Resource Use in Mariculture: A Case Study in Southeastern China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Survey Data
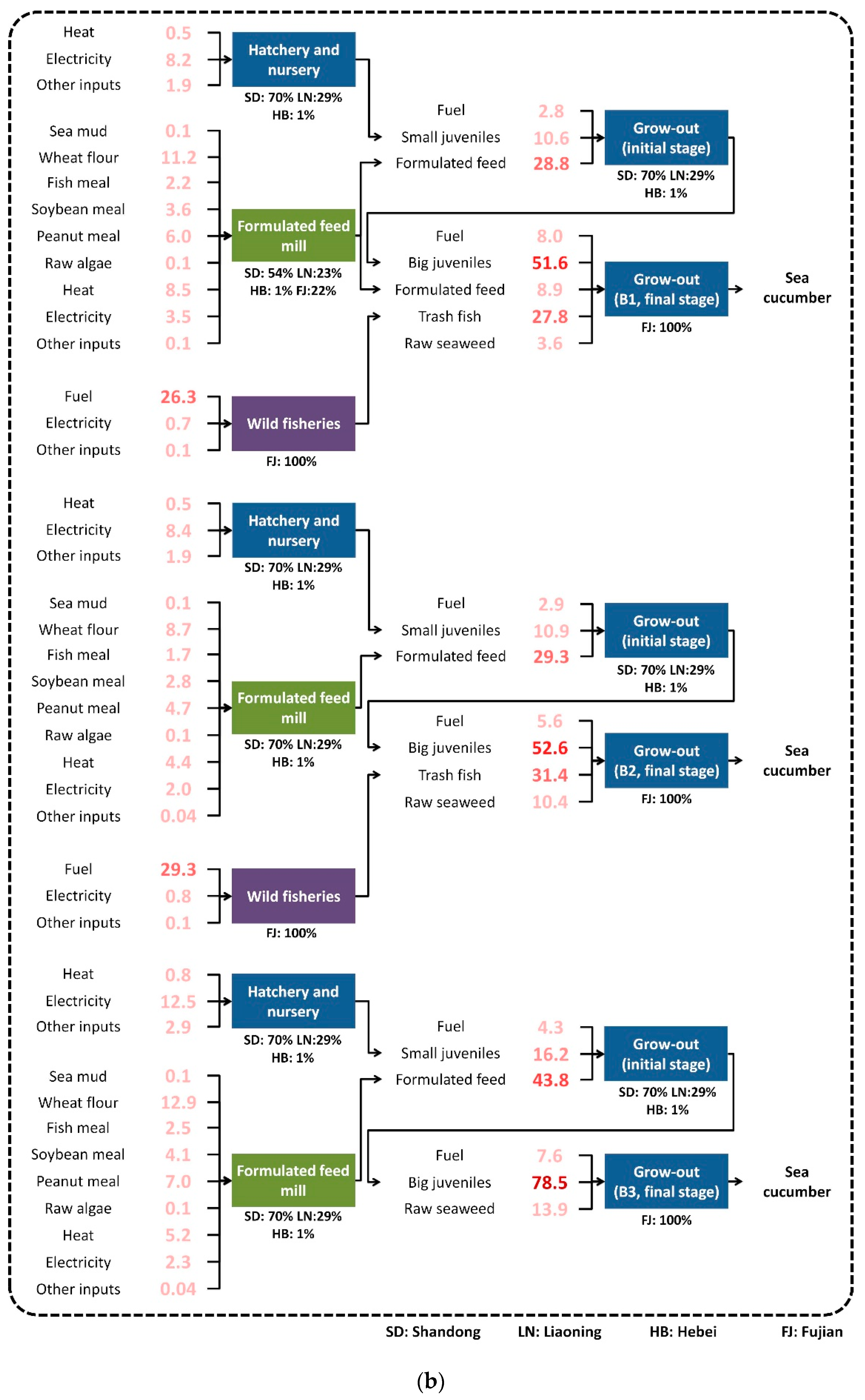
2.3. Description of the Aquaculture Production Process
2.4. Life Cycle Assessment Model
2.5. Statistical Analysis
3. Results
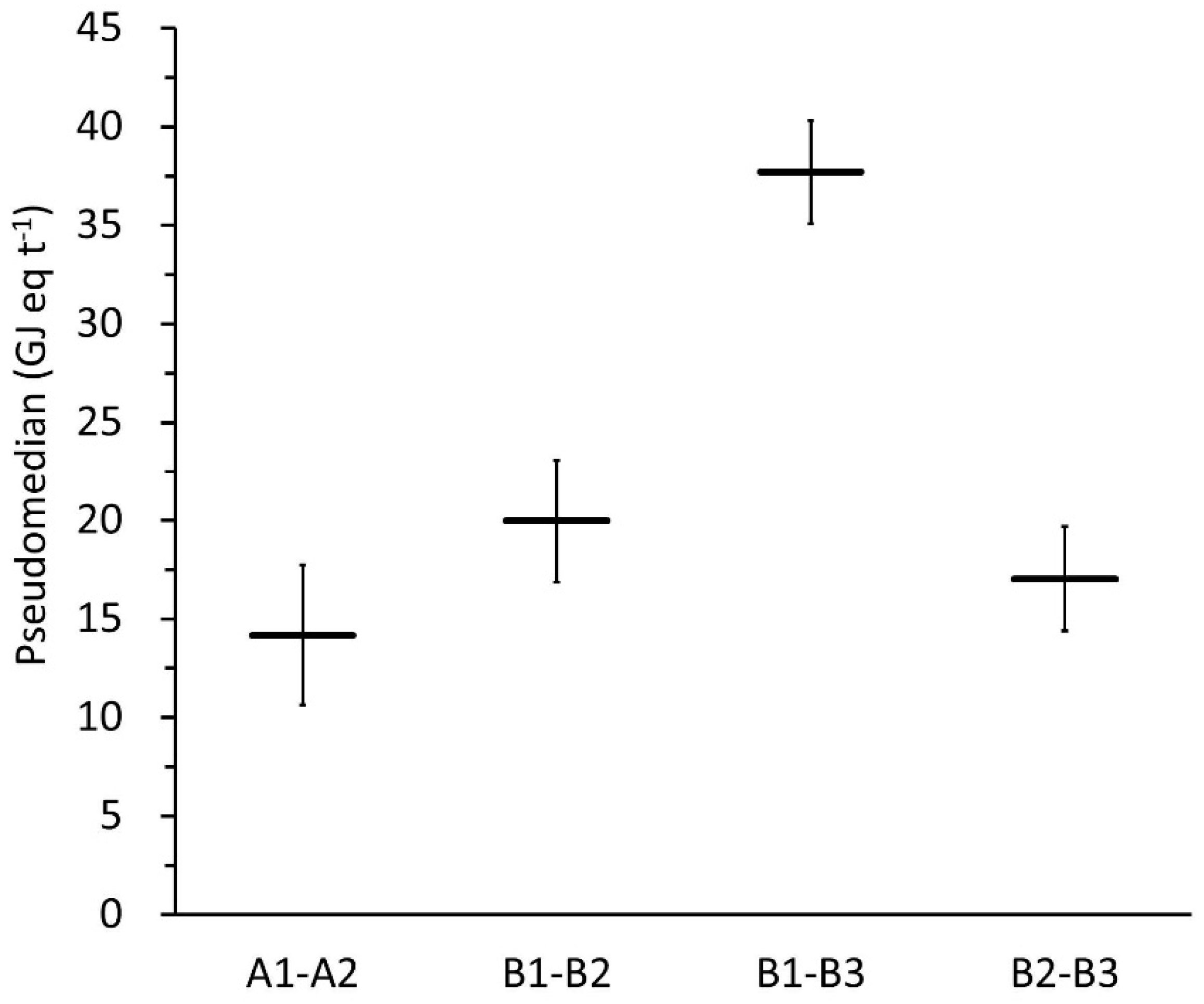
3.1. Comparison between Correlated and Uncorrelated Model Versions
3.2. Life Cycle Inventory
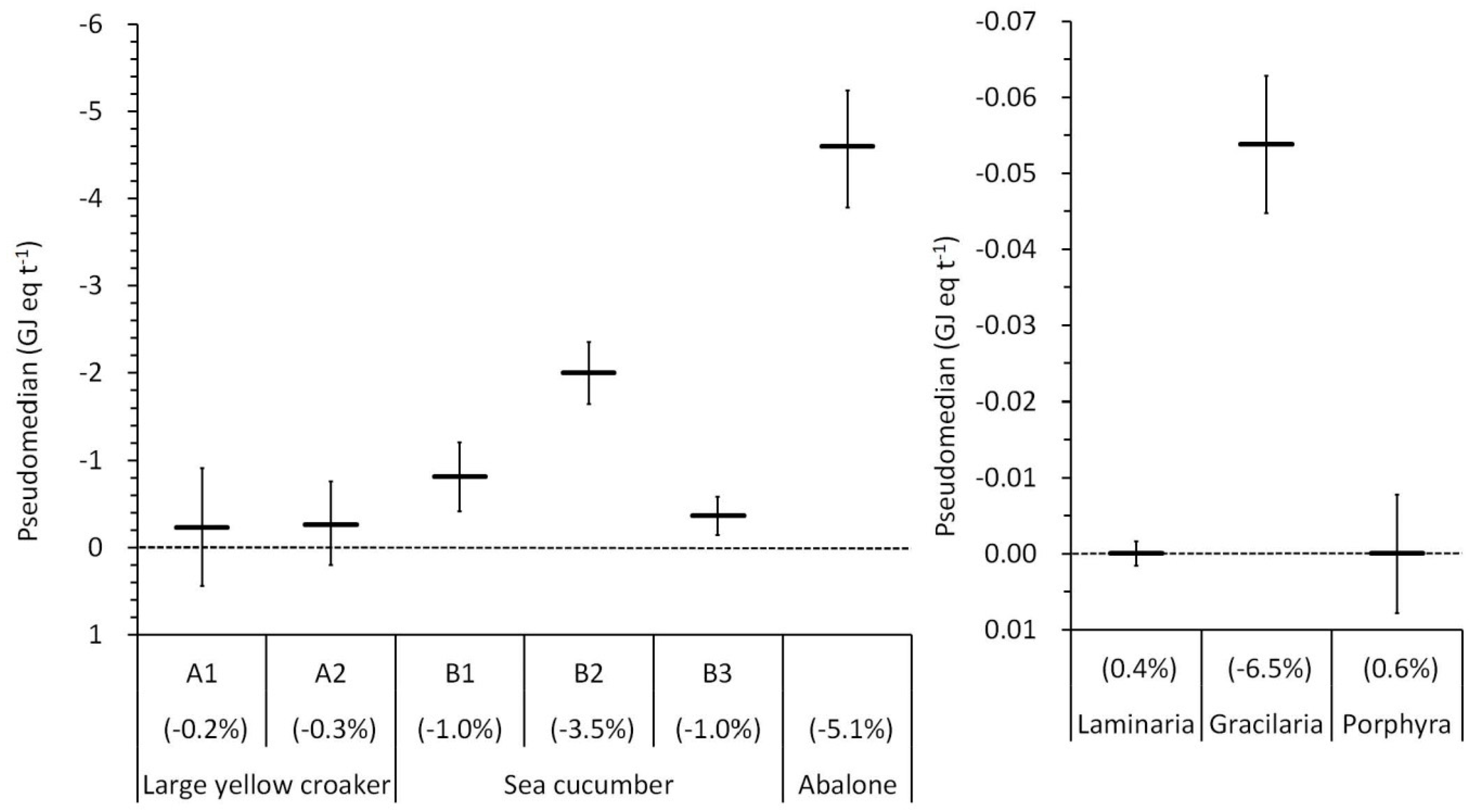
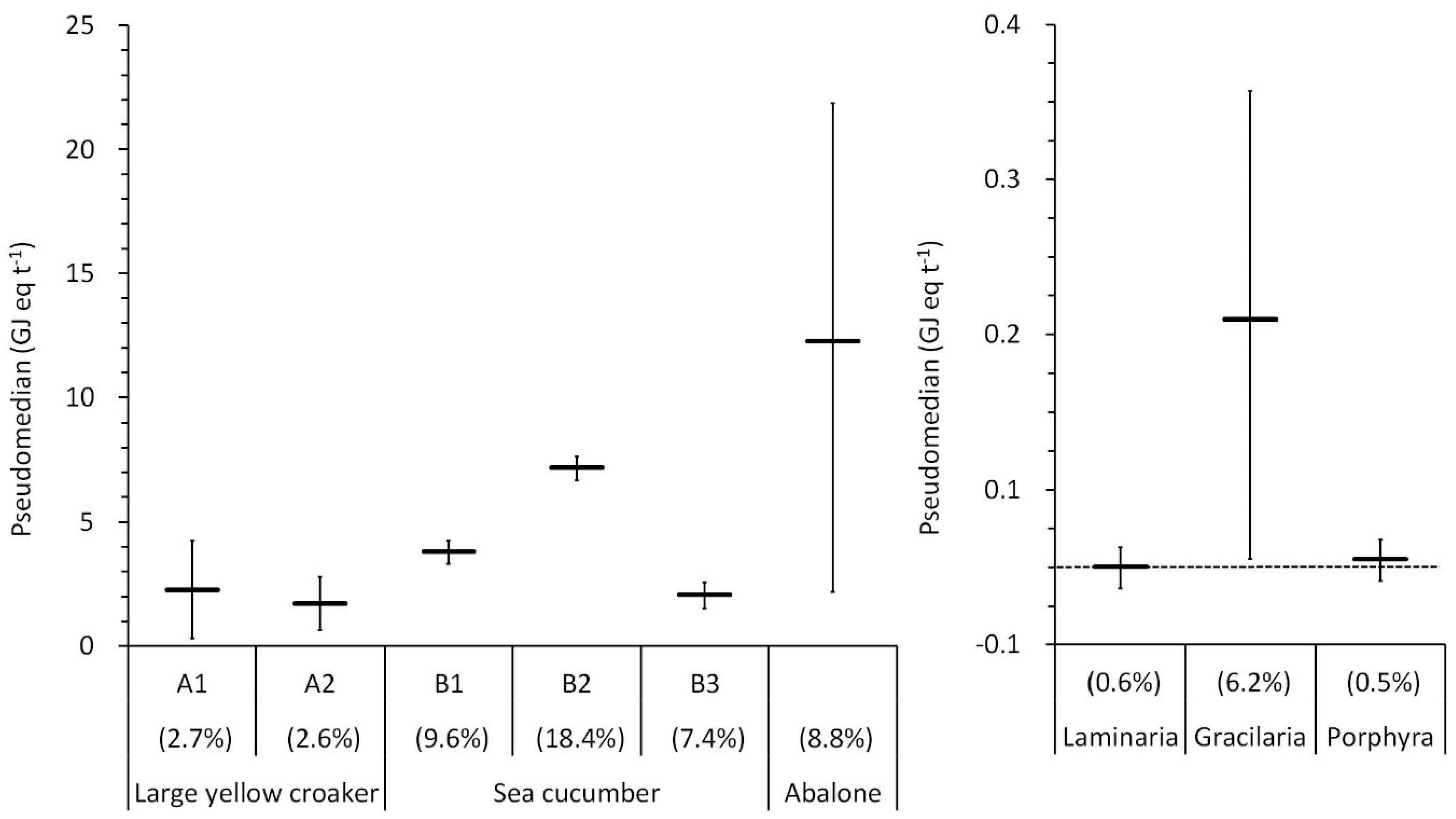
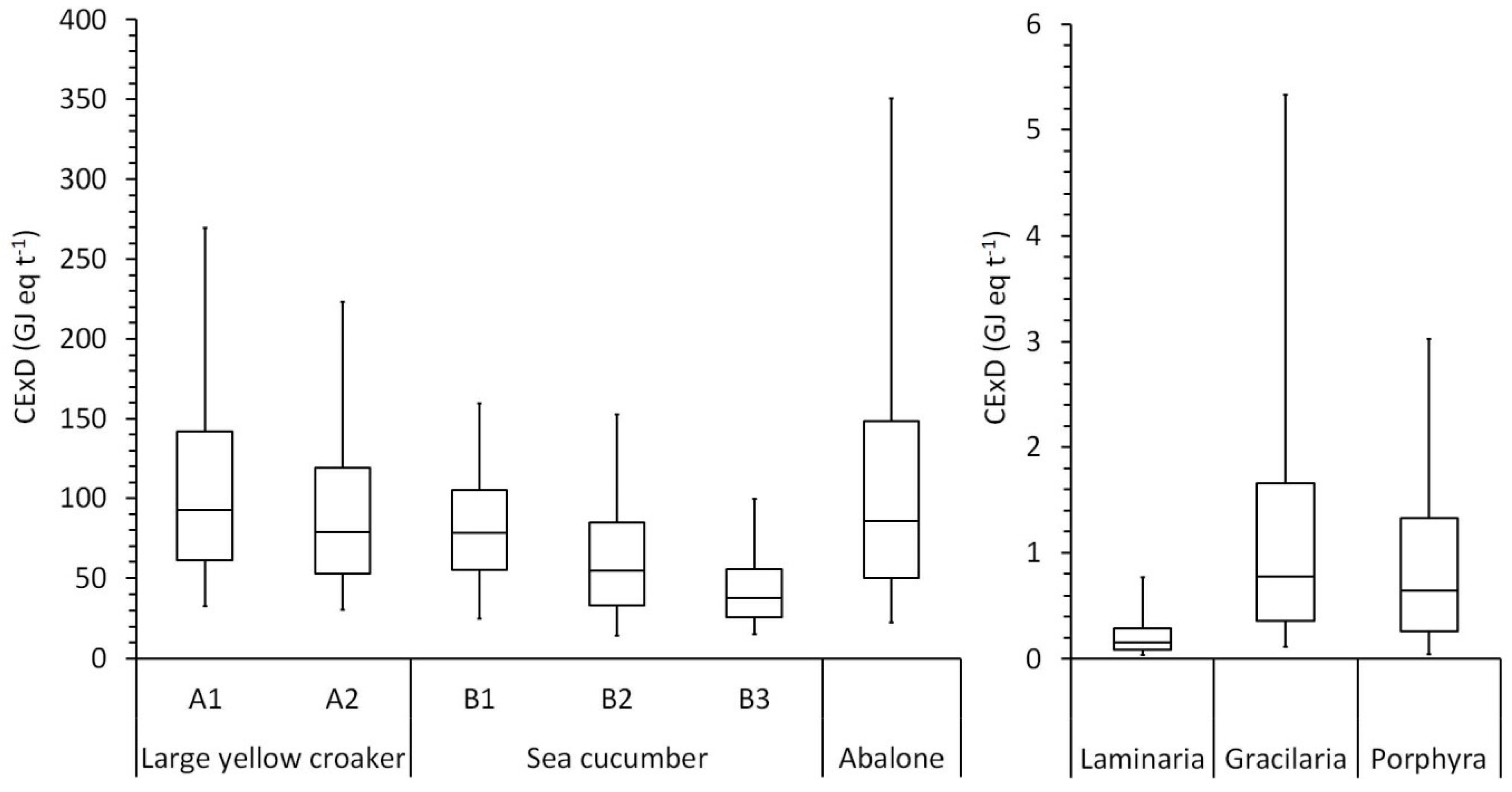
3.3. Life Cycle Impact Assessment
4. Discussion
4.1. Local and Global Impacts of Resource Use
4.2. Input Management
4.3. Correlation among Unit Process Variables
4.4. Limitations
4.5. Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2018—Meeting the Sustainable Development Goals; FAO: Roma, Italy, 2018. [Google Scholar]
- Barbier, E.B.; Hacker, S.D.; Kennedy, C.; Koch, E.W.; Stier, A.C.; Silliman, B.R. The value of estuarine and coastal ecosystem services. Ecol. Monogr. 2011, 81, 169–193. [Google Scholar] [CrossRef] [Green Version]
- Kaufman, L.; Dayton, P. Impacts of marine resource extraction on ecosystem services and sustainability. In Nature’s Services: Societal Dependence on Natural Ecosystems; Daily, G.C., Ed.; Island Press: Washington, DC, USA, 1997. [Google Scholar]
- Peterson, C.H.; Lubchenco, J. Marine Ecosystem Services. In Nature’s Services: Societal Dependence on Natural Ecosystems; Daily, G.C., Ed.; Island Press: Washington, DC, USA, 1997. [Google Scholar]
- Little, D.C.; Newton, R.W.; Beveridge, M.C.M. Aquaculture: A rapidly growing and significant source of sustainable food? Status, transitions and potential. Proc. Nutr. Soc. 2016, 75, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, P.S.; Ehrlich, P.R. Pervasive Externalities at the Population, Consumption, and Environment Nexus. Science 2013, 340, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, W.; Li, S. Framing ecosystem services in the telecoupled Anthropocene. Front. Ecol. Environ. 2016, 14, 27–36. [Google Scholar] [CrossRef]
- Sumaila, U.R.; Bellmann, C.; Tipping, A. Fishing for the future: An overview of challenges and opportunities. Mar. Policy 2016, 69, 173–180. [Google Scholar] [CrossRef]
- Liang, Y.X.; Cheng, X.W.; Zhu, H.; Shutes, B.; Yan, B.X.; Zhou, Q.W.; Yu, X.F. Historical Evolution of Mariculture in China During Past 40 Years and Its Impacts on Eco-environment. Chin. Geogr. Sci. 2018, 28, 363–373. [Google Scholar] [CrossRef]
- Garcia, F.; Kimpara, J.M.; Valenti, W.C.; Ambrosio, L.A. Emergy assessment of tilapia cage farming in a hydroelectric reservoir. Ecol. Eng. 2014, 68, 72–79. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, H.P.; Qin, P.; Zhou, J.; Wang, G.X. Comparisons of ecosystem services among three conversion systems in Yancheng National Nature Reserve. Ecol. Eng. 2009, 35, 609–629. [Google Scholar] [CrossRef]
- Zhang, L.X.; Song, B.; Chen, B. Emergy-based analysis of four farming systems: Insight into agricultural diversification in rural China. J. Clean. Prod. 2012, 28, 33–44. [Google Scholar] [CrossRef]
- Lu, H.F.; Tan, Y.W.; Zhang, W.S.; Qiao, Y.C.; Campbell, D.E.; Zhou, L.; Ren, H. Integrated emergy and economic evaluation of lotus-root production systems on reclaimed wetlands surrounding the Pearl River Estuary, China. J. Clean. Prod. 2017, 158, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.H.; Zheng, W.; Zhang, X.L.; Zhu, M.Y.; Ding, D.W. Ecological-economic assessment of monoculture and integrated multi-trophic aquaculture in Sanggou Bay of China. Aquaculture 2013, 410, 172–178. [Google Scholar] [CrossRef]
- Cao, L.; Diana, J.S.; Keoleian, G.A.; Lai, Q. Life Cycle Assessment of Chinese Shrimp Farming Systems Targeted for Export and Domestic Sales. Environ. Sci. Technol. 2011, 45, 6531–6538. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Naylor, R.; Henriksson, P.; Leadbitter, D.; Metian, M.; Troell, M.; Zhang, W. China’s aquaculture and the world’s wild fisheries. Science 2015, 347, 133–135. [Google Scholar] [CrossRef] [PubMed]
- Bostock, J.; McAndrew, B.; Richards, R.; Jauncey, K.; Telfer, T.; Lorenzen, K.; Little, D.; Ross, L.; Handisyde, N.; Gatward, I.; et al. Aquaculture: Global status and trends. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2897–2912. [Google Scholar] [CrossRef] [PubMed]
- Volpe, J.; Gee, J.; Ethier, V.; Beck, M.; Wilson, A. Global Aquaculture Performance Index (GAPI): The First Global Environmental Assessment of Marine Fish Farming; University of Victoria: Victoria, BC, Canada, 2010. [Google Scholar]
- Fishery Policy and Administration Bureau, Ministry of Agriculture of China. China Fisheries Yearbook; China Agriculture Press: Beijing, China, 2012–2017. (In Chinese)
- Chen, J.X.; Guang, C.T.; Xu, H.; Chen, Z.X.; Xu, P.; Yan, X.M.; Wang, Y.T.; Liu, J.F. A review of cage and pen aquaculture: China. In Cage Aquaculture—Regional Reviews and Global Overview; Halwart, M., Soto, D., Arthur, J.R., Eds.; FAO Fisheries Technical Paper No. 498; FAO: Rome, Italy, 2007; 241p. [Google Scholar]
- Halpern, B.S.; Walbridge, S.; Selkoe, K.A.; Kappel, C.V.; Micheli, F.; D’Agrosa, C.; Bruno, J.F.; Casey, K.S.; Ebert, C.; Fox, H.E.; et al. A global map of human impact on marine ecosystems. Science 2008, 319, 948–952. [Google Scholar] [CrossRef] [PubMed]
- Ningde Municipal Statistical Bureau. Ningde Statistical Yearbook; China Statistical Press: Beijing, China, 2017.
- Han, Q.; Keesing, J.K.; Liu, D. A Review of Sea Cucumber Aquaculture, Ranching, and Stock Enhancement in China. Rev. Fish. Sci. Aquac. 2016, 24, 326–341. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.; Zhang, G. Pacific Abalone Farming in China: Recent Innovations and Challenges. J. Shellfish Res. 2007, 35, 703–710. [Google Scholar] [CrossRef]
- Jiang, S.; Ren, Y.; Tang, B.; Li, C.; Jiang, C. Development status and countermeasures of Apostichopus japonicus culture industry in China. J. Agric. Sci. Technol. 2017, 19, 15–23. [Google Scholar]
- International Organization for Standardization. ISO 14044:2006 Environmental Management—Life Cycle Assessment—Requirements and Guidelines; International Organization for Standardization: Geneva, Switzerland, 2006. [Google Scholar]
- International Organization for Standardization. ISO 14040:2006 Environmental Management—Life Cycle Assessment—Principles and Framework; International Organization for Standardization: Geneva, Switzerland, 2006. [Google Scholar]
- Cao, L.; Diana, J.S.; Keoleian, G.A. Role of life cycle assessment in sustainable aquaculture. Rev. Aquac. 2013, 5, 61–71. [Google Scholar] [CrossRef] [Green Version]
- Tyedmers, P.; Pelletier, N. Biophysical accounting in aquaculture: Insights from current practice and the need for methodological development. In Comparative Assessment of the Environmental Costs of Aquaculture and Other Food Production Sectors: Methods for Meaningful Comparisons, Proceedings of the FAO/WFT Expert Workshop, Vancouver, BC, Canada, 24–28 April 2006; Bartley, D.M., Brugère, C., Soto, D., Gerber, P., Harvey, B., Eds.; FAO: Roma, Italy, 2006; pp. 229–241. [Google Scholar]
- Henriksson, P.J.G.; Guinee, J.B.; Kleijn, R.; de Snoo, G.R. Life cycle assessment of aquaculture systems-a review of methodologies. Int. J. Life Cycle Assess. 2012, 17, 304–313. [Google Scholar] [CrossRef] [PubMed]
- British Standards Institution. Assessment of Life Cycle Greenhouse Gas Emissions. Supplementary Requirements for the Application of PAS 2050:2011 to Seafood and Other Aquatic Products; BSI Standards Limited: London, UK, 2012. [Google Scholar]
- Boesch, M.E.; Hellweg, S.; Huijbregts, M.A.J.; Frischknecht, R. Applying cumulative exergy demand (CExD) indicators to the ecoinvent database. Int. J. Life Cycle Assess. 2007, 12, 181–190. [Google Scholar] [CrossRef]
- Bosma, R.; Anh, P.T.; Potting, J. Life cycle assessment of intensive striped catfish farming in the Mekong Delta for screening hotspots as input to environmental policy and research agenda. Int. J. Life Cycle Assess. 2011, 16, 903–915. [Google Scholar] [CrossRef] [Green Version]
- Huysveld, S.; Schaubroeck, T.; De Meester, S.; Sorgeloos, P.; Van Langenhove, H.; Van Linden, V.; Dewulf, J. Resource use analysis of Pangasius aquaculture in the Mekong Delta in Vietnam using Exergetic Life Cycle Assessment. J. Clean. Prod. 2013, 51, 225–233. [Google Scholar] [CrossRef]
- Nhu, T.T.; Dewulf, J.; Serruys, P.; Huysveld, S.; Nguyen, C.V.; Sorgeloos, P.; Schaubroeck, T. Resource usage of integrated Pig-Biogas-Fish system: Partitioning and substitution within attributional life cycle assessment. Resour. Conserv. Recycl. 2015, 102, 27–38. [Google Scholar] [CrossRef]
- Nhu, T.T.; Schaubroeck, T.; Henriksson, P.J.G.; Bosma, R.; Sorgeloos, P.; Dewulf, J. Environmental impact of non-certified versus certified (ASC) intensive Pangasius aquaculture in Vietnam, a comparison based on a statistically supported LCA. Environ. Pollut. 2016, 219, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Taelman, S.E.; De Meester, S.; Roef, L.; Michiels, M.; Dewulf, J. The environmental sustainability of microalgae as feed for aquaculture: A life cycle perspective. Bioresour. Technol. 2013, 150, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Wernet, G.; Bauer, C.; Steubing, B.; Reinhard, J.; Moreno-Ruiz, E.; Weidema, B. The ecoinvent database version 3 (part I): Overview and methodology. Int. J. Life Cycle Assess. 2016, 21, 1218–1230. [Google Scholar] [CrossRef]
- Dewulf, J.; Boesch, M.E.; De Meester, B.; Van der Vorst, G.; Van Langenhove, H.; Hellweg, S.; Huijbregts, M.A.J. Cumulative exergy extraction from the natural environment (CEENE): A comprehensive life cycle impact assessment method for resource accounting. Environ. Sci. Technol. 2007, 41, 8477–8483. [Google Scholar] [CrossRef] [PubMed]
- Groen, E.A.; Heijungs, R.; Bokkers, E.A.M.; de Boer, I.J.M. Methods for uncertainty propagation in life cycle assessment. Environ. Model. Softw. 2014, 62, 316–325. [Google Scholar] [CrossRef]
- The R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 1 February 2019).
- Henriksson, P.J.G.; Guinee, J.B.; Heijungs, R.; de Koning, A.; Green, D.M. A protocol for horizontal averaging of unit process data-including estimates for uncertainty. Int. J. Life Cycle Assess. 2014, 19, 429–436. [Google Scholar] [CrossRef]
- Muller, S.; Lesage, P.; Ciroth, A.; Mutel, C.; Weidema, B.P.; Samson, R. The application of the pedigree approach to the distributions foreseen in ecoinvent v3. Int. J. Life Cycle Assess. 2016, 21, 1327–1337. [Google Scholar] [CrossRef]
- Weidema, B.; Bauer, C.; Hischier, R.; Mutel, C.; Nemecek, T.; Reinhard, J.; Vadenbo, C.; Wernet, G. Overview and Methodology. Data Quality Guideline for the Ecoinvent Database Version 3; Ecoinvent Report 1(v3); The Ecoinvent Centre: St. Gallen, Switzerland, 2013. [Google Scholar]
- Henriksson, P.J.G.; Heijungs, R.; Dao, H.M.; Phan, L.T.; de Snoo, G.R.; Guinee, J.B. Product Carbon Footprints and Their Uncertainties in Comparative Decision Contexts. PLoS ONE 2015, 10, e0121221. [Google Scholar] [CrossRef] [PubMed]
- Hofert, M.; Kojadinovic, I.; Maechler, M.; Yan, J. Copula: Multivariate Dependence with Copulas. R Package Version 0.999-18. Available online: https://CRAN.R-project.org/package=copula (accessed on 1 February 2019).
- Nelsen, R. An Introduction to Copulas; Springer: New York, NY, USA, 2006. [Google Scholar]
- Bojaca, C.R.; Schrevens, E. Parameter uncertainty in LCA: Stochastic sampling under correlation. Int. J. Life Cycle Assess. 2010, 15, 238–246. [Google Scholar] [CrossRef]
- Sugiyama, H.; Fukushima, Y.; Hirao, M.; Hellweg, S.; Hungerbuhler, K. Using standard statistics to consider uncertainty in industry-based life cycle inventory databases. Int. J. Life Cycle Assess. 2005, 10, 399–405. [Google Scholar] [CrossRef]
- Chen, H. Large yellow croaker seedling production technology. Fisher. Sci. Technol. Inf. 2001, 28, 212–214. (In Chinese) [Google Scholar]
- Mercier, A.; Hamel, J.F. Sea cucumber aquaculture: Hatchery production, juvenile growth and industry challenges. In Advances in Aquaculture Hatchery Technology; Allan, G., Burnell, G., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2013; pp. 431–454. [Google Scholar] [CrossRef]
- Liu, H.; Xu, Q.; Liu, S.; Zhang, L.; Yang, H. Evaluation of body weight of sea cucumber Apostichopus japonicus by computer vision. Chin. J. Oceanol. Limnol. 2015, 33, 114–120. [Google Scholar] [CrossRef]
- Mardones, A.; Augsburger, A.; Vega, R.; de Los Rios-Escalante, P. Growth rates of Haliotis rufescens and Haliotis discus hannai in tank culture systems in southern Chile (41.5 degrees S). Latin Am. J. Aquat. Res. 2013, 41, 959–967. [Google Scholar] [CrossRef]
- FAO. Training Manual on Artificial Breeding of Abalone (Haliotis discus hannai) in Korea DPR; Training Manual 7; FAO: Roma, Italy, 1990. [Google Scholar]
- Sun, H.; Liang, M.; Yan, J.; Chen, B. Nutrient requirements and growth of the sea cucumber, Apostichopus japonicus. In Advances in Sea Cucumber Aquaculture and Management; Lovatelli, A., Conand, C., Purcell, S., Uthicke, S., Hamel, J.F., Mercier, A., Eds.; FAO Fisheries Technical Paper No. 463; FAO: Rome, Italy, 2004; pp. 327–331. [Google Scholar]
- Han, C. General Secretary of the Ningde Fishery Society: Next Year the Large Yellow Croaker Branch Will Have a Promising Set Up. Mod. Aquac. 2013, 10, 46–47. (In Chinese) [Google Scholar]
- Frischknecht, R.; Jungbluth, N.; Althaus, H.; Bauer, C.; Doka, G.; Dones, R.; Hischier, R.; Hellweg, S.; Humbert, S.; Köllner, T.; et al. Implementation of Life Cycle Impact Assessment Methods. Data (v2.0); Swiss Centre for Life Cycle Inventories: Edinburgh, UK, 2007. [Google Scholar]
- Abdou, K.; Aubin, J.; Romdhane, M.S.; Le Loc’h, F.; Lasrama, F.B.R. Environmental assessment of seabass (Dicentrarchus labrax) and seabream (Sparus aurata) farming from a life cycle perspective: A case study of a Tunisian aquaculture farm. Aquaculture 2017, 471, 204–212. [Google Scholar] [CrossRef]
- Aubin, J.; Papatryphon, E.; van der Werf, H.M.G.; Chatzifotis, S. Assessment of the environmental impact of carnivorous finfish production systems using life cycle assessment. J. Clean. Prod. 2009, 17, 354–361. [Google Scholar] [CrossRef]
- Boissy, J.; Aubin, J.; Drissi, A.; van der Werf, H.M.G.; Bell, G.J.; Kaushik, S.J. Environmental impacts of plant-based salmonid diets at feed and farm scales. Aquaculture 2011, 321, 61–70. [Google Scholar] [CrossRef]
- Guan, Y.; Hu, B.; Ai, C.; Zhang, J. Research and development of formulated feed and the feeding technology strategy on large yellow croaker. Feed Ind. 2014, 35, 27–32. (In Chinese) [Google Scholar]
- Hu, B. Application status of formulated feed for large yellow croaker. Chin. Aquac. 2015, 3, 48–50. (In Chinese) [Google Scholar]
- Henriksson, P.J.G.; Zhang, W.; Nahid, S.; Newton, R.; Phan, L.; Dao, H.; Zhang, Z.; Jaithiang, J.; Andong, R.; Chaimanuskul, K.; et al. Primary Data and Literature Sources Adopted in the SEAT LCA Studies; SEAT: Martorell, Spain, 2014. [Google Scholar]
- Chatvijitkul, S.; Boyd, C.E.; Davis, D.A.; McNevin, A.A. Embodied Resources in Fish and Shrimp Feeds. J. World Aquac. Soc. 2017, 48, 7–19. [Google Scholar] [CrossRef]
- Medeiros, M.V.; Aubin, J.; Camargo, A.F.M. Life cycle assessment of fish and prawn production: Comparison of monoculture and polyculture freshwater systems in Brazil. J. Clean. Prod. 2017, 156, 528–537. [Google Scholar] [CrossRef]
- Pelletier, N.; Tyedmers, P.; Sonesson, U.; Scholz, A.; Ziegler, F.; Flysjo, A.; Kruse, S.; Cancino, B.; Silverman, H. Not All Salmon Are Created Equal: Life Cycle Assessment (LCA) of Global Salmon Farming Systems. Environ. Sci. Technol. 2009, 43, 8730–8736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angel, D.L.; Katz, T.; Eden, N.; Spanier, E.; Black, K.D. Damage control in the coastal zone: Improving water quality by harvesting aquaculture-derived nutrients. In Strategic Management of Marine Ecosystems; Levner, E., Linkov, I., Proth, J.M., Eds.; Springer: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Soto, D.; Manjarrez, J.A.; Brummet, R. Aquaculture zoning, site selection and area management under the ecosystem approach to aquaculture. IEEE Trans. Power Appar. Syst. 2015, 50, 593–597. [Google Scholar]
- Mungkung, R.; Aubin, J.; Prihadi, T.H.; Slernbrouck, J.; van der Werf, H.M.G.; Legendre, M. Life Cycle Assessment for environmentally sustainable aquaculture management: A case study of combined aquaculture systems for carp and tilapia. J. Clean. Prod. 2013, 57, 249–256. [Google Scholar] [CrossRef]
- Langlois, J.; Freon, P.; Steyer, J.-P.; Delgenes, J.-P.; Helias, A. Sea-use impact category in life cycle assessment: State of the art and perspectives. Int. J. Life Cycle Assess. 2014, 19, 994–1006. [Google Scholar] [CrossRef]
- Taelman, S.E.; De Meester, S.; Schaubroeck, T.; Sakshaug, E.; Alvarenga, R.A.F.; Dewulf, J. Accounting for the occupation of the marine environment as a natural resource in life cycle assessment: An exergy based approach. Resour. Conserv. Recycl. 2014, 91, 1–10. [Google Scholar] [CrossRef]
- Draganovic, V.; Jorgensen, S.E.; Boom, R.; Jonkers, J.; Riesen, G.; van der Goot, A.J. Sustainability assessment of salmonid feed using energy, classical exergy and eco-exergy analysis. Ecol. Indic. 2013, 34, 277–289. [Google Scholar] [CrossRef]
- Papatryphon, E.; Petit, J.; Kaushik, S.J.; van der Werf, H.M.G. Environmental impact assessment of salmonid feeds using Life Cycle Assessment (LCA). Ambio 2004, 33, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Dietz, T.; Carpenter, S.R.; Folke, C.; Alberti, M.; Redman, C.L.; Schneider, S.H.; Ostrom, E.; Pell, A.N.; Lubchenco, J.; et al. Coupled human and natural systems. Ambio 2007, 36, 639–649. [Google Scholar] [CrossRef]
- Liu, J.; Mooney, H.; Hull, V.; Davis, S.J.; Gaskell, J.; Hertel, T.; Lubchenco, J.; Seto, K.C.; Gleick, P.; Kremen, C.; et al. Systems integration for global sustainability. Science 2015, 347. [Google Scholar] [CrossRef] [PubMed]




| Product | Data Points (318 Households) | Product Proportion (292 Households) | Data Points Used in Modeling * |
|---|---|---|---|
| Large yellow croaker | 115 | 27.3 | 84 (A1); 18 (A2) |
| Sea cucumber | 44 | 10.5 | 10 (B1); 15 (B2); 11 (B3) |
| Abalone | 52 | 12.4 | 49 |
| Laminaria | 67 | 15.9 | 61 |
| Gracilaria | 61 | 14.5 | 61 |
| Porphyra | 39 | 9.3 | 36 |
| Other finfish | 43 | 10.1 | Not included |
| Other products ** | 57 | Not included | Not included |
| Stage | Output | Large Yellow Croaker | Sea Cucumber | Abalone | |||
|---|---|---|---|---|---|---|---|
| Weight (g/unit) | Total Residence Time (months) | Weight (g/unit) | Total Residence Time (months) | Weight (g/unit) | Total Residence Time (months) | ||
| Hatchery/ nursery | Small juvenile | 0.39–2.16 * | 2 [50] | 0.20–2.50 [51,52] | 3 [51] | 0.04–1.21 [24,53] | 8 [54] |
| Initial grow-out (1) | Intermediate juvenile | - | - | 34.00–110.00 * [52] | 10 [55] | 2.10–5.00 * [24,53] | 12 [54] |
| Initial grow-out (2) | Big juvenile | - | - | - | - | 15.00–22.00 * [24,53] | 20 [54] |
| Final grow-out | Market-size product | 250.00–350.00 * [56] | 18 * | 138.00–169.00 * [52] | 16 * | 39.00–44.00 * [24,53] | 30 [54] |
| Stage | Inputs/Outputs | Yellow Croaker | Sea Cucumber | Abalone | Unit | |||
|---|---|---|---|---|---|---|---|---|
| A1 | A2 | B1 | B2 | B3 | ||||
| Seed production inputs | Heat | 378.50 (1.49) | 486.70 (1.47) | 180.70 (1.62) | 118.20 (1.89) | 141.60 (1.68) | 681.50 (1.86) | MJ |
| Electricity | 287.20 (1.50) | 369.60 (1.47) | 402.00 (0.79) | 249.50 (1.04) | 288.60 (0.87) | 1386.20 (1.03) | kWh | |
| Fry feed | 1.10 (1.52) | 1.40 (1.49) | - | - | - | - | kg | |
| Liquid oxygen | 0.60 (1.53) | 0.80 (1.48) | 3.10 (0.84) | 1.90 (1.09) | 2.30 (0.92) | 10.80 (1.08) | kg | |
| Calcium carbonate | 11.10 (1.51) | 14.40 (1.50) | 57.10 (0.84) | 35.70 (1.09) | 41.40 (0.92) | 199.50 (1.08) | kg | |
| Chlorine | 0.01 (1.52) | 0.01 (1.50) | 0.04 (0.80) | 0.03 (1.04) | 0.03 (0.88) | 0.14 (1.04) | kg | |
| Intermediate inputs and exchanges | Juvenile transport to local farm | - | - | 2.70 (0.79) | 1.70 (1.04) | 2.00 (0.88) | 9.40 (1.03) | t km |
| Small juveniles | - | - | 14.20 (0.75) | 8.70 (0.99) | 10.10 (0.83) | 48.40 (0.99) | kg | |
| Initial grow-out inputs | Fuel | - | - | 18.60 (1.92) | 12.20 (2.17) | 14.60 (1.97) | 70.80 (2.05) | kg |
| Formulated feed | - | - | 1010.00 (0.63) | 618.00 (0.88) | 687.80 (0.72) | - | kg | |
| Raw seaweed (laminaria) | - | - | - | - | - | 1847.00 (1.10) | kg | |
| Raw seaweed (gracilaria) | - | - | - | - | - | 10842.90 (1.09) | kg | |
| Juvenile transport between farms (intermediate) | - | - | - | - | - | 633.30 (1.03) | t km | |
| Intermediate inputs and exchanges | Juvenile transport to Fujian province | - | - | 937.60 (0.72) | 585.10 (0.97) | 659.00 (0.80) | 378.70 (1.57) | t km |
| Juvenile transport to local farm | 0.50 (1.50) | 0.70 (1.47) | 105.20 (0.66) | 65.10 (0.91) | 72.90 (0.75) | 249.10 (0.78) | t km | |
| Big juveniles | 2.70 (1.46) | 3.50 (1.43) | 550.00 (0.61) | 335.40 (0.87) | 372.60 (0.70) | 1273.70 (0.73) | kg | |
| Final grow-out inputs | Fuel | 30.50 (0.78) | 33.60 (0.85) | 68.50 (1.29) | 41.60 (1.05) | 41.00 (1.00) | 58.50 (1.57) | kg |
| Formulated feed | 428.90 (1.59) | - | 280.20 (0.68) | - | - | - | kg | |
| Trash fish | 3588.40 (0.63) | 4197.90 (0.45) | 986.60 (0.84) | 889.50 (0.80) | - | - | kg | |
| Raw seaweed (laminaria) | - | - | 3097.00 (0.57) | 7041.00 (0.54) | 5130.50 (0.98) | 3154.20 (0.99) | kg | |
| Raw seaweed (gracilaria) | - | - | 753.40 (0.63) | 1714.90 (0.60) | 1258.10 (1.04) | 18627.10 (0.96) | kg | |
| Chemicals | 1.00 (2.05) | 0.40 (1.49) | - | - | - | - | kg | |
| System output parameters | Market-size product | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | t |
| Economic feed conversion ratio | 4.40 (0.58) | 4.20 (0.45) | 6.40 (0.50) | 10.60 (0.53) | 7.30 (0.91) | 38.80 (0.81) | - | |
| Stage | Inputs/Outputs | Laminaria | Gracilaria | Porphyra | Unit |
|---|---|---|---|---|---|
| Seed production inputs | Heat | 8.00 × 10−4 (0.80) | - | 1.22 × 10−2 (0.31) | MJ |
| Electricity | 4.00 × 10−4 (0.80) | - | 6.60 × 10−3 (0.31) | kWh | |
| Intermediate inputs and exchanges | Seed transport to Fujian province | - | - | 0.87 (0.28) | t km |
| Seed transport to local farm | 0.01 (0.72) | - | 0.20 (0.21) | t km | |
| Seeds | 0.06 (0.70) | 145.32 (1.96) | 2.46 (0.85) | kg | |
| Grow-out inputs | Fuel | 2.32 (1.38) | 9.47 (2.01) | 10.92 (1.07) | kg |
| Chemicals | 0.01 (0.53) | - | - | kg | |
| System output parameters | Market-size product | 1.00 | 1.00 | 1.00 | t |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marín, T.; Wu, J.; Wu, X.; Ying, Z.; Lu, Q.; Hong, Y.; Wang, X.; Yang, W. Resource Use in Mariculture: A Case Study in Southeastern China. Sustainability 2019, 11, 1396. https://doi.org/10.3390/su11051396
Marín T, Wu J, Wu X, Ying Z, Lu Q, Hong Y, Wang X, Yang W. Resource Use in Mariculture: A Case Study in Southeastern China. Sustainability. 2019; 11(5):1396. https://doi.org/10.3390/su11051396
Chicago/Turabian StyleMarín, Tomás, Jing Wu, Xu Wu, Zimin Ying, Qiaoling Lu, Yiyuan Hong, Xiaoyan Wang, and Wu Yang. 2019. "Resource Use in Mariculture: A Case Study in Southeastern China" Sustainability 11, no. 5: 1396. https://doi.org/10.3390/su11051396





