addition of Rhodovulum sulfidophilum for Milkfish (Chanos chanos) Coastal Aquaculture" Sustainability 11, no. 7: 1880. https://doi.org/10.3390/su11071880