Experimental Investigation and Numerical Simulation of CO2–Brine–Rock Interactions during CO2 Sequestration in a Deep Saline Aquifer
Abstract
:1. Introduction
2. Experimental Set-Up
2.1. Experimental Materials
2.2. Experimental Procedure
2.3. Analytical Methods
2.4. Experimental Results
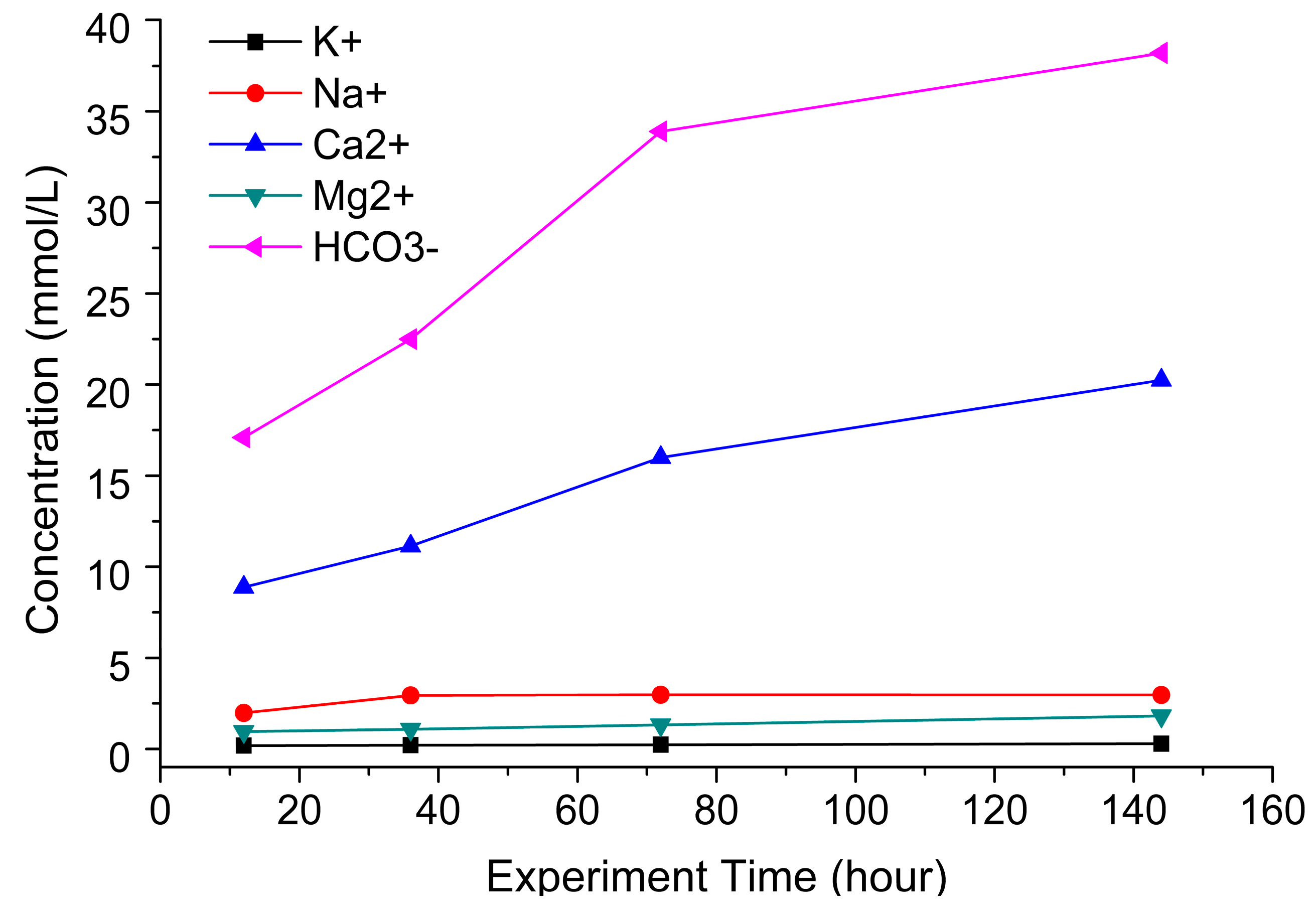
2.4.1. Effect of Experiment Time on CO2–Brine–Rock Interaction
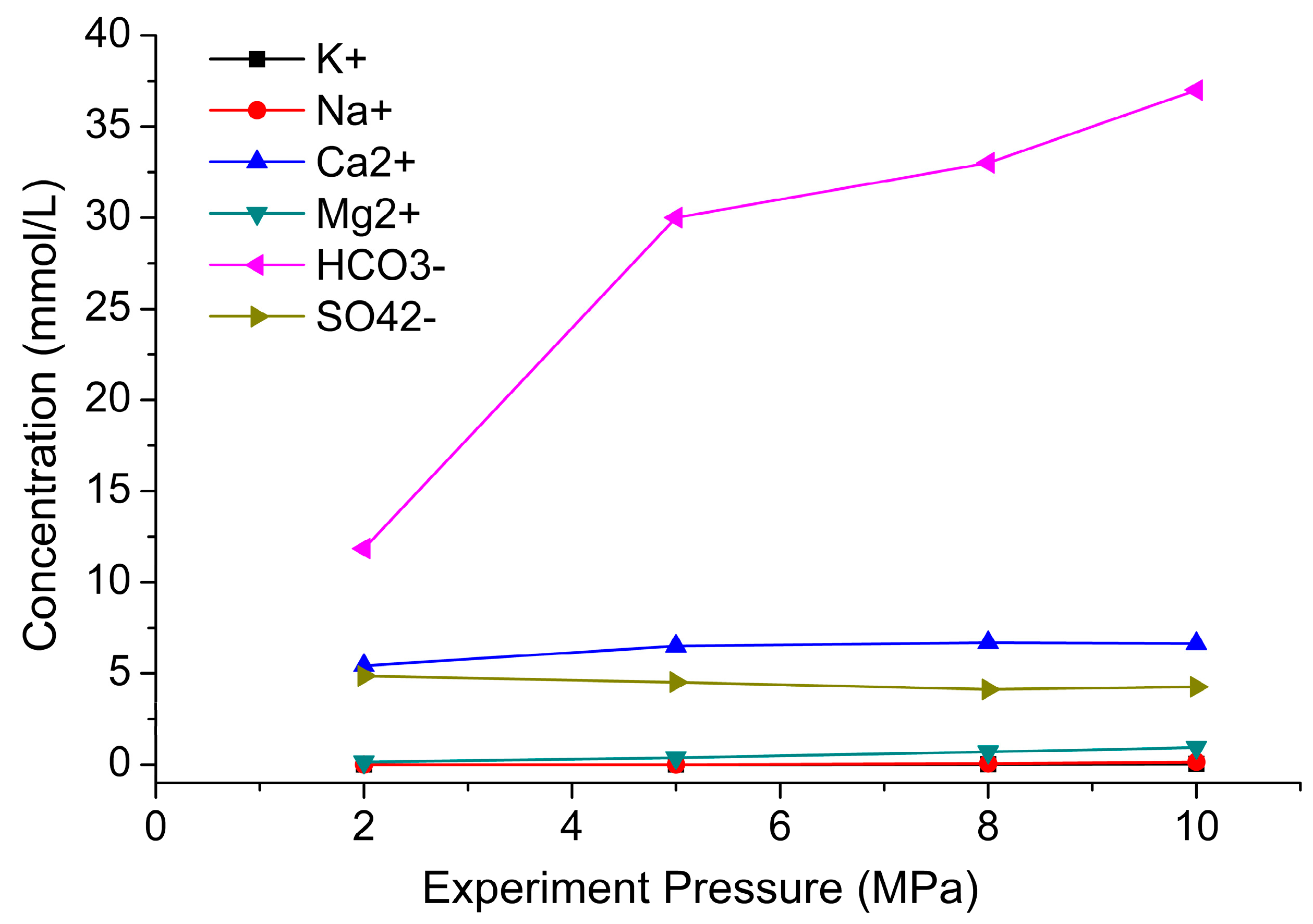
2.4.2. Effect of Experimental Pressure on CO2–Brine–Rock Interaction
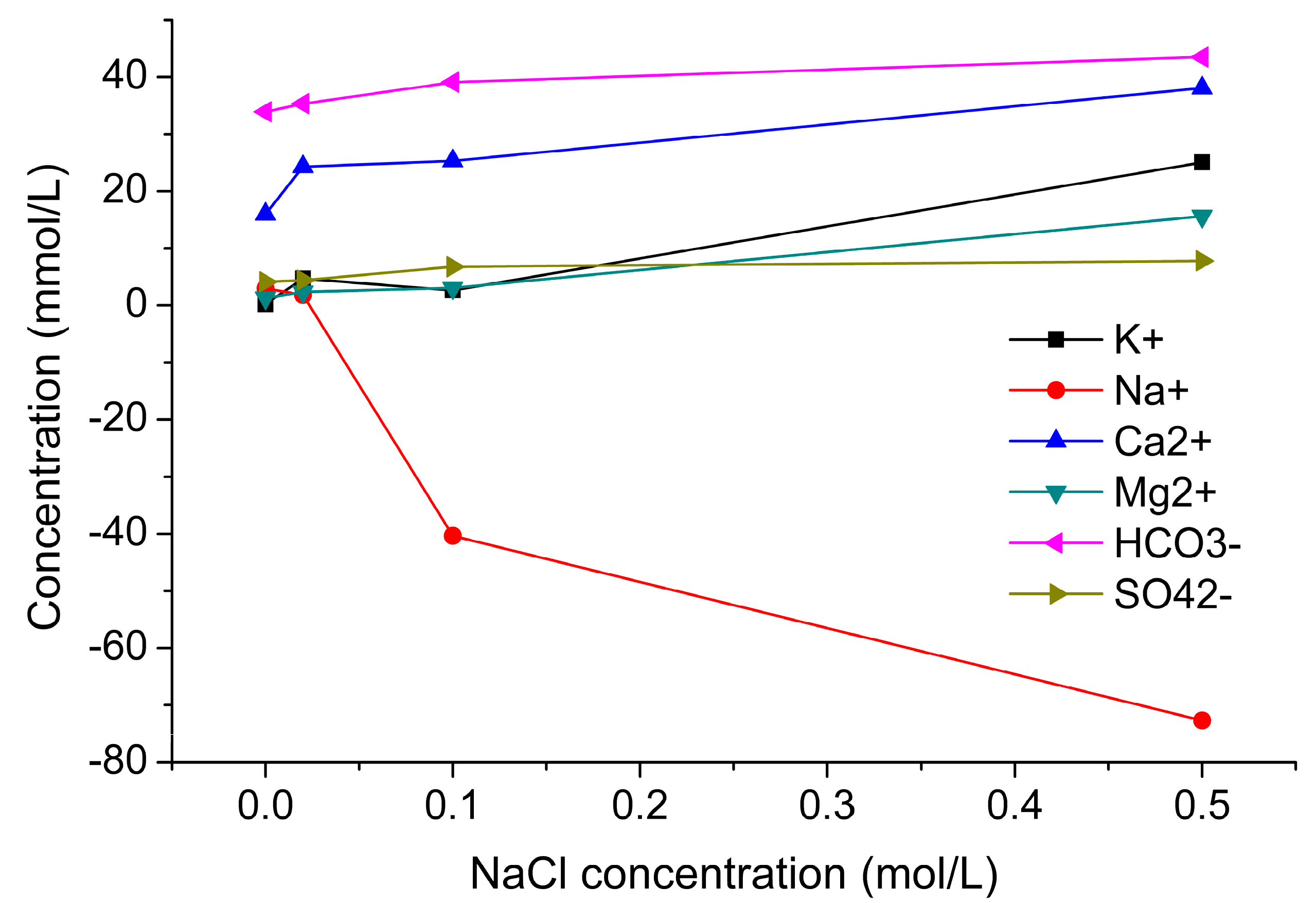
2.4.3. Effect of NaCl Concentration on CO2–Brine–Rock Interaction
2.4.4. Effect of CO2–Brine–Rock Interaction on Rock Minerals
3. Simulation Evaluation
3.1. Balanced Simulation by PHREEQC
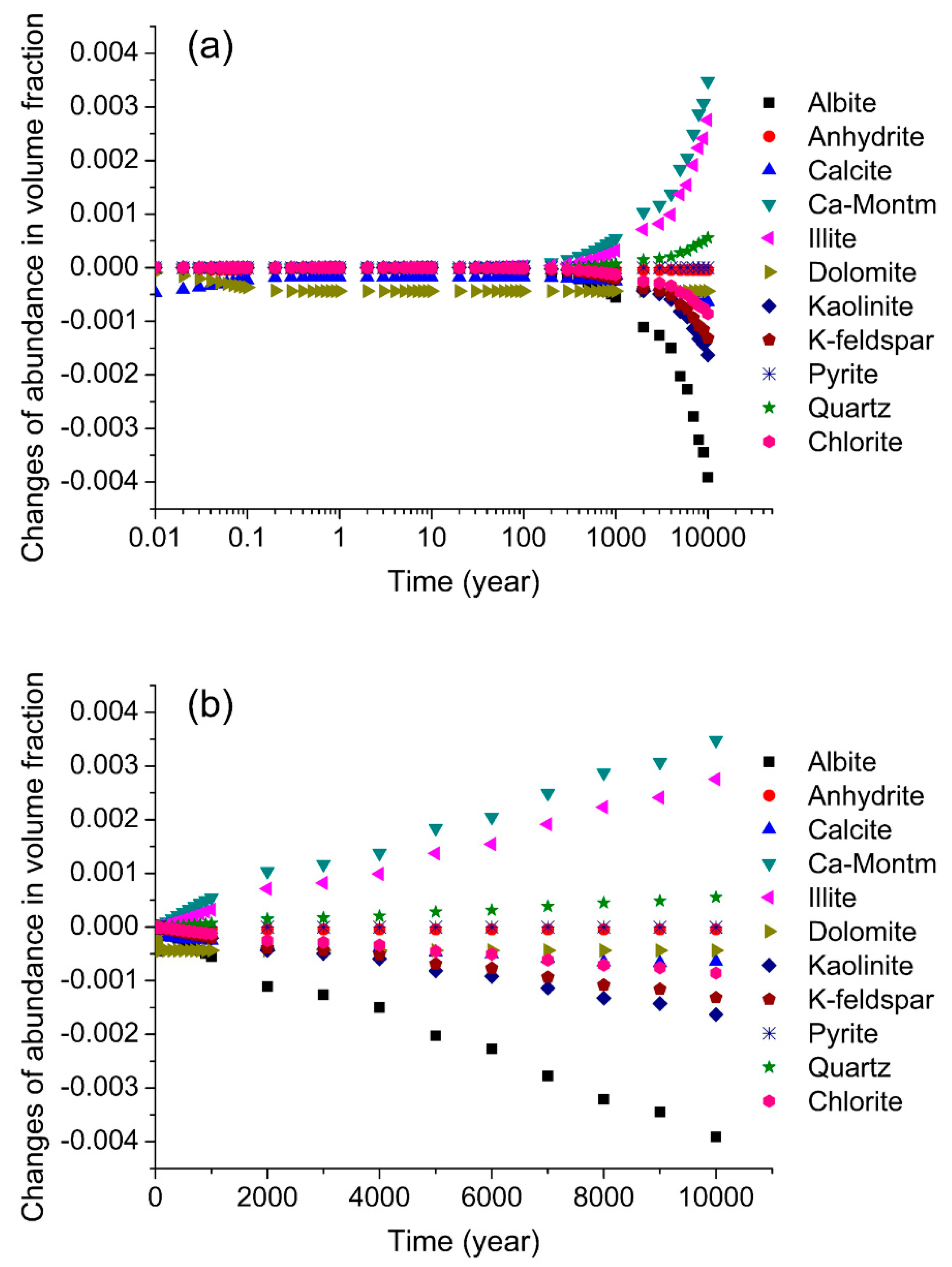
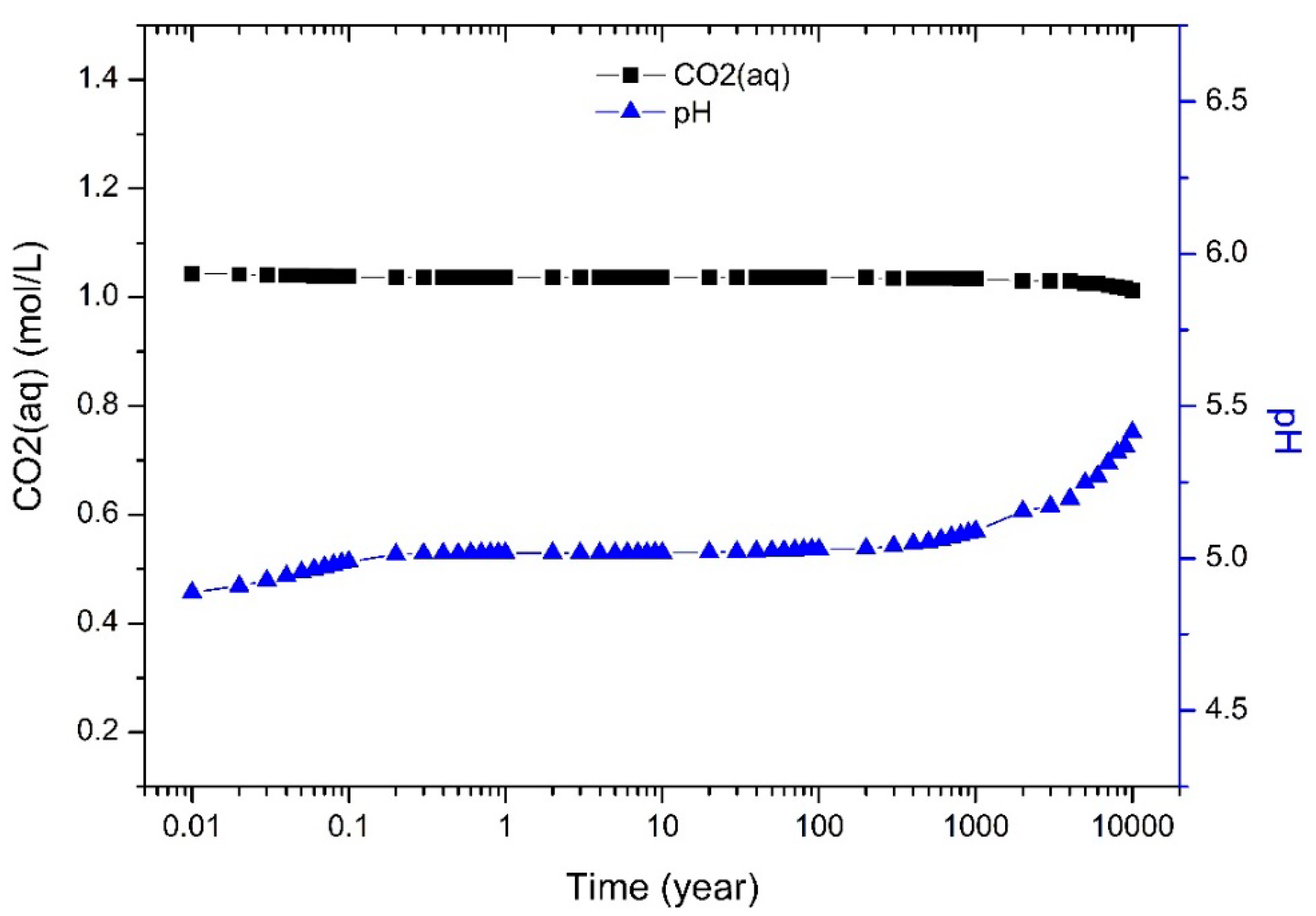
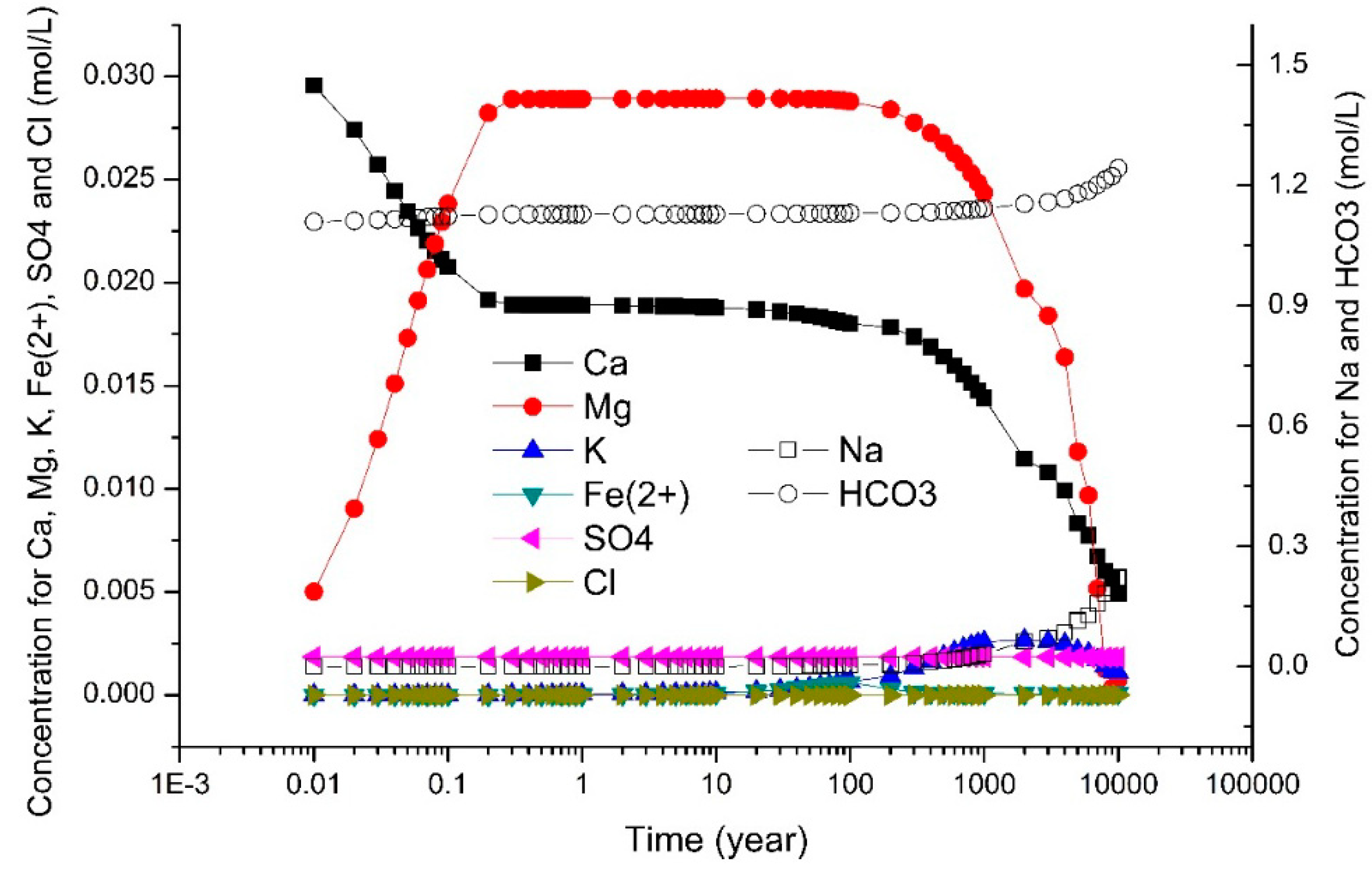
3.2. Dynamic Simulation by TOUGHREACT
4. Discussion
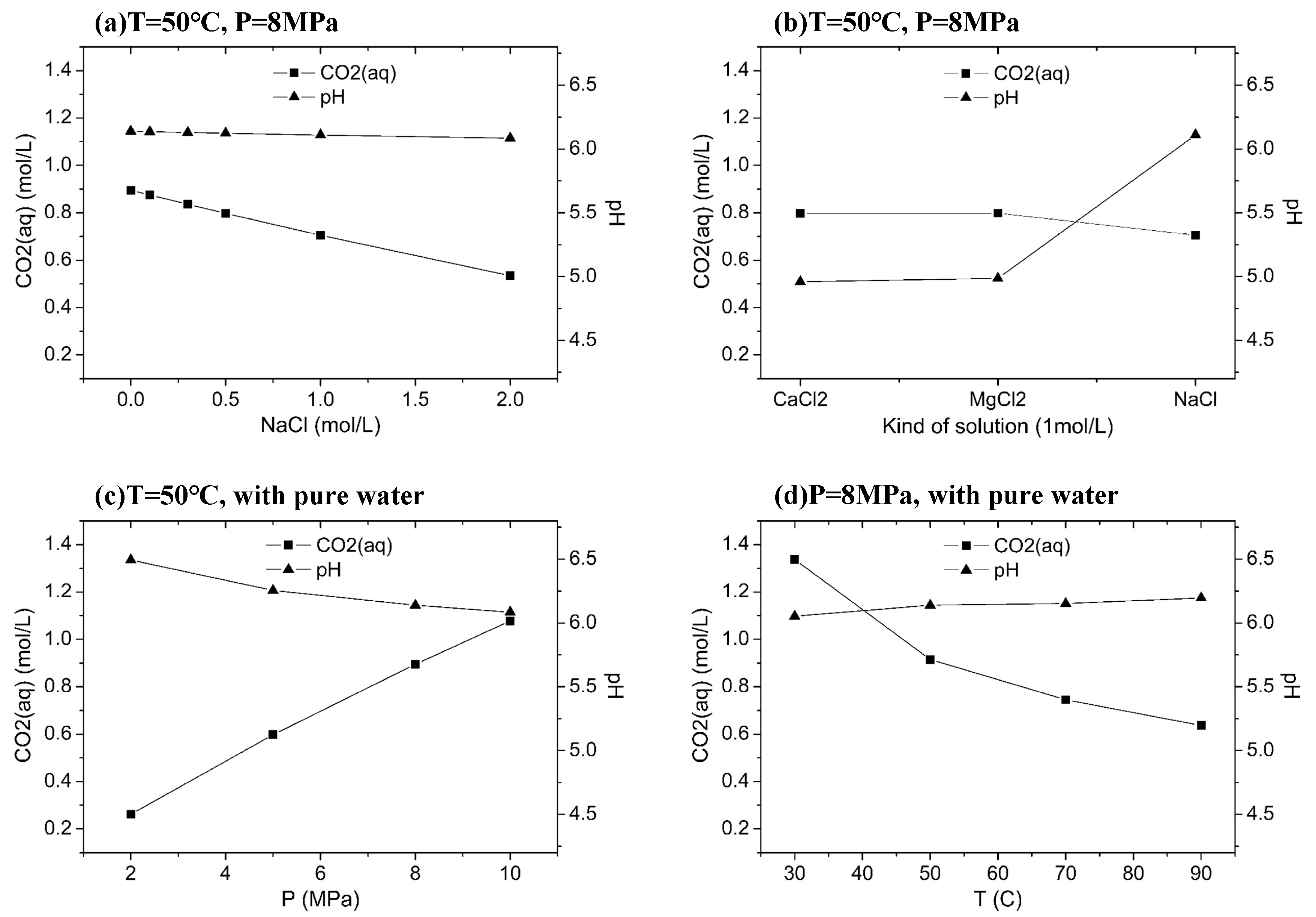
4.1. CO2 Dissolution and pH
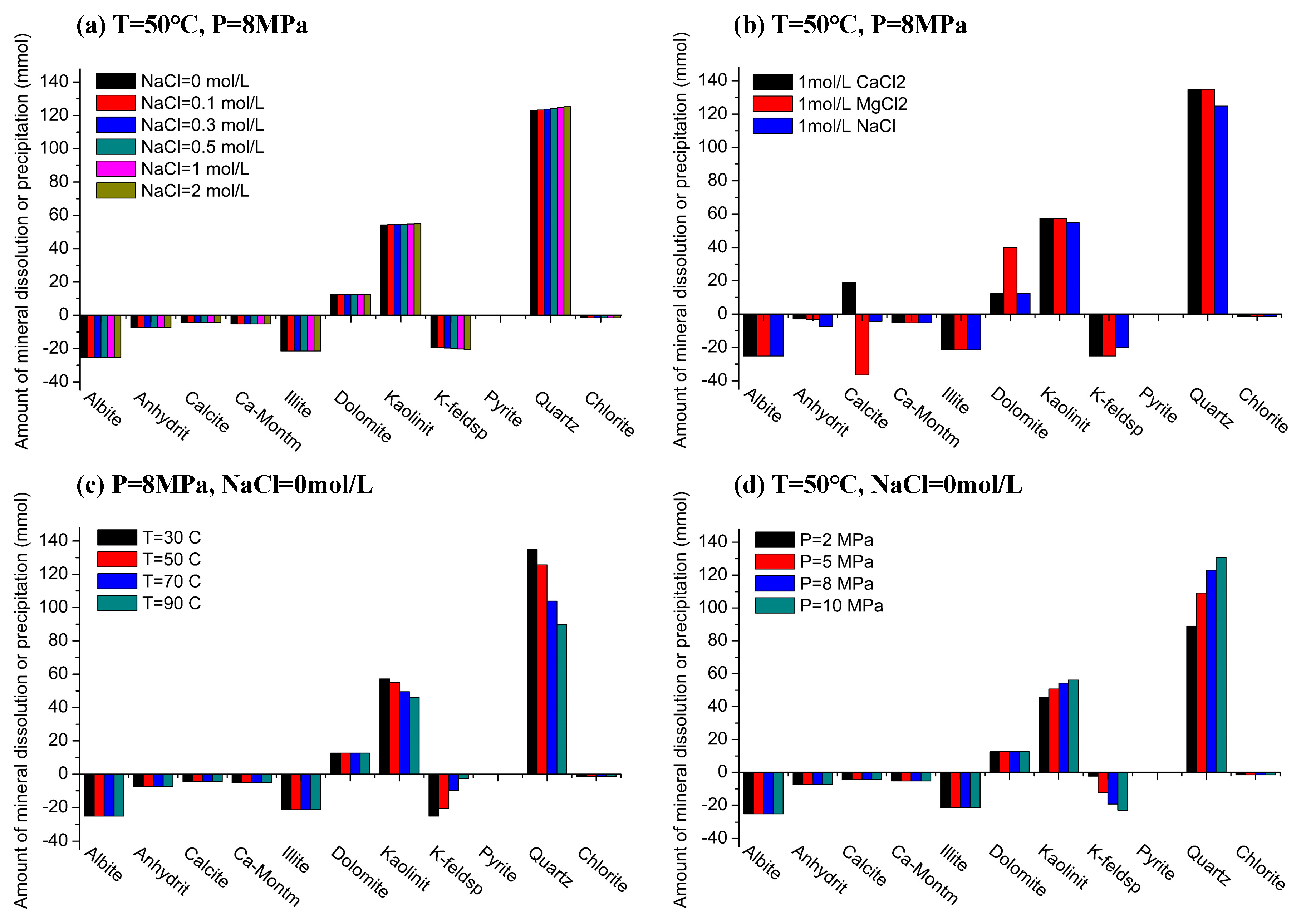
4.2. Minerals Dissolution and Precipitation
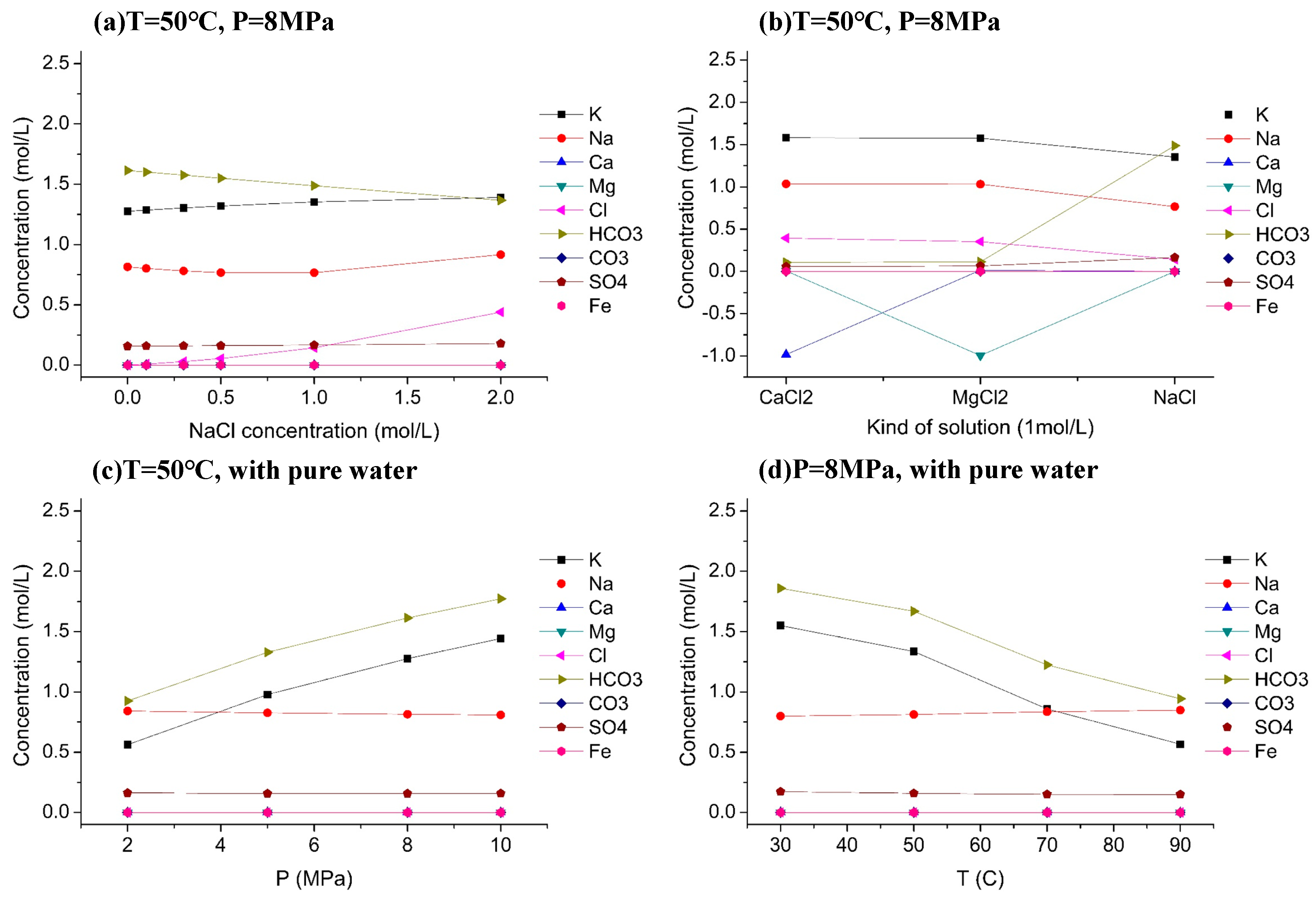
4.3. Main Ions in Solution
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, K.; Xu, T.; Wang, F.; Tian, H. Experimental study of CO2-brine-rock interaction during CO2 sequestration in deep coal seams. Int. J. Coal Geol. 2016, 154, 265–274. [Google Scholar] [CrossRef]
- Allen, M.R.; Stott, P.A.; Mitchell, J.F.B.; Schnur, R.; Dehworth, T.L. Quantifying the uncertainty in forecasts of anthropogenic climate change. Nature 2000, 407, 617. [Google Scholar] [CrossRef] [PubMed]
- Palmer, T.N.; Räisänen, J. Quantifying the risk of extreme seasonal precipitation events in a changing climate. Nature 2002, 415, 512–514. [Google Scholar] [CrossRef] [PubMed]
- Stocker, T.F.; Schmittner, A. Influence of CO2 emission rates on the stability of the thermohaline circulation. Nature 1997, 388, 862–865. [Google Scholar] [CrossRef]
- Bradshaw, J.; Bachu, S.; Bonijoly, D.; Burruss, R.; Holloway, S.; Christensen, N.P.; Mathiassen, O.M. CO2 storage capacity estimation: Issues and development of standards. Int. J. Greenh. Gas Control 2007, 1, 62–68. [Google Scholar] [CrossRef]
- Du, S.; Su, X.; Xu, W. Assessment of CO2 geological storage capacity in the oilfields of the Songliao Basin, northeastern China. Geosci. J. 2016, 20, 247–257. [Google Scholar] [CrossRef]
- Khudaida, K.J. Geological Carbon Sequestration in the Context of Two-Phase Flow in Porous Media: A Review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1105–1147. [Google Scholar]
- Bachu, S. Sequestration of CO2 in geological media: Criteria and approach for site selection in response to climate change. Energy Convers. Manag. 2000, 41, 953–970. [Google Scholar] [CrossRef]
- Bachu, S. CO2 storage in geological media: Role, means, status and barriers to deployment. Prog. Energy Combust. Sci. 2008, 34, 254–273. [Google Scholar] [CrossRef]
- Bachu, S.; Adams, J.J. Sequestration of CO2 in geological media in response to climate change: Capacity of deep saline aquifers to sequester CO2 in solution. Energy Convers Manag. Energy Convers. Manag. 2003, 44, 3151–3175. [Google Scholar] [CrossRef]
- Zahid, U.; Lim, Y.; Jung, J.; Han, C. CO2 geological storage: A review on present and future prospects. Korean J. Chem. Eng. 2011, 28, 674–685. [Google Scholar] [CrossRef]
- Zheng, F.; Shi, X.Q.; Wu, J.C.; Chen, Y.; Xu, H.X. Global Sensitivity Analysis of Reactive Transport Modeling of CO2 Geological Storage in a Saline Aquifer. Procedia Earth Planet. Sci. 2013, 7, 798–801. [Google Scholar] [CrossRef]
- Xu, T.; Apps, J.A.; Pruess, K. Mineral sequestration of carbon dioxide in a sandstone–shale system. Chem. Geol. 2005, 217, 295–318. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Y.; Xu, T.; Cheng, H.; Zheng, Y.; Xiong, P. Long-term Variations of CO2 Trapped in Different Mechanisms in Deep Saline Formations: A Case Study of the Songliao Basin, China. Int. J. Greenh. Gas Control 2009, 3, 161–180. [Google Scholar] [CrossRef]
- Maroto-Valer, M.M.; Garcia, S.; Kaminska, A. Underground carbon dioxide storage in saline formations. Waste Resour. Manag. 2010, 163, 77–88. [Google Scholar]
- Steel, L.; Mackay, E.; Maroto-Valer, M.M. Experimental investigation of CO2-brine-calcite interactions under reservoir conditions. Fuel Process. Technol. 2018, 169, 122–131. [Google Scholar] [CrossRef]
- Benson, S.M.; Cole, D.R. CO2 Sequestration in Deep Sedimentary Formations. Elements 2008, 4, 325–331. [Google Scholar] [CrossRef]
- Kampman, N.; Bickle, M.; Wigley, M.; Dubacq, B. Fluid flow and CO2-fluid-mineral interactions during CO2-storage in sedimentary basins. Chem. Geol. 2014, 369, 22–50. [Google Scholar] [CrossRef]
- Liu, F.Y.; Lu, P.; Griffith, C.; Hedges, S.W.; Soong, Y.; Hellevang, H.; Zhu, C. CO2-brine-caprock interaction: Reactivity experiments on Eau Claire shale and a review of relevant literature. Int. J. Greenh. Gas Control 2012, 7, 153–167. [Google Scholar] [CrossRef]
- Maskell, A.; Kampman, N.; Chapman, H.; Condon, D.J.; Bickle, M. Kinetics of CO2-fluid-rock reactions in a basalt aquifer, Soda Springs, Idaho. Appl. Geochem. 2015, 61, 272–283. [Google Scholar] [CrossRef]
- Ostertag-Henning, C.; Risse, A.; Thomas, B.; Rosenbauer, R.; Rochelle, C.; Purser, G.; Kilpatrick, A.; Rosenqvist, J.; Yardley, B.; Karamalidis, A.; et al. GaMin’11-an international inter-laboratory comparison for geochemical CO2-saline fluid-mineral interaction experiments. In 12th International Conference on Greenhouse Gas Control Technologies, Ghgt-12, Austin; Dixon, T., Herzog, H., Twinning, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 63, pp. 5538–5543. [Google Scholar]
- Pearce, J.K.; Kirste, D.M.; Dawson, G.K.W.; Farquhar, S.M.; Biddle, D.; Golding, S.D.; Rudolph, V. SO2 impurity impacts on experimental and simulated CO2-water-reservoir rock reactions at carbon storage conditions. Chem. Geol. 2015, 399, 65–86. [Google Scholar] [CrossRef]
- Tarkowski, R.; Wdowin, M.; Manecki, M. Petrophysical examination of CO2-brine-rock interactions-results of the first stage of long-term experiments in the potential Zaosie Anticline reservoir (central Poland) for CO2 storage. Environ. Monit. Assess. 2015, 187, 4215. [Google Scholar] [CrossRef] [PubMed]
- Waldmann, S.; Busch, A.; van Ojik, K.; Gaupp, R. Importance of mineral surface areas in Rotliegend sandstones for modeling CO2-water-rock interactions. Chem. Geol. 2014, 378, 89–109. [Google Scholar] [CrossRef]
- Zhao, D.F.; Liao, X.W.; Yin, D.D. An experimental study for the effect of CO2-brine-rock interaction on reservoir physical properties. J. Energy Inst. 2015, 88, 27–35. [Google Scholar] [CrossRef]
- Rathnaweera, T.D.; Ranjith, P.G.; Perera, M.S.A.; Ranathunga, A.S.; Wanniarachchi, W.A.M.; Yang, S.Q.; Lashin, A.; Arifi, N.A. An Experimental Investigation of Coupled Chemico-mineralogical and Mechanical Changes in Varyingly-cemented Sandstones upon CO2 Injection in Deep Saline Aquifer Environments. Energy 2017, 133, 404–414. [Google Scholar] [CrossRef]
- Wang, S.; Clarens, A.F. The effects of CO2-brine rheology on leakage processes in geologic carbon sequestration. Water Resour. Res. 2012, 48, 8518. [Google Scholar] [CrossRef]
- Liu, B.; Xu, J.; Li, Z.; Malekian, R.; Xu, Z. Modeling of CO2 transport and pressure buildup in reservoirs during CO2 storage in saline aquifers: A case in Dongying Depression in China. Environ. Earth Sci. 2018, 77, 158. [Google Scholar] [CrossRef]
- Rathnaweera, T.D.; Ranjith, P.G.; Perera, M.S.A.; Bandara, K.M.A.S.; Wanniarachchi, W.A.M.; Ranathunga, A.S. Investigation of relative flow characteristics of brine-saturated reservoir formation: A numerical study of the Hawkesbury formation. J. Nat. Gas Sci. Eng. 2017, 45, 609–624. [Google Scholar] [CrossRef]
- Emberley, S.; Hutcheon, I.; Shevalier, M.; Durocher, K.; Gunter, W.D.; Perkins, E.H. Geochemical monitoring of fluid-rock interaction and CO2 storage at the Weyburn CO2-injection enhanced oil recovery site, Saskatchewan, Canada. Energy 2004, 29, 1393–1401. [Google Scholar] [CrossRef]
- Duan, Z.; Sun, R. An improved model calculating CO2 solubility in pure water and aqueous NaCl solutions from 273 to 533 K and from 0 to 2000 bar. Chem. Geol. 2003, 193, 257–271. [Google Scholar] [CrossRef]
- Rosenbauer, R.J.; Koksalan, T. Experimental multi-phase CO2-brine-rock interactions at elevated temperature and pressure: Implications for CO2 sequestration in deep-saline aquifers. Abstr. Pap. Am. Chem. Soc. 2004, 227, U1098. [Google Scholar]
- Takenouchi, S.; Kennedy, G.C. The binary system H2O–CO2 at high temperatures and pressures. Am. J. Sci. 1964, 262, 1055–1074. [Google Scholar] [CrossRef]
- Xu, T.; Feng, G.; Shi, Y. On fluid–rock chemical interaction in CO2-based geothermal systems. J. Geochem. Explor. 2014, 144, 179–193. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, H.H.; Dijke, M.I.J.V.; Geiger, S.; Agar, S.M. Constitutive Relations for Reactive Transport Modeling: Effects of Chemical Reactions on Multi-phase Flow Properties. Transp. Porous Media 2016, 114, 795–814. [Google Scholar] [CrossRef]
- Lasaga, A.C. Chemical Kinetics of Water-Rock Interaction. J. Geophys. Res. 1984, 89, 4009–4025. [Google Scholar] [CrossRef]
- Rosenbauer, R.J.; Koksalan, T.; Palandri, J.L. Experimental investigation of CO2-brine-rock interactions at elevated temperature and pressure: Implications for CO2 sequestration in deep-saline aquifers. Fuel Process. Technol. 2005, 86, 1581–1597. [Google Scholar] [CrossRef]
- Cui, G.; Zhang, L.; Tan, C.; Ren, S.; Zhuang, Y.; Enechukwu, C. Injection of supercritical CO2 for geothermal exploitation from sandstone and carbonate reservoirs: CO2-water-rock interactions and their effects. J. Co2 Util. 2017, 20, 113–128. [Google Scholar] [CrossRef]
- Gysi, A.P.; Stefansson, A. CO2-water-basalt interaction. Low temperature experiments and implications for CO2 sequestration into basalts. Geochim. Cosmochim. Acta 2012, 81, 129–152. [Google Scholar] [CrossRef]
- Gundogan, O.; Mackay, E.; Todd, A. Comparison of numerical codes for geochemical modelling of CO2 storage in target sandstone reservoirs. Chem. Eng. Res. Des. 2011, 89, 1805–1816. [Google Scholar] [CrossRef]
- Davila, G.; Luquot, L.; Soler, J.M.; Cama, J. Interaction between a fractured marl caprock and CO2-rich sulfate solution under supercritical CO2 conditions. Int. J. Greenh. Gas Control 2016, 48, 105–119. [Google Scholar] [CrossRef]
- Xu, T.; Spycher, N.; Sonnenthal, E.; Zhang, G.; Zheng, L.; Pruess, K. TOUGHREACT Version 2.0: A simulator for subsurface reactive transport under non-isothermal multiphase flow conditions. Comput. Geosci. 2011, 37, 763–774. [Google Scholar] [CrossRef] [Green Version]
- Tian, H.; Xu, T.; Wang, F.; Patil, V.V.; Sun, Y.; Yue, G. A numerical study of mineral alteration and self-sealing efficiency of a caprock for CO2 geological storage. Acta Geotech. 2014, 9, 87–100. [Google Scholar] [CrossRef]
- Andre, L.; Audigane, P.; Azaroual, M.; Menjoz, A. Numerical modeling of fluid-rock chemical interactions at the supercritical CO2-liquid interface during CO2 injection into a carbonate reservoir, the Dogger aquifer (Paris Basin, France). Energy Convers. Manag. 2007, 48, 1782–1797. [Google Scholar] [CrossRef]
- Labus, K.; Tarkowski, R.; Wdowin, M. Modeling gas-rock-water interactions in carbon dioxide storage capacity assessment: A case study of Jurassic sandstones in Poland. Int. J. Environ. Sci. Technol. 2015, 12, 2493–2502. [Google Scholar] [CrossRef]
- Liu, Q. Investigation of Mineral Trapping of Carbon Dioxide Sequestration in Brines. Ph.D. Thesis, University of Nottingham, Nottingham, UK, 2012. [Google Scholar]
- Merkel, B.J.; Planer-Friedrich, B.; Nordstrom, D.K. Groundwater Geochemistry: A Practical Guide to Modeling of Natural and Contaminated Aquatic Systems; Springer-Verlag Berlin Heidelberg: Berlin, Germany, 2008. [Google Scholar]
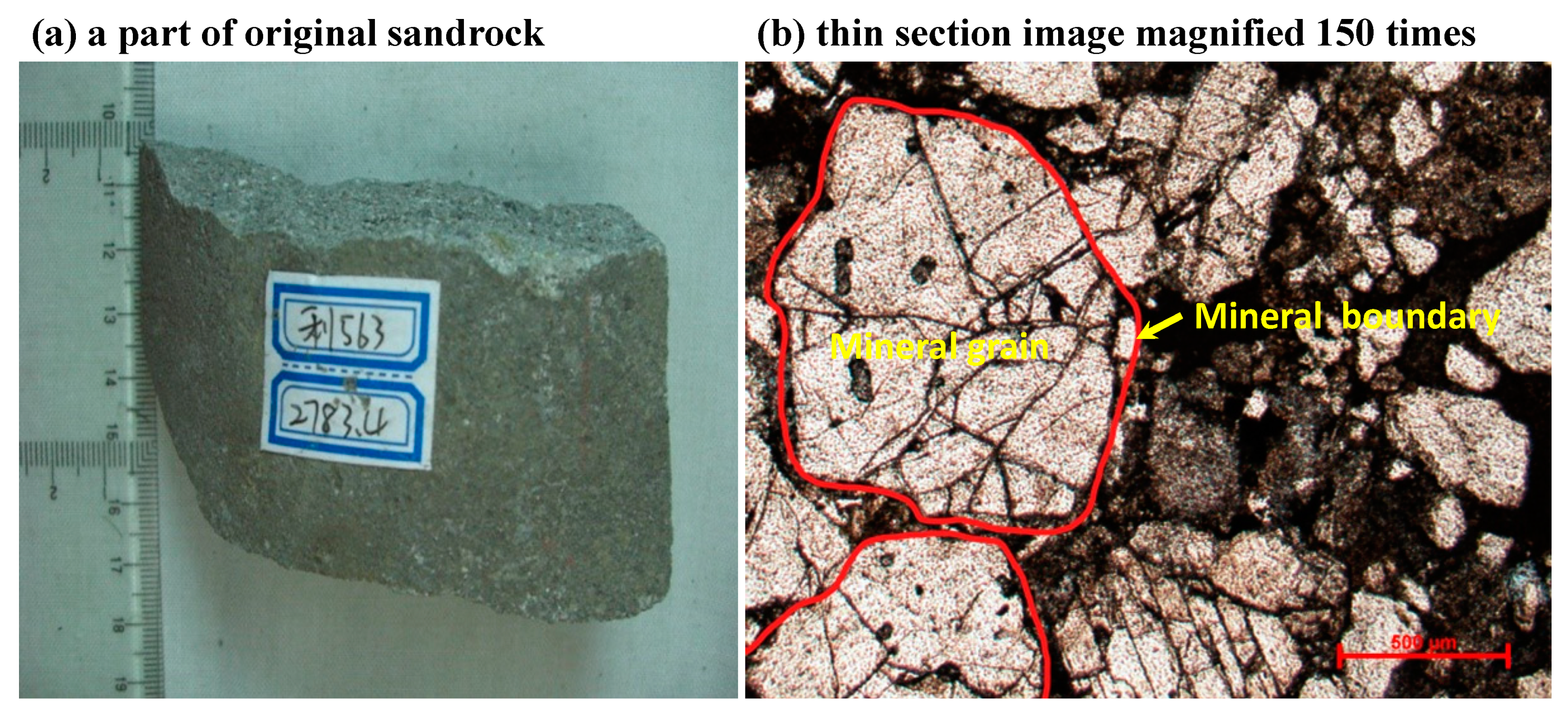
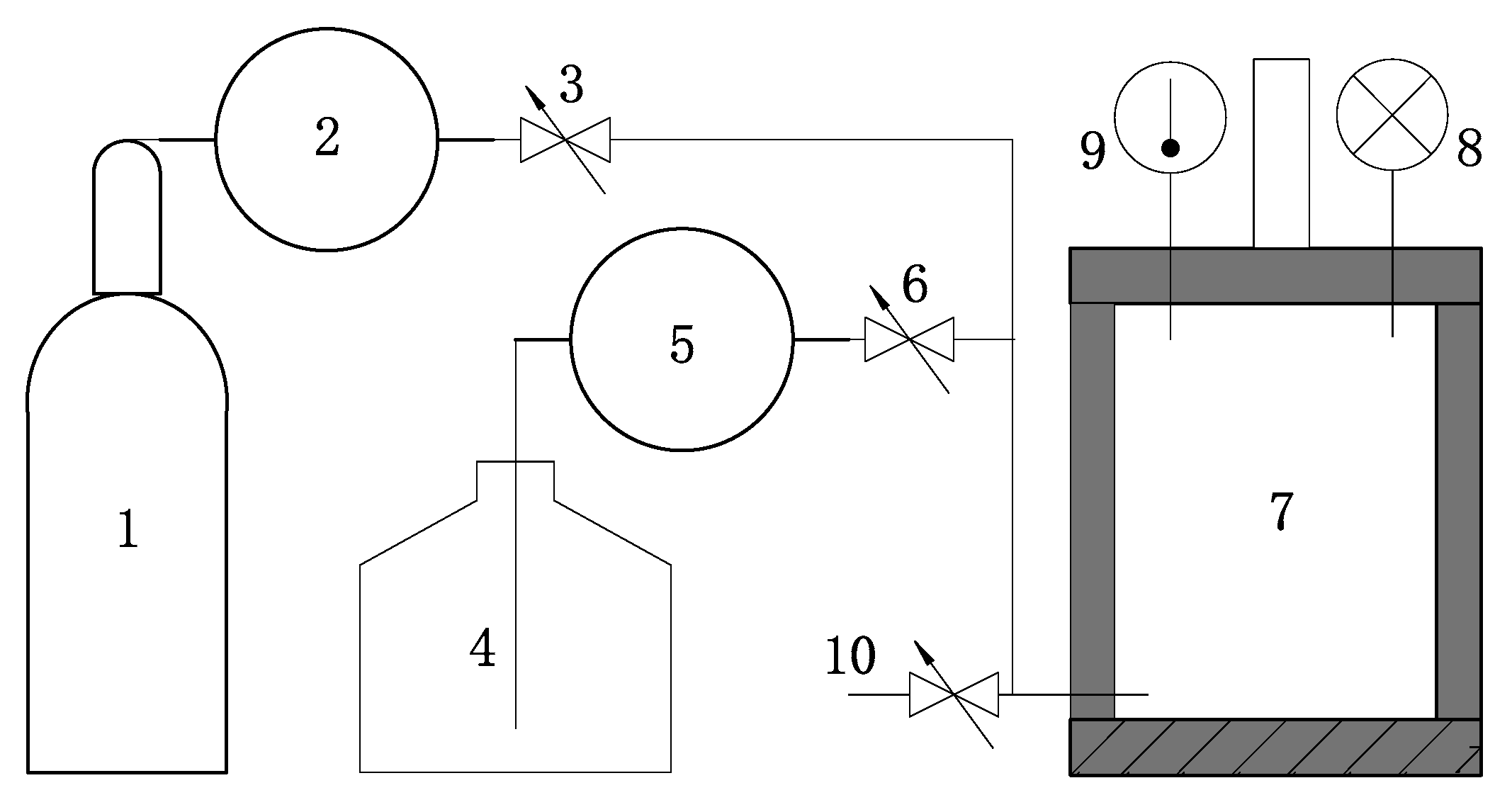

| Mineral | Chemical Formulae | Mass Fraction (%) 1 | Amount (mol) 2 |
|---|---|---|---|
| Quartz | SiO2 | 35.3 | 0.588 |
| Calcite | CaCO3 | 13.3 | 0.133 |
| K-feldspar | KAlSi3O8 | 7.0 | 0.025 |
| Albite | NaAlSi3O8 | 6.6 | 0.025 |
| Dolomite | CaMg(CO3)2 | 15.3 | 0.083 |
| Pyrite | FeS2 | 1.3 | 0.011 |
| Anhydrite | CaSO4 | 1.0 | 0.007 |
| Ca-Montmorillonite | Ca0.165Al2.33Si3.67O10(OH)2 | 1.9 | 0.005 |
| Kaolinite | Al2Si2O5(OH)4 | 9.3 | 0.036 |
| Chlorite | Mg5Al2Si3O10(OH)8 | 0.8 | 0.001 |
| Illite | K0.6Mg0.25Al2.3Si3.5O10(OH)2 | 8.2 | 0.021 |
| Experiment Time (h) | NaCl Solution Concentration (mol/L) | Experiment Pressure (MPa) |
|---|---|---|
| 12 | 0 * | 2 |
| 24 | 0.02 | 5 |
| 72 * | 0.1 | 8 * |
| 144 | 0.5 | 10 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Zhao, F.; Xu, J.; Qi, Y. Experimental Investigation and Numerical Simulation of CO2–Brine–Rock Interactions during CO2 Sequestration in a Deep Saline Aquifer. Sustainability 2019, 11, 317. https://doi.org/10.3390/su11020317
Liu B, Zhao F, Xu J, Qi Y. Experimental Investigation and Numerical Simulation of CO2–Brine–Rock Interactions during CO2 Sequestration in a Deep Saline Aquifer. Sustainability. 2019; 11(2):317. https://doi.org/10.3390/su11020317
Chicago/Turabian StyleLiu, Bo, Fangyuan Zhao, Jinpeng Xu, and Yueming Qi. 2019. "Experimental Investigation and Numerical Simulation of CO2–Brine–Rock Interactions during CO2 Sequestration in a Deep Saline Aquifer" Sustainability 11, no. 2: 317. https://doi.org/10.3390/su11020317




