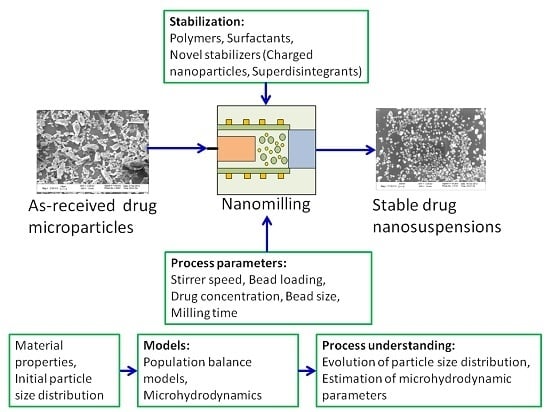
Nanomilling of Drugs for Bioavailability Enhancement: A Holistic Formulation-Process Perspective
Abstract
:1. Introduction
2. Formulation Aspects in the Preparation of Stable Drug Nanosuspensions
2.1. Impact of the Physicochemical Properties of Drugs
2.2. Impact of Polymers as Stabilizers
2.3. Impact of Surfactants as Stabilizers
2.4. Synergistic Stabilization via Combination of Polymers–Surfactants
2.5. Novel Stabilizers
2.5.1. Colloidal Superdisintegrants
2.5.2. Charged Nanoparticles
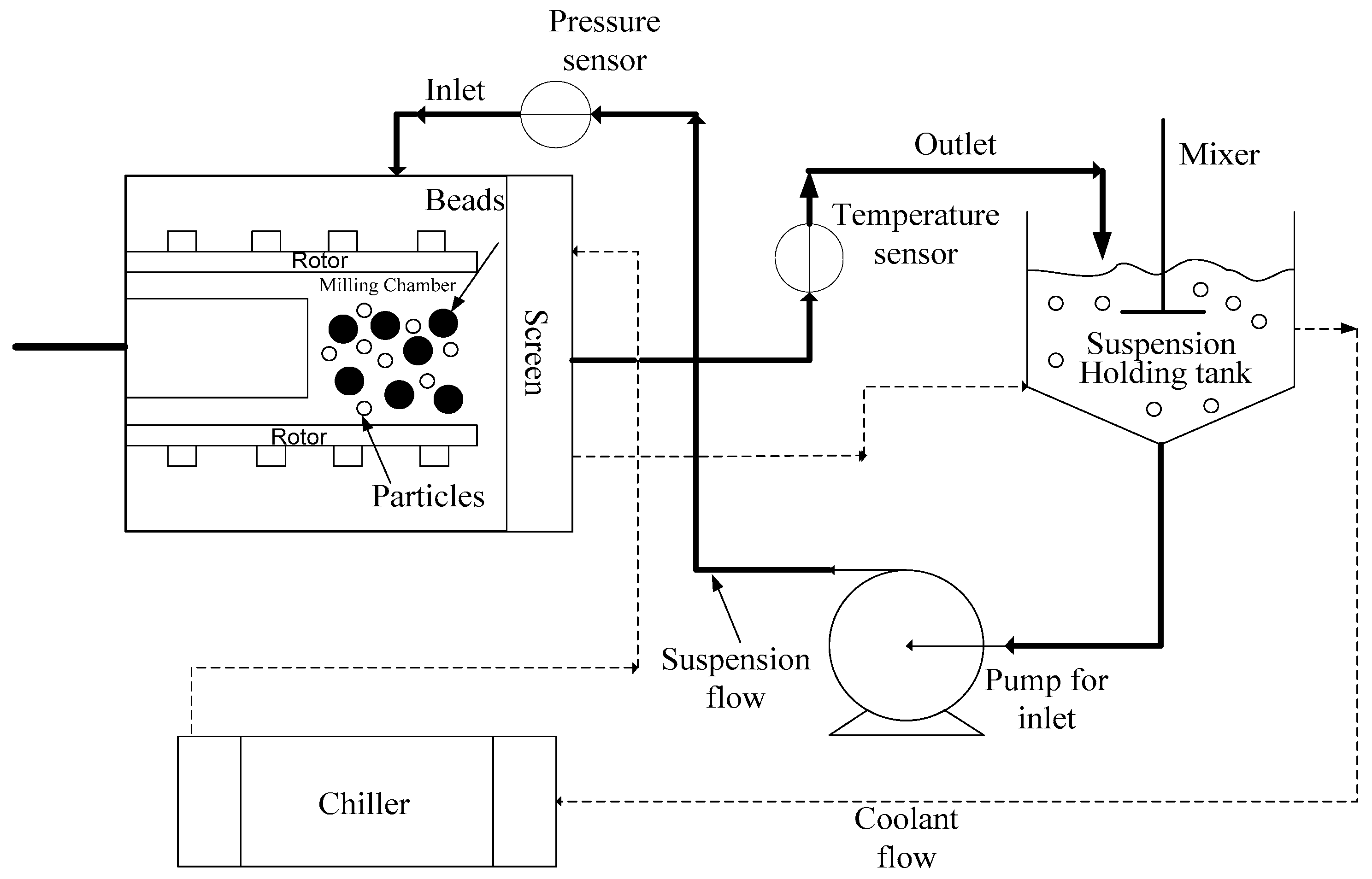
3. Processing: Impact of Process Parameters, Bead Material-Size, and Material Properties of Drug
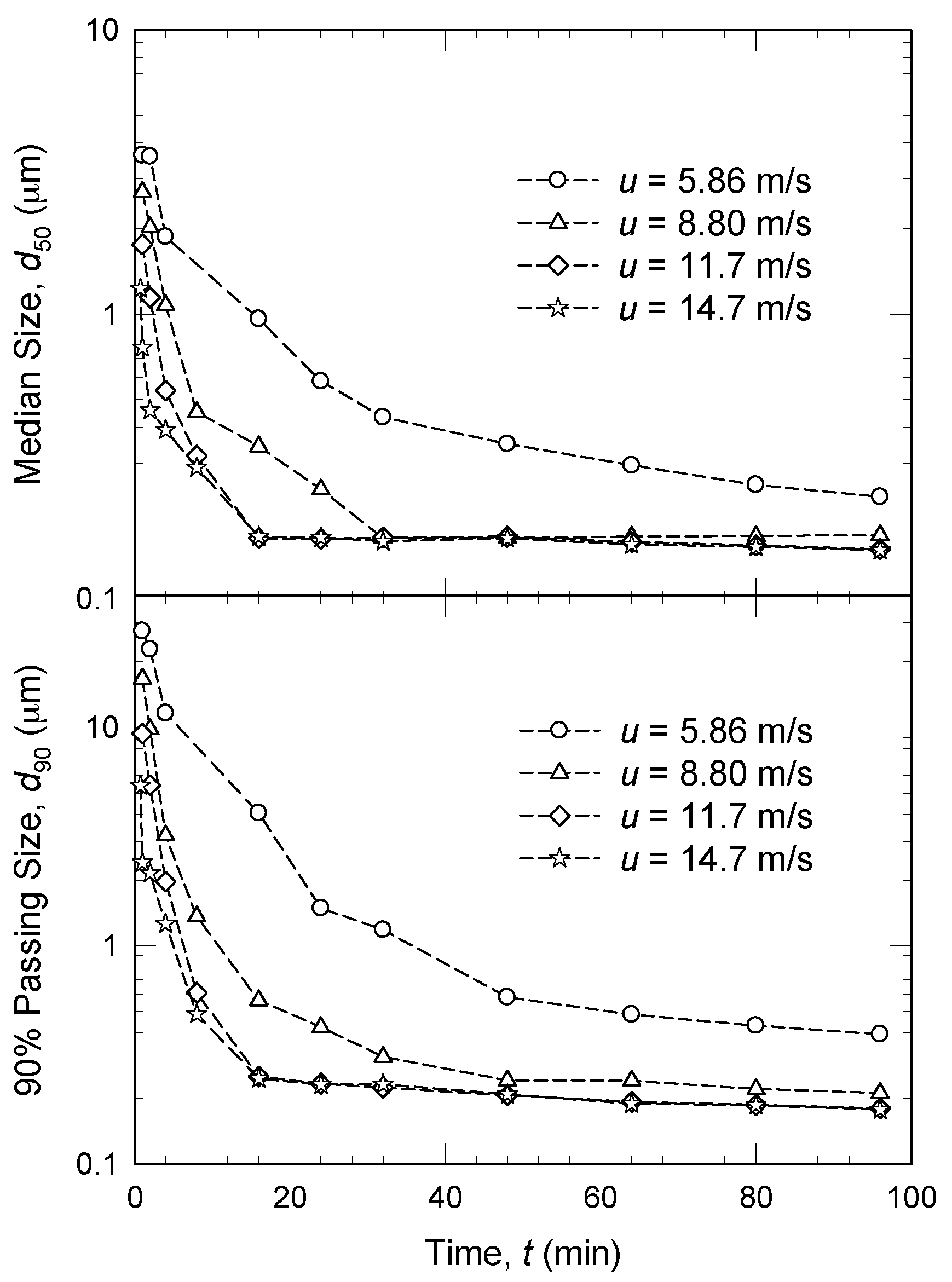
3.1. Stirrer/Agitation Speed
3.2. Bead Loading
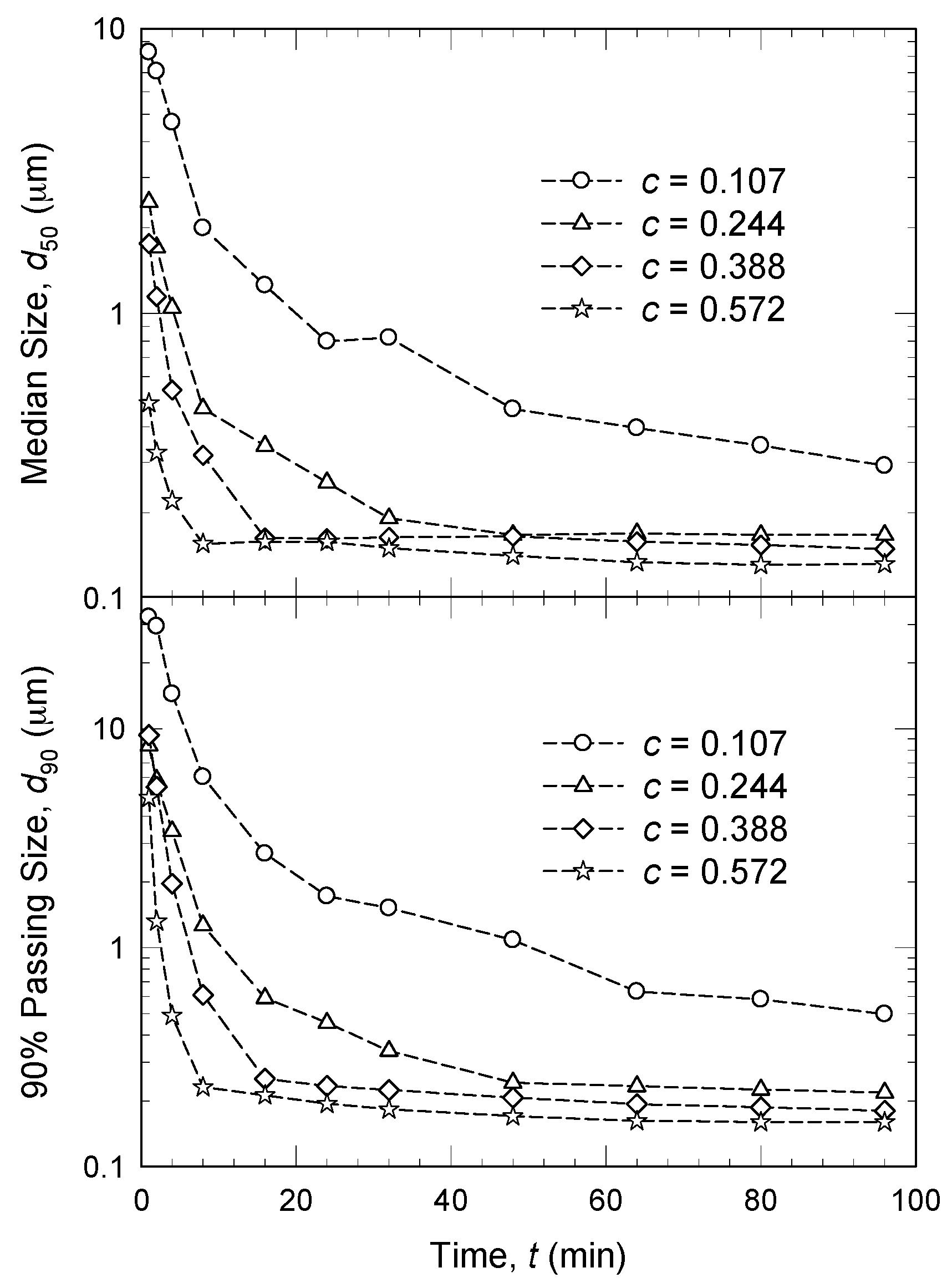
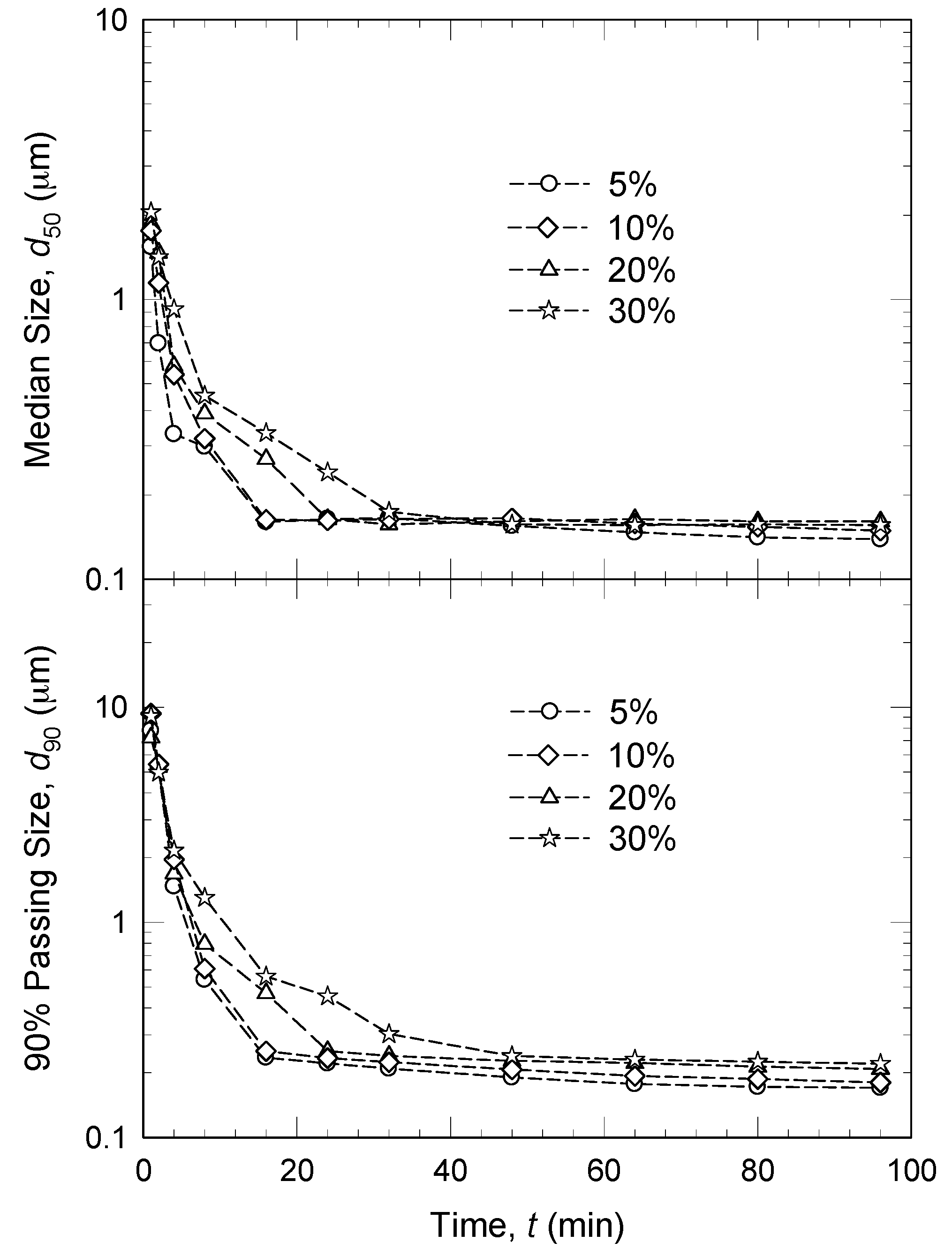
3.3. Drug Concentration
3.4. Size and Material of Construction of the Beads
3.5. Milling Time
3.6. Material Properties of Drugs
4. Models for Enhanced Process Understanding
4.1. Purely Descriptive Dynamic Models
4.2. Population Balance Models (PBMs)
4.3. Microhydrodynamic Models
- Bilgili and Afolabi [69] found that there exits an optimal HPC concentration in WSMM of griseofulvin suspensions in the presence of HPC–SDS, which was explained by a combined microhydrodynamics-adsorption analysis. An increase in HPC concentration had two counteracting effects: reduction in θ at higher suspension viscosity (viscous dampening) and higher HPC adsorption on drug nanoparticles.
- Upon an increase in stirrer speed u, more mechanical energy was imparted and all microhydrodynamic parameters increased monotonically, i.e., higher u led to higher θ, ν, a, ub, σbmax, and F [45,61,120]. In other words, higher u led to more frequent and energetic/forceful bead–bead collisions and more frequent drug particle compressions.
- An increase in volumetric bead concentration c led to two counteracting effects: ν and a increased, whereas θ, ub, and σbmax decreased [45,61,120]. In other words, higher c led to more bead–bead collisions and drug particle compressions, but less energetic/forceful collisions/compressions. Overall positive impact, i.e., faster breakage of the drug particles, was explained by an increase in the milling intensity factor F.
- Similar to c, there were also two major counteracting effects of db. A decrease in db led to lower θ, ub, σbmax and higher ν and a [61], i.e., more bead–bead collisions with less energy. The overall effect of db could not be explained by F alone; other microhydrodynamic parameters such as ν and a seem to explain the bead size impact better than F. While F can successfully explain the impact of all process parameters [45], it may be inadequate to explain the impact of bead size, which is usually regarded as an equipment parameter in media milling.
5. Challenges and Outlook
5.1. Preparation of Sub-100 nm Drug Particles
5.2. Solid-State Changes
5.3. Contamination due to Media (Bead) Wear
5.4. Continuous Processing
5.5. Scale-up
5.6. Combined Methods
6. Summary
Acknowledgments
Author Contributions
Conflicts of Interest
Symbols Used
Greek Letters
Indices
References
- Kipp, J.E. The role of solid nanoparticle technology in the parenteral delivery of poorly water-soluble drugs. Int. J. Pharm. 2004, 284, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A. Poor aqueous solubility: An industry wide problem in drug discovery. Am. Pharm. Rev. 2002, 5, 82–85. [Google Scholar]
- Chawla, G.; Bansal, A.K. A comparative assessment of solubility advantage from glassy and crystalline forms of a water-insoluble drug. Eur. J. Pharm. Sci. 2007, 32, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.; Kakumanu, V.K.; Bansal, A.K. Analytical techniques for quantification of amorphous/crystalline phases in pharmaceutical solids. J. Pharm. Sci. 2006, 95, 1641–1665. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Srinivasan, K.; Gowthamarajan, K.; Singare, D.S.; Prakash, D.; Gaikwad, N.B. Investigation of preparation parameters of nanosuspension by top-down media milling to improve the dissolution of poorly water-soluble glyburide. Eur. J. Pharm. Biopharm. 2011, 78, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Inkyo, M.; Yumoto, R.; Nagai, J.; Takano, M.; Nagata, S. Nanoparticulation of probucol, a poorly water-soluble drug, using a novel wet-milling process to improve in vitro dissolution and in vivo oral absorption. Drug Dev. Ind. Pharm. 2012, 38, 1015–1023. [Google Scholar] [CrossRef] [PubMed]
- Tuomela, A.; Liu, P.; Puranen, J.; Rönkkö, S.; Laaksonen, T.; Kalesnykas, G.; Oksala, O.; Ilkka, J.; Laru, J.; Järvinen, K.; et al. Brinzolamide nanocrystal formulations for ophthalmic delivery: Reduction of elevated intraocular pressure in vivo. Int. J. Pharm. 2014, 467, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Noyes, A.A.; Whitney, W.R. The rate of solution of solid substances in their own solutions. J. Am. Chem. Soc. 1897, 19, 930–934. [Google Scholar] [CrossRef]
- Müller, R.H.; Böhm, B.H. Nanosuspensions. In Emulsions and Nanosuspensions for the Formulation of Poorly Soluble Drugs; Benita, S., Böhm, B.H., Eds.; Medpharm Scientific: Stuttgart, Germany, 1998; pp. 149–173. [Google Scholar]
- Möschwitzer, J.; Müller, R.H.; Hunter, R.; Preedy, V. Nanocrystal formulations for improved delivery of poorly soluble drugs. In Nanomedicine in Health and Disease; Hunter, R.J., Preedy, V.R., Eds.; CRC Press: New York, NY, USA, 2011; pp. 79–99. [Google Scholar]
- Kumar, P.; Krishna, K.G. Nanosuspensions: The solution to deliver hydrophobic drugs. Int. J. Drug Deliv. 2012, 3, 546–557. [Google Scholar]
- Patravale, V.; Kulkarni, R. Nanosuspensions: A promising drug delivery strategy. J. Pharm. Pharmacol. 2004, 56, 827–840. [Google Scholar] [CrossRef] [PubMed]
- Rao, B.S.; Dubey, S.S. Nano fabricated drug delivery devises. Int. J. Pharm. Technol. 2012, 4, 1974–1986. [Google Scholar]
- Sun, B.; Yeo, Y. Nanocrystals for the parenteral delivery of poorly water-soluble drugs. Curr. Opin. Solid State Mater. Sci. 2012, 16, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Wacker, M. Nanocarriers for intravenous injection—The long hard road to the market. Int. J. Pharm. 2013, 457, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, L.; Chan, H.-K.; Watanabe, W. Formation, characterization, and fate of inhaled drug nanoparticles. Adv. Drug Deliv. Rev. 2011, 63, 441–455. [Google Scholar] [CrossRef] [PubMed]
- Chingunpituk, J. Nanosuspension technology for drug delivery. Walailak J. Sci. Technol. 2011, 4, 139–153. [Google Scholar]
- Date, A.A.; Patravale, V. Current strategies for engineering drug nanoparticles. Curr. Opin. Colloid Interface Sci. 2004, 9, 222–235. [Google Scholar] [CrossRef]
- Leleux, J.; Williams, R.O. Recent advancements in mechanical reduction methods: Particulate systems. Drug Dev. Ind. Pharm. 2014, 40, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Paradkar, A.; Ambike, A.A.; Jadhav, B.K.; Mahadik, K.R. Characterization of curcumin-PVP solid dispersion obtained by spray drying. Int. J. Pharm. 2004, 271, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Kesisoglou, F.; Panmai, S.; Wu, Y. Application of nanoparticles in oral delivery of immediate release formulations. Curr. Nanosci. 2007, 3, 183–190. [Google Scholar] [CrossRef]
- Merisko-Liversidge, E.M.; Liversidge, G.G. Drug nanoparticles: Formulating poorly water-soluble compounds. Toxicol. Pathol. 2008, 36, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Van Eerdenbrugh, B.; Froyen, L.; van Humbeeck, J.; Martens, J.A.; Augustijns, P.; van den Mooter, G. Drying of crystalline drug nanosuspensions—The importance of surface hydrophobicity on dissolution behavior upon redispersion. Eur. J. Pharm. Sci. 2008, 35, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.H.; Jacobs, C.; Kayser, O. Nanosuspensions as particulate drug formulations in therapy: Rationale for development and what we can expect for the future. Adv. Drug Deliv. Rev. 2001, 47, 3–19. [Google Scholar] [CrossRef]
- Müller, R.H.; Peters, K. Nanosuspensions for the formulation of poorly soluble drugs: I. Preparation by a size-reduction technique. Int. J. Pharm. 1998, 160, 229–237. [Google Scholar] [CrossRef]
- Rabinow, B.E. Nanosuspensions in drug delivery. Nat. Rev. Drug Discov. 2004, 3, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Cooper, E.R. Nanoparticles: A personal experience for formulating poorly water soluble drugs. J. Control. Release 2010, 141, 300–302. [Google Scholar] [CrossRef] [PubMed]
- Basa, S.; Muniyappan, T.; Karatgi, P.; Prabhu, R.; Pillai, R. Production and in vitro characterization of solid dosage form incorporating drug nanoparticles. Drug Dev. Ind. Pharm. 2008, 34, 1209–1218. [Google Scholar] [CrossRef] [PubMed]
- Bhakay, A.; Azad, M.; Vizzotti, E.; Dave, R.N.; Bilgili, E. Enhanced recovery and dissolution of griseofulvin nanoparticles from surfactant-free nanocomposite microparticles incorporating wet-milled swellable dispersants. Drug Dev. Ind. Pharm. 2014, 40, 1509–1522. [Google Scholar] [CrossRef] [PubMed]
- Lee, J. Drug nano- and microparticles processed into solid dosage forms: Physical properties. J. Pharm. Sci. 2003, 92, 2057–2068. [Google Scholar] [CrossRef] [PubMed]
- Van Eerdenbrugh, B.; van den Mooter, G.; Augustijns, P. Top-down production of drug nanocrystals: Nanosuspension stabilization, miniaturization and transformation into solid products. Int. J. Pharm. 2008, 364, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Knieke, C.; Azad, M.A.; To, D.; Bilgili, E.; Davé, R.N. Sub-100 micron fast dissolving nanocomposite drug powders. Powder Technol. 2015, 271, 49–60. [Google Scholar] [CrossRef]
- Tuomela, A.; Laaksonen, T.; Laru, J.; Antikainen, O.; Kiesvaara, J.; Ilkka, J.; Oksala, O.; Rönkkö, S.; Järvinen, K.; Hirvonen, J.; et al. Solid formulations by a nanocrystal approach: Critical process parameters regarding scale-ability of nanocrystals for tableting applications. Int. J. Pharm. 2015, 485, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Munjal, B.; Bansal, A.K. Differential effect of buffering agents on the crystallization of gemcitabine hydrochloride in frozen solutions. Int. J. Pharm. 2014, 471, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.; Arteaga, C.; Abdelmalek, B.; Davé, R.; Bilgili, E. Spray drying of drug-swellable dispersant suspensions for preparation of fast-dissolving, high drug-loaded, surfactant-free nanocomposites. Drug Dev. Ind. Pharm. 2015, 1617–1631. [Google Scholar] [CrossRef] [PubMed]
- Sievens-Figueroa, L.; Bhakay, A.; Jerez-Rozo, J.I.; Pandya, N.; Romañach, R.J.; Michniak-Kohn, B.; Iqbal, Z.; Bilgili, E.; Davé, R.N. Preparation and characterization of hydroxypropyl methyl cellulose films containing stable BCS Class II drug nanoparticles for pharmaceutical applications. Int. J. Pharm. 2012, 423, 496–508. [Google Scholar] [CrossRef] [PubMed]
- Krull, S.M.; Susarla, R.; Afolabi, A.; Li, M.; Ying, Y.; Iqbal, Z.; Bilgili, E.; Davé, R.N. Polymer strip films as a robust, surfactant-free platform for delivery of BCS Class II drug nanoparticles. Int. J. Pharm. 2015, 489, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Susarla, R.; Afolabi, A.; Patel, D.; Bilgili, E.; Davé, R.N. Novel use of superdisintegrants as viscosity enhancing agents in biocompatible polymer films containing griseofulvin nanoparticles. Powder Technol. 2015, 285, 25–33. [Google Scholar] [CrossRef]
- Bhakay, A.; Vizzotti, E.; Li, M.; Davé, R.; Bilgili, E. Incorporation of fenofibrate nanoparticles prepared by melt emulsification into polymeric films. J. Pharm. Innov. 2016, 11, 53–63. [Google Scholar] [CrossRef]
- Krull, S.M.; Ma, Z.; Li, M.; Davé, R.N.; Bilgili, E. Preparation and characterization of fast dissolving pullulan films containing BCS Class II drug nanoparticles for bioavailability enhancement. Drug Dev. Ind. Pharm. 2016, 42, 1073–1085. [Google Scholar] [CrossRef] [PubMed]
- Kwade, A. Wet comminution in stirred media mills—Research and its practical application. Powder Technol. 1999, 105, 14–20. [Google Scholar] [CrossRef]
- Merisko-Liversidge, E.; Liversidge, G.G.; Cooper, E.R. Nanosizing: A formulation approach for poorly-water-soluble compounds. Eur. J. Pharm. Sci. 2003, 18, 113–120. [Google Scholar] [CrossRef]
- Brough, C.; Williams, R. Amorphous solid dispersions and nano-crystal technologies for poorly water-soluble drug delivery. Int. J. Pharm. 2013, 453, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Möschwitzer, J.P. Drug nanocrystals in the commercial pharmaceutical development process. Int. J. Pharm. 2013, 453, 142–156. [Google Scholar] [CrossRef] [PubMed]
- Afolabi, A.; Akinlabi, O.; Bilgili, E. Impact of process parameters on the breakage kinetics of poorly water-soluble drugs during wet stirred media milling: A microhydrodynamic view. Eur. J. Pharm. Sci. 2014, 51, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Cerdeira, A.M.; Gander, B.; Mazzotti, M. Role of milling parameters and particle stabilization on nanogrinding of drug substances of similar mechanical properties. Chem. Eng. Technol. 2011, 34, 1427–1438. [Google Scholar] [CrossRef]
- Ghosh, I.; Schenck, D.; Bose, S.; Ruegger, C. Optimization of formulation and process parameters for the production of nanosuspension by wet media milling technique: Effect of Vitamin E TPGs and nanocrystal particle size on oral absorption. Eur. J. Pharm. Sci. 2012, 47, 718–728. [Google Scholar] [CrossRef] [PubMed]
- Merisko-Liversidge, E.; Liversidge, G.G. Nanosizing for oral and parenteral drug delivery: A perspective on formulating poorly-water soluble compounds using wet media milling technology. Adv. Drug Deliv. Rev. 2011, 63, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Eskin, D.; Zhupanska, O.; Hamey, R.; Moudgil, B.; Scarlett, B. Microhydrodynamics of stirred media milling. Powder Technol. 2005, 156, 95–102. [Google Scholar] [CrossRef]
- Bernotat, S.; Schönert, K. Size reduction. In Ullmann’s Encyclopedia of Industrial Chemistry; Springer: Berlin, Germany, 1988; pp. 199–905. [Google Scholar]
- Kesisoglou, F.; Panmai, S.; Wu, Y. Nanosizing—Oral formulation development and biopharmaceutical evaluation. Adv. Drug Deliv. Rev. 2007, 59, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Shegokar, R.; Müller, R.H. Nanocrystals: Industrially feasible multifunctional formulation technology for poorly soluble actives. Int. J. Pharm. 2010, 399, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, J.; Watanabe, W. Physical and chemical stability of drug nanoparticles. Adv. Drug Deliv. Rev. 2011, 63, 456–469. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zheng, Y.; Zhang, L.; Wang, Q.; Zhang, D. Stability of nanosuspensions in drug delivery. J. Control. Release 2013, 172, 1126–1141. [Google Scholar] [CrossRef] [PubMed]
- Junyaprasert, V.B.; Morakul, B. Nanocrystals for enhancement of oral bioavailability of poorly water-soluble drugs. Asian J. Pharm. Sci. 2015, 10, 13–23. [Google Scholar] [CrossRef]
- Peltonen, L.; Hirvonen, J. Pharmaceutical nanocrystals by nanomilling: Critical process parameters, particle fracturing and stabilization methods. J. Pharm. Pharmacol. 2010, 62, 1569–1579. [Google Scholar] [CrossRef] [PubMed]
- Cerdeira, A.M.; Mazzotti, M.; Gander, B. Miconazole nanosuspensions: Influence of formulation variables on particle size reduction and physical stability. Int. J. Pharm. 2010, 396, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Bhakay, A.; Davé, R.; Bilgili, E. Recovery of BCS Class II drugs during aqueous redispersion of core-shell type nanocomposite particles produced via fluidized bed coating. Powder Technol. 2013, 236, 221–234. [Google Scholar] [CrossRef]
- Bitterlich, A.; Laabs, C.; Busmann, E.; Grandeury, A.; Juhnke, M.; Bunjes, H.; Kwade, A. Challenges in nanogrinding of active pharmaceutical ingredients. Chem. Eng. Technol. 2014, 37, 840–846. [Google Scholar] [CrossRef]
- Colombo, I.; Grassi, G.; Grassi, M. Drug mechanochemical activation. J. Pharm. Sci. 2009, 98, 3961–3986. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yaragudi, N.; Afolabi, A.; Dave, R.; Bilgili, E. Sub-100 nm drug particle suspensions prepared via wet milling with low bead contamination through novel process intensification. Chem. Eng. Sci. 2015, 130, 207–220. [Google Scholar] [CrossRef]
- Chin, C.P.; Wu, H.S.; Wang, S.S. New approach to pesticide delivery using nanosuspensions: Research and applications. Ind. Eng. Chem. Res. 2011, 50, 7637–7643. [Google Scholar] [CrossRef]
- Juhnke, M.; Berghausen, J.; Timpe, C. Accelerated formulation development for nanomilled active pharmaceutical ingredients using a screening approach. Chem. Eng. Technol. 2010, 33, 1412–1418. [Google Scholar] [CrossRef]
- Knieke, C.; Azad, M.; Davé, R.; Bilgili, E. A study of the physical stability of wet media-milled fenofibrate suspensions using dynamic equilibrium curves. Chem. Eng. Res. Des. 2013, 91, 1245–1258. [Google Scholar] [CrossRef]
- Bhakay, A.; Merwade, M.; Bilgili, E.; Dave, R.N. Novel aspects of wet milling for the production of microsuspensions and nanosuspensions of poorly water-soluble drugs. Drug Dev. Ind. Pharm. 2011, 37, 963–976. [Google Scholar] [CrossRef] [PubMed]
- Opoczky, L.; Farnady, F. Fine grinding and states of equilibrium. Powder Technol. 1984, 39, 107–115. [Google Scholar] [CrossRef]
- Leung, D.H.; Lamberto, D.J.; Liu, L.; Kwong, E.; Nelson, T.; Rhodes, T.; Bak, A. A new and improved method for the preparation of drug nanosuspension formulations using acoustic mixing technology. Int. J. Pharm. 2014, 473, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, L.; Davé, R.; Bilgili, E. An intensified vibratory milling process for enhancing the breakage kinetics during the preparation of drug nanosuspensions. AAPS PharmSciTech 2016, 17, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Bilgili, E.; Afolabi, A. A combined microhydrodynamics-polymer adsorption analysis for elucidation of the roles of stabilizers in wet stirred media milling. Int. J. Pharm. 2012, 439, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Bilgili, E.; Li, M.; Afolabi, A. Is the combination of cellulosic polymers and anionic surfactants a good strategy for ensuring physical stability of BCS Class II drug nanosuspensions? Pharm. Dev. Technol. 2016, 21, 199–510. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Huey, B.D.; Burgess, D.J. Scanning probe microscopy method for nanosuspension stabilizer selection. Langmuir 2009, 25, 12481–12487. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, D.J.; Sepassi, S.; King, S.M.; Holland, S.J.; Martini, L.G.; Lawrence, M.J. Characterization of polymer adsorption onto drug nanoparticles using depletion measurements and small-angle neutron scattering. Mol. Pharm. 2013, 10, 4146–4158. [Google Scholar] [CrossRef] [PubMed]
- Bitterlich, A.; Laabs, C.; Krautstrunk, I.; Dengler, M.; Juhnke, M.; Grandeury, A.; Bunjes, H.; Kwade, A. Process parameter dependent growth phenomena of naproxen nanosuspension manufactured by wet media milling. Eur. J. Pharm. Biopharm. 2015, 92, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.; Wang, X.; Wang, H.; Zhang, X.; Wang, Y.; Wu, B. Elucidating the in vivo fate of nanocrystals using a physiologically based pharmacokinetic model: A case study with the anticancer agent SNX-2112. Int. J. Nanomed. 2015, 10, 2521–2535. [Google Scholar]
- Kumar, S.; Jog, R.; Shen, J.; Zolnik, B.; Sadrieh, N.; Burgess, D.J. Formulation and performance of danazol nano-crystalline suspensions and spray dried powders. Pharm. Res. 2015, 32, 1694–1703. [Google Scholar] [CrossRef] [PubMed]
- Bhakay, A.; Azad, M.; Bilgili, E.; Dave, R. Redispersible fast dissolving nanocomposite microparticles of poorly water-soluble drugs. Int. J. Pharm. 2014, 461, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Komasaka, T.; Fujimura, H.; Tagawa, T.; Sugiyama, A.; Kitano, Y. Practical method for preparing nanosuspension formulations for toxicology studies in the discovery stage: Formulation optimization and in vitro/in vivo evaluation of nanosized poorly water-soluble compounds. Chem. Pharm. Bull. 2014, 62, 1073–1082. [Google Scholar] [CrossRef] [PubMed]
- Leng, D.; Chen, H.; Li, G.; Guo, M.; Zhu, Z.; Xu, L.; Wang, Y. Development and comparison of intramuscularly long-acting paliperidone palmitate nanosuspensions with different particle size. Int. J. Pharm. 2014, 472, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Mahesh, K.V.; Singh, S.K.; Gulati, M. A comparative study of top-down and bottom-up approaches for the preparation of nanosuspensions of glipizide. Powder Technol. 2014, 256, 436–449. [Google Scholar] [CrossRef]
- Shah, S.R.; Parikh, R.H.; Chavda, J.R.; Sheth, N.R. Glibenclamide nanocrystals for bioavailability enhancement: Formulation design, process optimization, and pharmacodynamic evaluation. J. Pharm. Innov. 2014, 9, 227–237. [Google Scholar] [CrossRef]
- Sarnes, A.; Kovalainen, M.; Häkkinen, M.R.; Laaksonen, T.; Laru, J.; Kiesvaara, J.; Ilkka, J.; Oksala, O.; Rönkkö, S.; Järvinen, K.; et al. Nanocrystal-based per-oral itraconazole delivery: Superior in vitro dissolution enhancement vs. sporanox® is not realized in in vivo drug absorption. J. Control. Release 2014, 180, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Yuminoki, K.; Seko, F.; Horii, S.; Takeuchi, H.; Teramoto, K.; Nakada, Y.; Hashimoto, N. Preparation and evaluation of high dispersion stable nanocrystal formulation of poorly water-soluble compounds by using povacoat. J. Pharm. Sci. 2014, 103, 3772–3781. [Google Scholar] [CrossRef] [PubMed]
- Cerdeira, A.M.; Mazzotti, M.; Gander, B. Formulation and drying of miconazole and itraconazole nanosuspensions. Int. J. Pharm. 2013, 443, 209–220. [Google Scholar] [CrossRef] [PubMed]
- George, M.; Ghosh, I. Identifying the correlation between drug/stabilizer properties and critical quality attributes (CQAs) of nanosuspension formulation prepared by wet media milling technology. Eur. J. Pharm. Sci. 2013, 48, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, A.; Afolabi, A.; Bilgili, E. Continuous production of drug nanoparticle suspensions via wet stirred media milling: A fresh look at the rehbinder effect. Drug Dev. Ind. Pharm. 2013, 39, 266–283. [Google Scholar] [CrossRef] [PubMed]
- Niwa, T.; Danjo, K. Design of self-dispersible dry nanosuspension through wet milling and spray freeze-drying for poorly water-soluble drugs. Eur. J. Pharm. Sci. 2013, 50, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.S.M.; York, P.; Ali, A.M.A.; Blagden, N. Hydrocortisone nanosuspensions for ophthalmic delivery: A comparative study between microfluidic nanoprecipitation and wet milling. J. Control. Release 2011, 149, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, I.; Bose, S.; Vippagunta, R.; Harmon, F. Nanosuspension for improving the bioavailability of a poorly soluble drug and screening of stabilizing agents to inhibit crystal growth. Int. J. Pharm. 2011, 409, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Rong, X.; Laru, J.; van Veen, B.; Kiesvaara, J.; Hirvonen, J.; Laaksonen, T.; Peltonen, L. Nanosuspensions of poorly soluble drugs: Preparation and development by wet milling. Int. J. Pharm. 2011, 411, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.J.; Patel, J.K.; Pandya, V.M. Improvement in the dissolution of poorly water soluble drug using media milling technique. Thai J. Pharm. Sci. 2010, 34, 155–164. [Google Scholar]
- Baert, L.; van ‘t Klooster, G.; Dries, W.; François, M.; Wouters, A.; Basstanie, E.; Iterbeke, K.; Stappers, F.; Stevens, P.; Schueller, L.; et al. Development of a long-acting injectable formulation with nanoparticles of rilpivirine (TMC278) for HIV treatment. Eur. J. Pharm. Biopharm. 2009, 72, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Fakes, M.G.; Vakkalagadda, B.J.; Qian, F.; Desikan, S.; Gandhi, R.B.; Lai, C.; Hsieh, A.; Franchini, M.K.; Toale, H.; Brown, J. Enhancement of oral bioavailability of an HIV-attachment inhibitor by nanosizing and amorphous formulation approaches. Int. J. Pharm. 2009, 370, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Inkyo, M.; Yumoto, R.; Nagai, J.; Takano, M.; Nagata, S. Nanoparticulation of poorly water soluble drugs using a wet-mill process and physicochemical properties of the nanopowders. Chem. Pharm. Bull. 2009, 57, 1050–1057. [Google Scholar] [CrossRef] [PubMed]
- Van Eerdenbrugh, B.; Vermant, J.; Martens, J.A.; Froyen, L.; van Humbeeck, J.; Augustijns, P.; van den Mooter, G. A screening study of surface stabilization during the production of drug nanocrystals. J. Pharm. Sci. 2009, 98, 2091–2103. [Google Scholar] [CrossRef] [PubMed]
- Ain-Ai, A.; Gupta, P.K. Effect of arginine hydrochloride and hydroxypropyl cellulose as stabilizers on the physical stability of high drug loading nanosuspensions of a poorly soluble compound. Int. J. Pharm. 2008, 351, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-Y.; Park, C.H.; Lee, J. Effect of polymer molecular weight on nanocomminution of poorly soluble drug. Drug Deliv. 2008, 15, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Xu, S.; Li, S. Understanding a relaxation behavior in a nanoparticle suspension for drug delivery applications. Int. J. Pharm. 2008, 351, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Choi, J.Y.; Park, C.H. Characteristics of polymers enabling nano-comminution of water-insoluble drugs. Int. J. Pharm. 2008, 355, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.-G.; Dong, L.C.; Song, Y.-Q. Nanosizing of a drug/carrageenan complex to increase solubility and dissolution rate. Int. J. Pharm. 2007, 342, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Sepassi, S.; Goodwin, D.J.; Drake, A.F.; Holland, S.; Leonard, G.; Martini, L.; Lawrence, M.J. Effect of polymer molecular weight on the production of drug nanoparticles. J. Pharm. Sci. 2007, 96, 2655–2666. [Google Scholar] [CrossRef] [PubMed]
- Van Eerdenbrugh, B.; Froyen, L.; Martens, J.; Blaton, N.; Augustijns, P.; Brewster, M.; van den Mooter, G. Characterization of physico-chemical properties and pharmaceutical performance of sucrose co-freeze-dried solid nanoparticulate powders of the anti-HIV agent loviride prepared by media milling. Int. J. Pharm. 2007, 338, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Jinno, J.-I.; Kamada, N.; Miyake, M.; Yamada, K.; Mukai, T.; Odomi, M.; Toguchi, H.; Liversidge, G.G.; Higaki, K.; Kimura, T. Effect of particle size reduction on dissolution and oral absorption of a poorly water-soluble drug, cilostazol, in beagle dogs. J. Control. Release 2006, 111, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-Y.; Yoo, J.Y.; Kwak, H.-S.; Uk Nam, B.; Lee, J. Role of polymeric stabilizers for drug nanocrystal dispersions. Curr. Appl. Phys. 2005, 5, 472–474. [Google Scholar] [CrossRef]
- Lee, M.K.; Kim, S.; Ahn, C.H.; Lee, J. Hydrophilic and hydrophobic amino acid copolymers for nano-comminution of poorly soluble drugs. Int. J. Pharm. 2010, 384, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Dalvi, S.V.; Dave, R.N. Analysis of nucleation kinetics of poorly water-soluble drugs in presence of ultrasound and hydroxypropyl methyl cellulose during antisolvent precipitation. Int. J. Pharm. 2010, 387, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Rasenack, N.; Hartenhauer, H.; Müller, B.W. Microcrystals for dissolution rate enhancement of poorly water-soluble drugs. Int. J. Pharm. 2003, 254, 137–145. [Google Scholar] [CrossRef]
- Azad, M.; Afolabi, A.; Bhakay, A.; Leonardi, J.; Davé, R.; Bilgili, E. Enhanced physical stabilization of fenofibrate nanosuspensions via wet co-milling with a superdisintegrant and an adsorbing polymer. Eur. J. Pharm. Biopharm. 2015, 94, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Onoue, S.; Takahashi, H.; Kawabata, Y.; Seto, Y.; Hatanaka, J.; Timmermann, B.; Yamada, S. Formulation design and photochemical studies on nanocrystal solid dispersion of curcumin with improved oral bioavailability. J. Pharm. Sci. 2010, 99, 1871–1881. [Google Scholar] [CrossRef] [PubMed]
- Berglund, K.D.; Przybycien, T.M.; Tilton, R.D. Coadsorption of sodium dodecyl sulfate with hydrophobically modified nonionic cellulose polymers. 1. Role of polymer hydrophobic modification. Langmuir 2003, 19, 2705–2713. [Google Scholar] [CrossRef]
- Evertsson, H.; Nilsson, S. Microviscosity in clusters of ethyl hydroxyethyl cellulose and sodium dodecyl sulfate formed in dilute aqueous solutions as determined with fluorescence probe techniques. Macromolecules 1997, 30, 2377–2385. [Google Scholar] [CrossRef]
- Winnik, F.M.; Winnik, M.A. The interaction of sodium dodecylsulfate with (hydroxypropyl) cellulose. Polym. J. 1990, 22, 482–488. [Google Scholar] [CrossRef]
- Shimabayashi, S.; Uno, T.; Oouchi, Y.; Komatsu, E. Interaction between hydroxypropylcellulose and surfactant and its effect on dispersion stability of kaolinite suspension in an aqueous phase. In Formation and Dynamics of Self-Organized Structures in Surfactants and Polymer Solutions; Springer: Berlin, Germany, 1997; pp. 136–140. [Google Scholar]
- Verma, S.; Kumar, S.; Gokhale, R.; Burgess, D.J. Physical stability of nanosuspensions: Investigation of the role of stabilizers on ostwald ripening. Int. J. Pharm. 2011, 406, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.-I.; Shim, Y.-H.; Kim, C.; Lim, G.-T.; Choi, K.-C.; Yoon, C. Effect of cryoprotectants on the reconstitution of surfactant-free nanoparticles of poly (dl-lactide-co-glycolide). J. Microencapsul. 2005, 22, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Lebhardt, T.; Roesler, S.; Uusitalo, H.P.; Kissel, T. Surfactant-free redispersible nanoparticles in fast-dissolving composite microcarriers for dry-powder inhalation. Eur. J. Pharm. Biopharm. 2011, 78, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Liversidge, G.G.; Cundy, K.C. Particle size reduction for improvement of oral bioavailability of hydrophobic drugs: I. Absolute oral bioavailability of nanocrystalline danazol in beagle dogs. Int. J. Pharm. 1995, 125, 91–97. [Google Scholar] [CrossRef]
- Azad, M.A.; Afolabi, A.; Patel, N.; Davé, R.; Bilgili, E. Preparation of stable colloidal suspensions of superdisintegrants via wet stirred media milling. Particuology 2014, 14, 76–82. [Google Scholar] [CrossRef]
- Juhnke, M.; John, E. Wet-media milling of colloidal drug suspensions stabilized by means of charged nanoparticles. Chem. Eng. Technol. 2012, 35, 1931–1940. [Google Scholar] [CrossRef]
- Kawatra, S.K. Advances in Comminution; Society for Mining, Metallurgy and Exploration: Englewood, CO, USA, 2006. [Google Scholar]
- Afolabi, A. Batch and Continuous Production of Stable Dense Suspensions of Drug Nanoparticles in a Wet Stirred Media Mill. Ph.D. Thesis, New Jersey Institute of Technology, Otto H. York Department of Chemical, Biological and Pharmaceutical Engineering, Newark, NJ, USA, 2013. [Google Scholar]
- Hennart, S.; Domingues, M.; Wildeboer, W.; van Hee, P.; Meesters, G. Study of the process of stirred ball milling of poorly water soluble organic products using factorial design. Powder Technol. 2010, 198, 56–60. [Google Scholar] [CrossRef]
- Kumar, S.; Burgess, D.J. Wet milling induced physical and chemical instabilities of naproxen nano-crystalline suspensions. Int. J. Pharm. 2014, 466, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Juhnke, M.; Märtin, D.; John, E. Generation of wear during the production of drug nanosuspensions by wet media milling. Eur. J. Pharm. Biopharm. 2012, 81, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Singare, D.S.; Marella, S.; Gowthamrajan, K.; Kulkarni, G.T.; Vooturi, R.; Rao, P.S. Optimization of formulation and process variable of nanosuspension: An industrial perspective. Int. J. Pharm. 2010, 402, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Bilgili, E.; Hamey, R.; Scarlett, B. Production of pigment nanoparticles using a wet stirred mill with polymeric media. China Particuol. 2004, 2, 93–100. [Google Scholar] [CrossRef]
- Knieke, C.; Sommer, M.; Peukert, W. Identifying the apparent and true grinding limit. Powder Technol. 2009, 195, 25–30. [Google Scholar] [CrossRef]
- Prasher, C.L. Crushing and Grinding Process Handbook; John Wiley & Sons Ltd.: Chichester, NY, USA, 1987. [Google Scholar]
- Gahn, C.; Mersmann, A. Brittle fracture in crystallization processes part A. Attrition and abrasion of brittle solids. Chem. Eng. Sci. 1999, 54, 1273–1282. [Google Scholar] [CrossRef]
- Gahn, C.; Mersmann, A. Brittle fracture in crystallization processes part B. Growth of fragments and scale-up of suspension crystallizers. Chem. Eng. Sci. 1999, 54, 1283–1292. [Google Scholar] [CrossRef]
- Ghadiri, M.; Zhang, Z. Impact attrition of particulate solids. Part 1: A theoretical model of chipping. Chem. Eng. Sci. 2002, 57, 3659–3669. [Google Scholar] [CrossRef]
- Vogel, L.; Peukert, W. Breakage behaviour of different materials—Construction of a mastercurve for the breakage probability. Powder Technol. 2003, 129, 101–110. [Google Scholar] [CrossRef]
- Meier, M.; John, E.; Wieckhusen, D.; Wirth, W.; Peukert, W. Influence of mechanical properties on impact fracture: Prediction of the milling behaviour of pharmaceutical powders by nanoindentation. Powder Technol. 2009, 188, 301–313. [Google Scholar] [CrossRef]
- Lawn, B.; Marshall, D. Hardness, toughness, and brittleness: An indentation analysis. J. Am. Ceram. Soc. 1979, 62, 347–350. [Google Scholar] [CrossRef]
- Meier, M.; John, E.; Wieckhusen, D.; Wirth, W.; Peukert, W. Generally applicable breakage functions derived from single particle comminution data. Powder Technol. 2009, 194, 33–41. [Google Scholar] [CrossRef]
- Box, G.E.P.; Wilson, K.B. On the experimental attainment of optimum conditions. J. R. Stat. Soc. Ser. B 1951, 13, 1–45. [Google Scholar]
- Bilgili, E.; Arastoopour, H.; Bernstein, B.; Hamey, R. Some novel applications of grinding and milling technologies: Milling of soft materials and nanomilling. In Fine Particle Technology and Characterization; Yekeler, M., Ed.; Research Signpost: Kerala, India, 2008; pp. 41–67. [Google Scholar]
- Capece, M.; Bilgili, E.; Davé, R.N. Formulation of a physically motivated specific breakage rate parameter for ball milling via the discrete element method. AIChE J. 2014, 60, 2404–2415. [Google Scholar] [CrossRef]
- Gudin, D.; Turczyn, R.; Mio, H.; Kano, J.; Saito, F. Simulation of the movement of beads by the dem with respect to the wet grinding process. AIChE J. 2006, 52, 3421–3426. [Google Scholar] [CrossRef]
- Knieke, C.; Steinborn, C.; Romeis, S.; Peukert, W.; Breitung-Faes, S.; Kwade, A. Nanoparticle production with stirred-media mills: Opportunities and limits. Chem. Eng. Technol. 2010, 33, 1401–1411. [Google Scholar] [CrossRef]
- Sommer, M.; Stenger, F.; Peukert, W.; Wagner, N.J. Agglomeration and breakage of nanoparticles in stirred media mills—A comparison of different methods and models. Chem. Eng. Sci. 2006, 61, 135–148. [Google Scholar] [CrossRef]
- Annapragada, A.; Adjei, A. Numerical simulation of milling processes as an aid to process design. Int. J. Pharm. 1996, 136, 1–11. [Google Scholar] [CrossRef]
- Kwade, A. Determination of the most important grinding mechanism in stirred media mills by calculating stress intensity and stress number. Powder Technol. 1999, 105, 382–388. [Google Scholar] [CrossRef]
- Tuzcu, E.T.; Rajamani, R.K. Modeling breakage rates in mills with impact energy spectra and ultra fast load cell data. Miner. Eng. 2011, 24, 252–260. [Google Scholar] [CrossRef]
- Graeme, L. CFD modelling of a stirred bead mill for fine grinding. In Proceedings of the Second International Conference on CFD in the Minerals and Process Industries, Melbourne, Australia, 6–8 December 1999; pp. 449–454.
- Gers, R.; Climent, E.; Legendre, D.; Anne-Archard, D.; Frances, C. Numerical modelling of grinding in a stirred media mill: Hydrodynamics and collision characteristic. Chem. Eng. Sci. 2010, 65, 2052–2064. [Google Scholar] [CrossRef] [Green Version]
- Gers, R.; Anne-Archard, D.; Climent, E.; Legendre, D.; Frances, C. Two colliding grinding beads: Experimental flow fields and particle capture efficiency. Chem. Eng. Technol. 2010, 33, 1438–1446. [Google Scholar] [CrossRef]
- Rosenkranz, S.; Breitung-Faes, S.; Kwade, A. Experimental investigations and modelling of the ball motion in planetary ball mills. Powder Technol. 2011, 212, 224–230. [Google Scholar] [CrossRef]
- Cundall, P.A.; Strack, O.D. A discrete numerical model for granular assemblies. Geotechnique 1979, 29, 47–65. [Google Scholar] [CrossRef]
- Khanal, M.; Schubert, W.; Tomas, J. Oblique impact simulations of high strength agglomerates. In Micro-Macro-Interaction; Springer: Berlin, Germany, 2008; pp. 243–253. [Google Scholar]
- Mishra, B. A review of computer simulation of tumbling mills by the discrete element method: Part II—Practical applications. Int. J. Miner. Process. 2003, 71, 95–112. [Google Scholar] [CrossRef]
- Mori, H.; Mio, H.; Kano, J.; Saito, F. Ball mill simulation in wet grinding using a tumbling mill and its correlation to grinding rate. Powder Technol. 2004, 143, 230–239. [Google Scholar] [CrossRef]
- Van Buijtenen, M.S.; Deen, N.G.; Heinrich, S.; Antonyuk, S.; Kuipers, J. A discrete element study of wet particle–particle interaction during granulation in a spout fluidized bed. Can. J. Chem. Eng. 2009, 87, 308–317. [Google Scholar] [CrossRef]
- Stražišar, J.; Runovc, F. Kinetics of comminution in micro-and sub-micrometer ranges. Int. J. Miner. Process. 1996, 44, 673–682. [Google Scholar] [CrossRef]
- Varinot, C.; Berthiaux, H.; Dodds, J. Prediction of the product size distribution in associations of stirred bead mills. Powder Technol. 1999, 105, 228–236. [Google Scholar] [CrossRef]
- Cho, H.; Waters, M.; Hogg, R. Investigation of the grind limit in stirred-media milling. Int. J. Miner. Process. 1996, 44, 607–615. [Google Scholar] [CrossRef]
- Bilgili, E.; Scarlett, B. Population balance modeling of non-linear effects in milling processes. Powder Technol. 2005, 153, 59–71. [Google Scholar] [CrossRef]
- Bilgili, E. On the consequences of non-first-order breakage kinetics in comminution processes: Absence of self-similar size spectra. Part. Part. Syst. Charact. 2007, 24, 12–17. [Google Scholar] [CrossRef]
- Bilgili, E.; Yepes, J.; Scarlett, B. Formulation of a non-linear framework for population balance modeling of batch grinding: Beyond first-order kinetics. Chem. Eng. Sci. 2006, 61, 33–44. [Google Scholar] [CrossRef]
- Capece, M.; Bilgili, E.; Dave, R. Identification of the breakage rate and distribution parameters in a non-linear population balance model for batch milling. Powder Technol. 2011, 208, 195–204. [Google Scholar] [CrossRef]
- Heim, A.; Leszczyniecki, R.; Amanowicz, K. Determination of parameters for wet-grinding model in perl mills. Powder Technol. 1985, 41, 173–179. [Google Scholar] [CrossRef]
- Hennart, S.; Wildeboer, W.; van Hee, P.; Meesters, G. Identification of the grinding mechanisms and their origin in a stirred ball mill using population balances. Chem. Eng. Sci. 2009, 64, 4123–4130. [Google Scholar] [CrossRef]
- Bilgili, E.; Hamey, R.; Scarlett, B. Nano-milling of pigment agglomerates using a wet stirred media mill: Elucidation of the kinetics and breakage mechanisms. Chem. Eng. Sci. 2006, 61, 149–157. [Google Scholar] [CrossRef]
- Hänchen, M.; Krevor, S.; Mazzotti, M.; Lackner, K.S. Validation of a population balance model for olivine dissolution. Chem. Eng. Sci. 2007, 62, 6412–6422. [Google Scholar] [CrossRef]
- Somasundaran, P.; Runkana, V. Modeling flocculation of colloidal mineral suspensions using population balances. Int. J. Miner. Process. 2003, 72, 33–55. [Google Scholar] [CrossRef]
- Hounslow, M. The population balance as a tool for understanding particle rate processes. KONA Powder Part J. 1998, 16, 179–193. [Google Scholar] [CrossRef]
- Varinot, C.; Hiltgun, S.; Pons, M.-N.; Dodds, J. Identification of the fragmentation mechanisms in wet-phase fine grinding in a stirred bead mill. Chem. Eng. Sci. 1997, 52, 3605–3612. [Google Scholar] [CrossRef]
- Eskin, D.; Zhupanska, O.; Hamey, R.; Moudgil, B.; Scarlett, B. Microhydrodynamic analysis of nanogrinding in stirred media mills. AIChE J. 2005, 51, 1346–1358. [Google Scholar] [CrossRef]
- Gidaspow, D. Multiphase Flow and Fluidization: Continuum and Kinetic Theory Descriptions; Academic Press: Salt Lake City, UT, USA, 1994. [Google Scholar]
- Eskin, D.; Miller, M.J. A model of non-newtonian slurry flow in a fracture. Powder Technol. 2008, 182, 313–322. [Google Scholar] [CrossRef]
- Hall, M. Encyclopedia of Nanoscience and Nanotechnology; American Scientific Publishers: Valencia, CA, USA, 2010. [Google Scholar]
- Xiong, R.; Lu, W.; Li, J.; Wang, P.; Xu, R.; Chen, T. Preparation and characterization of intravenously injectable nimodipine nanosuspension. Int. J. Pharm. 2008, 350, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Sinha, B.; Müller, R.H.; Möschwitzer, J.P. Bottom-up approaches for preparing drug nanocrystals: Formulations and factors affecting particle size. Int. J. Pharm. 2013, 453, 126–141. [Google Scholar] [CrossRef] [PubMed]
- Ajazuddin, S.S. Applications of novel drug delivery system for herbal formulations. Fitoterapia 2010, 81, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Mukerjee, A.; Vishwanatha, J.K. Formulation, characterization and evaluation of curcumin-loaded plga nanospheres for cancer therapy. Anticancer Res. 2009, 29, 3867–3875. [Google Scholar] [PubMed]
- Chamarthy, S.P.; Pinal, R. The nature of crystal disorder in milled pharmaceutical materials. Colloids Surf. A 2008, 331, 68–75. [Google Scholar] [CrossRef]
- Feng, T.; Pinal, R.; Carvajal, M.T. Process induced disorder in crystalline materials: Differentiating defective crystals from the amorphous form of griseofulvin. J. Pharm. Sci. 2008, 97, 3207–3221. [Google Scholar] [CrossRef] [PubMed]
- Otte, A.; Carvajal, M.T. Assessment of milling-induced disorder of two pharmaceutical compounds. J. Pharm. Sci. 2011, 100, 1793–1804. [Google Scholar] [CrossRef] [PubMed]
- Wildfong, P.L.; Hancock, B.C.; Moore, M.D.; Morris, K.R. Towards an understanding of the structurally based potential for mechanically activated disordering of small molecule organic crystals. J. Pharm. Sci. 2006, 95, 2645–2656. [Google Scholar] [CrossRef] [PubMed]
- Howorth, C.; Lee, W.; Rainforth, W.; Messer, P. Contamination rates from Ce-and Y-TZP ball milling media. Br. Ceram. Trans. J. 1991, 90, 18–21. [Google Scholar]
- Czekai, D.A.; Seaman, L.P. Continuously Feeding Therapeutical or Imaging Agents and Rigid Grinding Media to a Milling Chamber, Contacting the Agent with Grinding Media to Reduce Particle Size, Continuously Removing and Separating. U.S. Patent 5,718,388, 17 February 1998. [Google Scholar]
- Plumb, K. Continuous processing in the pharmaceutical industry: Changing the mind set. Chem. Eng. Res. Des. 2005, 83, 730–738. [Google Scholar] [CrossRef]
- Mannheim, V. Empirical and scale-up modeling in stirred ball mills. Chem. Eng. Res. Des. 2011, 89, 405–409. [Google Scholar] [CrossRef]
- Wagener, P.; Lau, M.; Breitung-Faes, S.; Kwade, A.; Barcikowski, S. Physical fabrication of colloidal ZnO nanoparticles combining wet-grinding and laser fragmentation. Appl. Phys. A 2012, 108, 793–799. [Google Scholar] [CrossRef]
- Patel, C.M.; Chakraborty, M.; Murthy, Z.V.P. Preparation of fenofibrate nanoparticles by combined stirred media milling and ultrasonication method. Ultrason. Sonochem. 2014, 21, 1100–1107. [Google Scholar] [CrossRef] [PubMed]
- Kipp, J.E.; Wong, J.C.T.; Doty, M.J.; Rebbeck, C.L. Microprecipitation Method for Preparing Submicron Suspensions. U.S. Patent 6,607,784, 19 August 2003. [Google Scholar]
- Rabinow, B.; Kipp, J.; Papadopoulos, P.; Wong, J.; Glosson, J.; Gass, J.; Sun, C.-S.; Wielgos, T.; White, R.; Cook, C. Itraconazole IV nanosuspension enhances efficacy through altered pharmacokinetics in the rat. Int. J. Pharm. 2007, 339, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Shelar, D.B.; Pawar, S.K.; Vavia, P.R. Fabrication of isradipine nanosuspension by anti-solvent microprecipitation-high-pressure homogenization method for enhancing dissolution rate and oral bioavailability. Drug Deliv. Transl. Res. 2013, 3, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R. Nanocrystals for Use in Topical Cosmetic Formulations and Method of Production Thereof. U.S. Patent 9,114,077, 25 August 2015. [Google Scholar]



| References (Year) | Drug | Drug Concentration (%) a | Stabilizer(s) b | Stabilizer Concentration (%) a | Reported Smallest Median or Mean Particle Size after Milling (nm) |
|---|---|---|---|---|---|
| Bitterlich et al. (2015) [73] | Naproxen | 5 | Poloxamer-188, PVP-K 30, PVA-Mowiol 3-85, Mowiol 4-88, PVP-VA64, HPC-LF, Polysorbate-80, TPGS, SDS, HPMC | 0.1–15 | ~150 |
| Dong et al. (2015) [74] | SNX-2112 | 1 | Poloxamer-188, Polysorbate-80 | 0.01–1 | 203 |
| Kumar et al. (2015) [75] | Danazol | 1 | PVP-K 30, 40, PVA, HPMC-E3, E5, E15, Methocel-A15, SDS, TPGS, Poloxamer-188, 407, Dowfax-2A1, HPC | 0.2 | 168 |
| Afolabi et al. (2014) [45] | Griseofulvin | 5–30 c | HPC-SL | 2.5 | 132 |
| SDS | 0.5 | ||||
| Bhakay et al. (2014) [76] | Griseofulvin, Azodicarbonamide | 10 c | HPC-SL, SDS | 0–2.5 | 160 |
| Bitterlich et al. (2014) [59] | Cinnarizine, Fenofibrate | 10 | DOSS | 0.25 | 276 |
| SDS | 0.1 | ||||
| Poloxamer-188, PVP-K30, PVP-VA64, PVA-Mowiol 3–85, Polysorbate-80, HPMC, Vit-E TPGS | 2.5 | ||||
| Komasaka et al. (2014) [77] | Cilostazol, Curucumin, Furosemide, Naproxen, Phenytoin, Nifedipine, Danazol, Spironolactone, Cinnarizine, Piroxicam, Indomethacin | 10, 20 | HPMC of different molecular weight, TC-5E, MC-400, Metolose, PVP-K17, Polysorbate-80, SDS, Cremophor-RH40, Poloxamer-188, Vit-E TPGS | 0.5 | ~120 |
| Leng et al. (2014) [78] | Paliperidone palmitate | 15 | Polysorbate-80 | NM f | 492 (±8) |
| Mahesh et al. (2014) [79] | Glipizide | 1.6 | SDS, Poloxamer-188, 407, Polysorbate-80 | 2.5–7.5 d | NM f |
| PVP-K30, HPMC | 2.5–5 d | ||||
| Shah et al. (2014) [80] | Glibenclamide | 0.5 | PVP-S630 D, Poloxamer-188, Polysorbate-80, HPMC, HPC, HEC, SDS | 0.25 | 329 |
| Sarnes et al. (2014) [81] | Itraconazole | 15 | Poloxamer-407 | 9–12 | 315 (±5) |
| Yuminoki et al. (2014) [82] | Griseofulvin, Hydrochlorothiazide, Tolbutamide, Acyclovir, Indomethacin, Diprydamole, Naproxen, Piroxicam, Phenytoin | 1–20 | HPC, PVP, POVA, PVA | 1–10 | 120 (±2) |
| Bhakay et al. (2013) [58] | Griseofulvin, Phenylbutazone | 10 c | HPC-SL, SDS | 0–2.5 | 145 |
| Mannitol | 10 | ||||
| Cerdeira et al. (2013) [83] | Miconazole, Itraconazole | 5–20 | SDS | 0–0.2 | 136 |
| HPC-LF | 1.25–5 | ||||
| HPMC-E15, Poloxamer-188, 407 | 5 | ||||
| George and Ghosh (2013) [84] | Naproxen, compound A, B, C, D and E from Novartis | 5 | Vit-E TPGS, Poloxamer-407, SDS, DOSS | 1 | <500 |
| HPMC | 2.5 | ||||
| Knieke et al. (2013) [64] | Fenofibrate | 2.5 | HPMC-E3 | 5–50 e | 151 |
| SDS | 5–20 e | ||||
| Monteiro et al. (2013) [85] | Griseofulvin, Naproxen | 10 c | HPC-SL | 2.5 | 138 |
| SDS | 0.0825, 0.5 | ||||
| Niwa and Danjo (2013) [86] | Phenytoin | 8 | PVP-K30 | 0.25–16 | 168 |
| SDS | 0.1 | ||||
| Ghosh et al. (2012) [47] | NVS-102 | 2, 5 | HPMC | 1 | 277 |
| Vit-E TPGS | 0.5–5 | ||||
| Tanaka et al. (2012) [6] | Probucol | 1 | Gelucire-44/14, Gelucire-50/13, Vit-E TPGS, Poloxamer-188, 338 | 1 | 77 |
| Sievens-Figueroa et al. (2012) [36] | Naproxen, Fenofibrate, Griseofulvin | 10c | HPMC-E15LV | 2.5 c | 144 |
| SDS | 0.075, 0.5c | ||||
| Ali et al. (2011) [87] | Hydrocortisone | 2 | PVP, Polysorbate-80 | 0.2 | 300 |
| HPMC | 0.5 | ||||
| Bhakay et al. (2011) [65] | Itraconazole, Fenofibrate, Griseofulvin, Ibuprofen, Azodicarbonamide, Sulfamethoxazole | 2c | SA, SDS, HPMC, Polysorbate-80 | 0.1 | 740 |
| HPMC-E15 LV | 0.2 | ||||
| Cerdeira et al. (2011) [46] | Miconazole, Itraconazole, Etravirine | 20 | HPC-LF | 5 | 129 |
| SDS | 0–0.2 | ||||
| Chin et al. (2011) [62] | Carbofuran | 40.6, 44 | Atlox-4913 | 4–7 | 29 |
| PVP-K30 | 1–3 | ||||
| Miglyol-812 | 1–3 | ||||
| Ghosh et al. (2011) [88] | Compound NVS-102 | 5 | Vit-E TPGS | 3, 5 | 230.2 |
| SDS, HPMC, PVP-K30 | 1 | ||||
| Poloxamer-188, 407 | 2 | ||||
| Liu et al. (2011) [89] | Indomethacin, Itraconazole | 40 | Polysorbate-80, PEG-6000, Poloxamer-188, 407 | 10–80 e | 345 |
| Cerdeira et al. (2010) [57] | Miconazole | 5–25 | HPC-LF | 1.25–6.25 | 140 |
| HPC-EF, PVP-30, Poloxamer-188, HPMC-E15 | 1.25, 2.5 | ||||
| SDS | 0.0125, 0.05, 0.2 | ||||
| DOSS (SD) | 0.1, 5 | ||||
| BKC | 0.1 | ||||
| Juhnke et al. (2010) [63] | Naproxen | 2 | HPC-LF | 0.5 | 151 |
| Compounds A and B, from Novartis | |||||
| Patel et al. (2010) [90] | Famotidine | 0.4 | HPMC-K15M, PVP-K30, Polysorbate-80, Poloxamer-188, 407 | 0.4, 0.8 | 244.6 |
| Baert et al. (2009) [91] | Rilpivirine (TMC278) | 12.5 | Poloxamer-338 | 3.125 | 200 |
| Vit-E TPGS | 3.125 | ||||
| Fakes et al. (2009) [92] | HIV-attachment inhibitor: BMS-488043 | 10 | HPC-SL | 1.25, 2.1 | 120 |
| SDS, DOSS | 0.1 | ||||
| Tanaka et al. (2009) [93] | Omeprazole, Albendazole, Danazol | 1 | Polysorbate-80, Poloxamer-188, 407 | 0.05–5 | 102 |
| Van Eerdenbrugh et al. (2009) [94] | Loviride, Itraconazole, Cinnarizine, Griseofulvin, Indomethacin, Mebendazole, Naproxen, Phenylbutazone, Phenytoin | 20 | PVP-K30, K90, PVA-PEG (K-IR), Poloxamer-188, Vit-E TPGS, PVA, Polysorbate-80 | 10–100 | >1000 |
| HPMC-E15, HEC, HPC, MC, NaCMC, NaAlg | 1–10 | ||||
| Ain-Ai and Gupta (2008) [95] | Naproxen | 10, 30 | HPC | 1–4 | 417 |
| AH | 0–1.2 | ||||
| Choi et al. (2008) [96] | Itraconazole | 8 | HPC of different molecular weights | 1.33 | 110 |
| Deng et al. (2008) [97] | Compound A | 15 | Plasdone S-630 | 3.5, 4.1 | 82 |
| SD | 0.25, 0.295 | ||||
| Lee et al. (2008) [98] | Ibuprofen, Glimepiride, Digitoxin, Naproxen, Biphenyl dimethyl dicarboxylate, Paclitaxel, Lipoic acid, Predinisolone acetate, Nifedipin, Hydrocortihydrocortisone acetate, Itraconazole | 8 | HPC, PVP, PEG , Poloxamer-188, 407 | 1.33 | 119 (±37) |
| SDS, Benzethonium chloride | 1 | ||||
| Van Eerdenbrugh et al. (2008) [23] | Loviride, Itraconazole, Cinnarizine, Griseofulvin, Indomethacin, Mebendazole, Naproxen, Phenylbutazone, Phenytoin | 20c | Vit-E TPGS | 25 e | 156 |
| Dai et al. (2007) [99] | Poorly water soluble compound/carrageenan complex | 5 | Poloxamer-407 | 0.75 | 300 |
| Tyloxapol, HPMC-2910, HPC-SL | 1.5, 2 | ||||
| PVP-K30 | 0.75, 2 | ||||
| Plasdone-S630 | 1.31, 2 | ||||
| DOSS | 0.15 | ||||
| Sepassi et al.(2007) [100] | Nabumetone, Halofantrine | 20 | HPMC-E3LV, E4M, PVP-K12, K30, K90 | 0.63–6.25 | 650 |
| Van Eerdenbrugh et al.(2007) [101] | Loviride | 20 | Polysorbate-80, Poloxamer-188 | 50 e | 264 (±14) |
| Jinno et al. (2006) [102] | Cilostazol | 0.25 | HPC | 16.5 | 220 |
| DOSS | 0.8 |
| References (Year) | Mill Type | Stirrer/Circumference Speed (rpm) | Suspension Flow Rate (mL/min) | Milling Time (h) | Bead Type a | Nominal or Median Bead Size (µm) | Bead Boading (%) b | Drug Concentration (%) c |
|---|---|---|---|---|---|---|---|---|
| Bitterlich et al. (2015) [73] | Planetary ball mill | 400 | NM f | 4 | Al2O3 | 100 | 50 | 5 |
| Al2O3 | 300 | |||||||
| ZrO2 | 100 | |||||||
| ZrO2 | 200 | |||||||
| ZrO2 | 300 | |||||||
| ZrO2 | 500 | |||||||
| Li et al. (2015) [68] | Vibratory media mill | 40%–90% d | NM f | 1.6 | ZrO2 | 50–1500 | 30–70 | 10 |
| Li et al. (2015) [61] | Wet stirred media mill | 11.7–14.7 e | 126–343 | 2–6 | ZrO2 | 50–800 | 62.5–93.75 | 10 |
| Afolabi et al. (2014) [45] | Wet stirred media mill | 5.86–14.7 e | 126 | 1.6 | ZrO2 | 430 | 17.5–93.75 | 5–30 |
| Kumar and Burgess (2014) [122] | Wet stirred media mill | 2000–3400 | NM f | 1–4 | ZrO2 | NM f | NM f | 1 |
| Shah et al.(2014) [80] | Wet media mill | 400–1100 | NM f | 3–11 | ZrO2 | 100–1000 | 50 f | 0.5 |
| Bitterlich et al.(2014) [59] | Planetary ball mill | 400 | NM f | 4 | ZrO2 | 325 | 50 | 10 g |
| Wet stirred media mill | 9 e | NM f | 6–24 | Al2O3 (irregular) | 185–320 | 70 h | ||
| Al2O3 (spherical) | 311 | |||||||
| ZrO2 | 185–475 | |||||||
| Monteiro et al. (2013) [85] | Wet stirred media mill | 13.2 e | 55–110 | ~1 | ZrO2 | 430 | 62.5 | 10 |
| Ghosh et al.(2012) [47] | Planetary mill | 150–400 | NM f | 4 | ZrO2 | 100–500 | NM f | 2–5 |
| Wet stirred media mill | 2500 | NM f | 1–4 | ZrO2 | 100–500 | NM f | ||
| Juhnke et al.(2012) [123] | Wet stirred media mill | 6–12 e | NM f | NM f | ZrO2 | 100–500 | 80 | 10 g |
| Tanaka et al.(2012) [6] | Wet stirred media mill | 8–12 e | NM f | NM f | ZrO2 | 15–50 | 500 i | 1 |
| Bhakay et al. (2011) [65] | Wet stirred media mill | 2.65 e | NM f | 0.5–1.3 | Crosslinked polystyrene | 200–350 | 50 | 2 |
| Attritor mode | 2.65–4.97 e | NM f | 1.3 | Zirconia rings | NM f | |||
| Cerdeira et al.(2011) [46] | High energy media mill | 2400–3600 | 97–183 j | 0.25–1 | ZrO2 | 400–800 | 81–85 | 20 g |
| Chin et al.(2011) [62] | High energy intensive ball mill | 3000 | NM f | 2 | ZrO2 | 100–800 | NM f | 40.6–44 g |
| Singh et al.(2011) [5] | Wet stirred media mill | 2500–3400 | 100 | 3–6.5 | ZrO2 | 200 | NM f | 4 |
| Hennart et al.(2010) [121] | Wet stirred media mill | 2000–6000 | NM f | 3 | ZrO2 | 300–800 | 80 | NM f |
| Juhnke et al. (2010) [63] | Planetary mill | 400 | NM f | 0.25–2 | ZrO2 | 200 | 60 | 2 g |
| Wet stirred media mill | 10 e | NM f | 8 | Crosslinked polystyrene | 360–500 | |||
| 6 e | 8 | ZrO2 | 100 | |||||
| Singare et al.(2010) [124] | Wet stirred media mill | 2500–3400 | 100 | 3–6 | ZrO2 | 200 | NM f | 6.4 |
| Deng et al.(2008) [97] | NanoMill-01 Systems milling apparatus | 1800–4400 | NM f | 0.67–1 | Cross-linked polystyrene | 500 | NM f | 15 g |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Azad, M.; Davé, R.; Bilgili, E. Nanomilling of Drugs for Bioavailability Enhancement: A Holistic Formulation-Process Perspective. Pharmaceutics 2016, 8, 17. https://doi.org/10.3390/pharmaceutics8020017
Li M, Azad M, Davé R, Bilgili E. Nanomilling of Drugs for Bioavailability Enhancement: A Holistic Formulation-Process Perspective. Pharmaceutics. 2016; 8(2):17. https://doi.org/10.3390/pharmaceutics8020017
Chicago/Turabian StyleLi, Meng, Mohammad Azad, Rajesh Davé, and Ecevit Bilgili. 2016. "Nanomilling of Drugs for Bioavailability Enhancement: A Holistic Formulation-Process Perspective" Pharmaceutics 8, no. 2: 17. https://doi.org/10.3390/pharmaceutics8020017
APA StyleLi, M., Azad, M., Davé, R., & Bilgili, E. (2016). Nanomilling of Drugs for Bioavailability Enhancement: A Holistic Formulation-Process Perspective. Pharmaceutics, 8(2), 17. https://doi.org/10.3390/pharmaceutics8020017