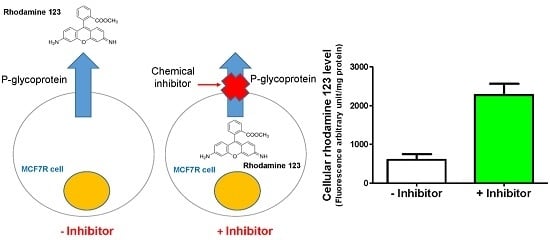
Evaluation of P-Glycoprotein Inhibitory Potential Using a Rhodamine 123 Accumulation Assay
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Culture
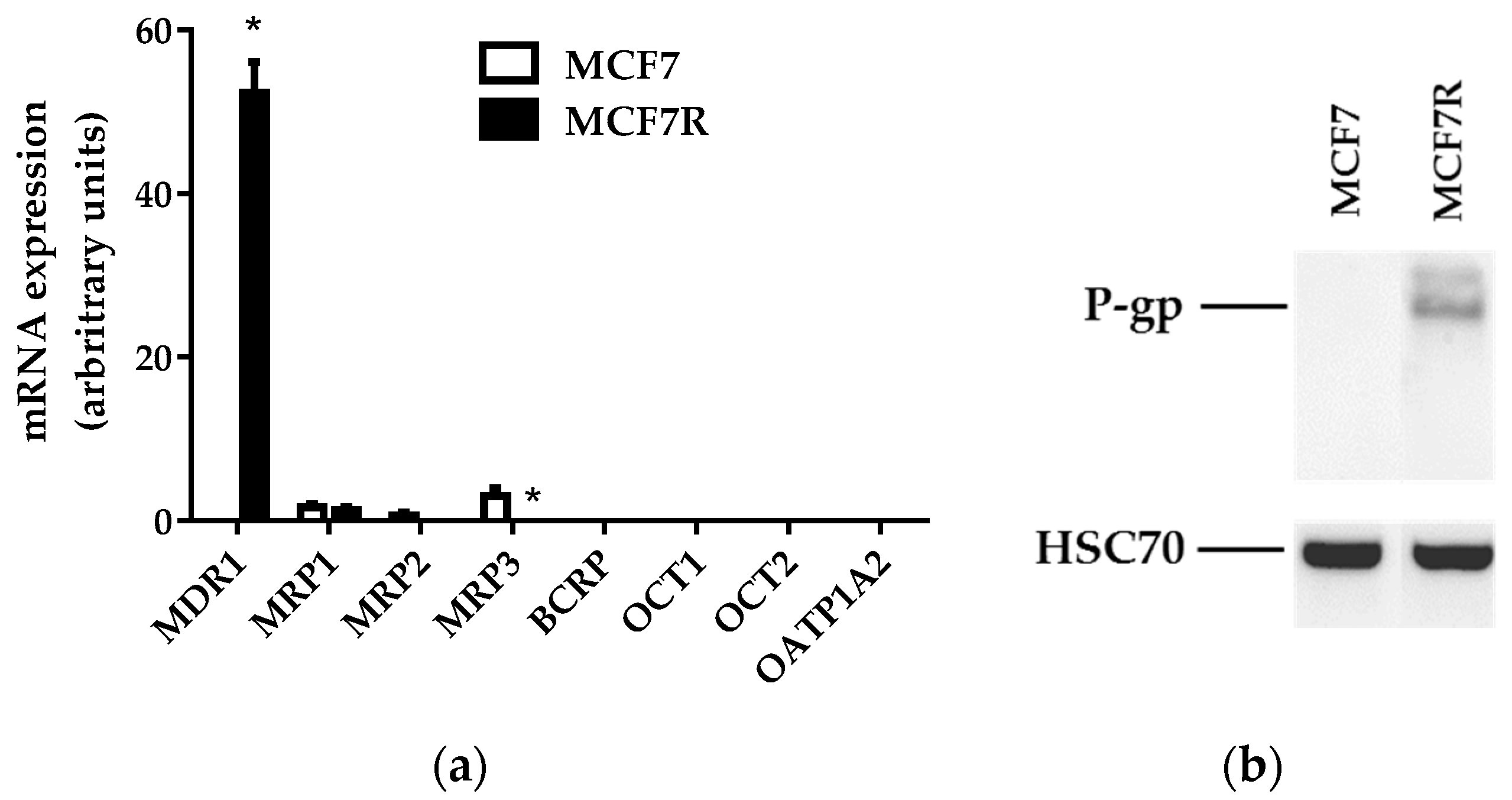
2.3. RNA Isolation and Analysis
2.4. Western Blot Analysis
2.5. Rhodamine 123 Accumulation Assay
2.6. Data Analysis
3. Results and Discussion
3.1. Expression of MDR1/P-gp in MCF7 and MCF7R Cells
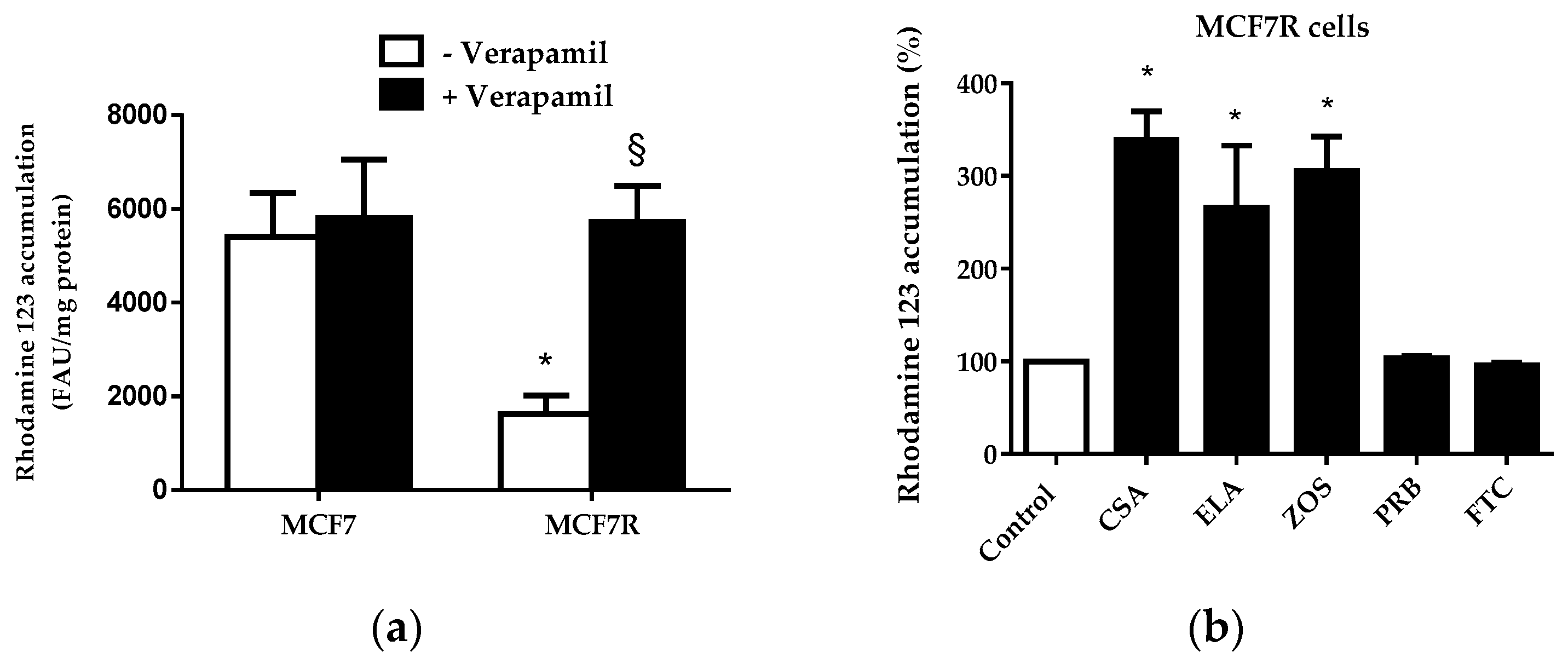
3.2. Rhodamine 123 Accumulation in MCF7 and MCF7R Cells
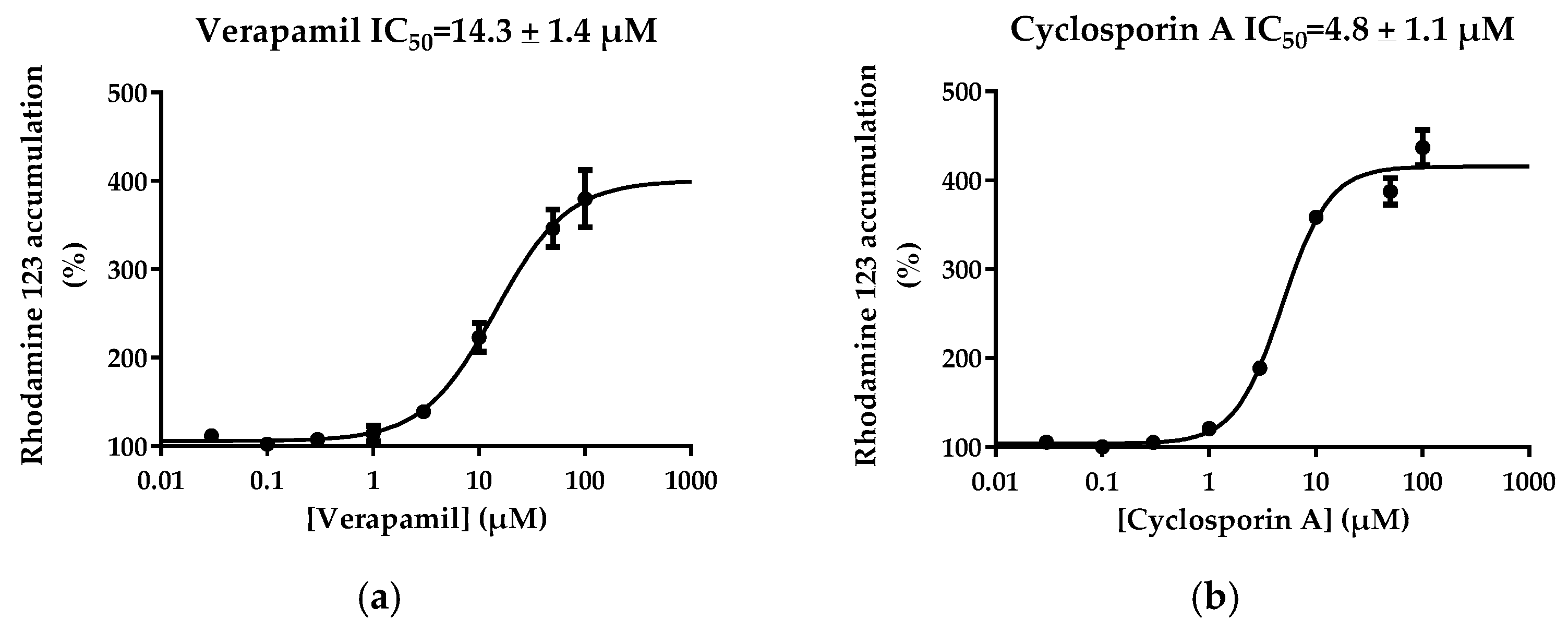
3.3. Determination of P-gp IC50 Values for a Range of Structurally-Unrelated Reference P-gp Inhibitors
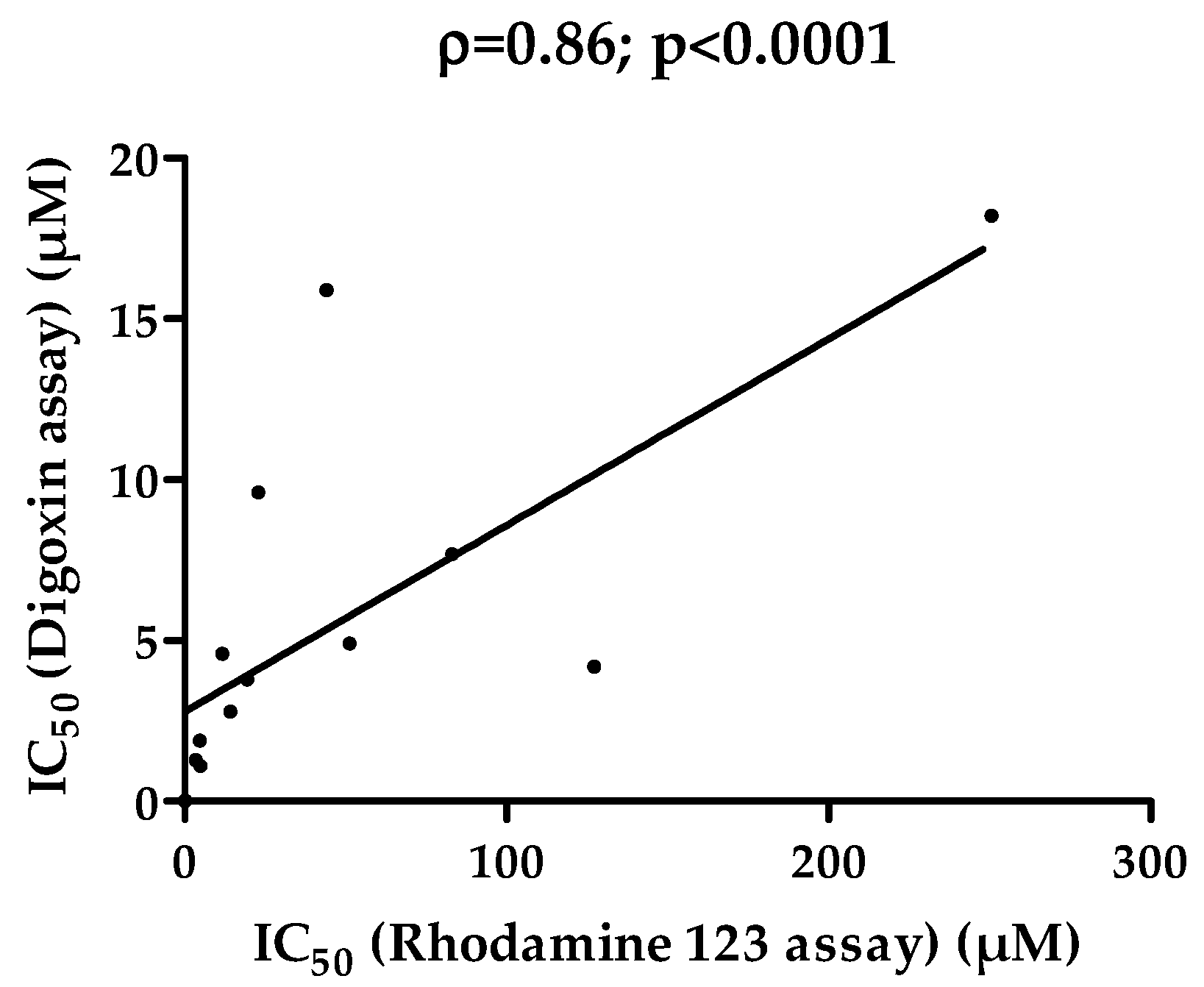
3.4. Comparison of IC50 Values for P-gp Inhibition Determined from Rhodamine 123 and Digoxin Transport Assays
3.5. Prediction of in Vivo P-gp Inhibition from Rhodamine 123 Accumulation Assays
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Gottesman, M.M.; Pastan, I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu. Rev. Biochem. 1993, 62, 385–427. [Google Scholar] [CrossRef] [PubMed]
- Fardel, O.; Lecureur, V.; Guillouzo, A. The P-glycoprotein multidrug transporter. Gen. Pharmacol. 1996, 27, 1283–1291. [Google Scholar] [CrossRef]
- Bradley, G.; Juranka, P.F.; Ling, V. Mechanism of multidrug resistance. Biochim. Biophys. Acta 1988, 948, 87–128. [Google Scholar] [CrossRef]
- Zhou, S.F. Structure, function and regulation of P-glycoprotein and its clinical relevance in drug disposition. Xenobiotica 2008, 38, 802–832. [Google Scholar] [CrossRef] [PubMed]
- Fenner, K.S.; Troutman, M.D.; Kempshall, S.; Cook, J.A.; Ware, J.A.; Smith, D.A.; Lee, C.A. Drug-drug interactions mediated through P-glycoprotein: Clinical relevance and in vitro-in vivo correlation using digoxin as a probe drug. Clin. Pharmacol. Ther. 2009, 85, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, Y.D.; Strong, J.M.; Reynolds, K.S.; Huang, S.M. A regulatory viewpoint on transporter-based drug interactions. Xenobiotica 2008, 38, 709–724. [Google Scholar] [CrossRef] [PubMed]
- Giacomini, K.M.; Huang, S.M.; Tweedie, D.J.; Benet, L.Z.; Brouwer, K.L.; Chu, X.; Dahlin, A.; Evers, R.; Fischer, V.; Hillgren, K.M.; et al. Membrane transporters in drug development. Nat. Rev. Drug Discov. 2010, 9, 215–236. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, K.L.; Keppler, D.; Hoffmaster, K.A.; Bow, D.A.; Cheng, Y.; Lai, Y.; Palm, J.E.; Stieger, B.; Evers, R. In vitro methods to support transporter evaluation in drug discovery and development. Clin. Pharmacol. Ther. 2013, 94, 95–112. [Google Scholar] [CrossRef] [PubMed]
- Rautio, J.; Humphreys, J.E.; Webster, L.O.; Balakrishnan, A.; Keogh, J.P.; Kunta, J.R.; Serabjit-Singh, C.J.; Polli, J.W. In vitro P-glycoprotein inhibition assays for assessment of clinical drug interaction potential of new drug candidates: A recommendation for probe substrates. Drug Metab. Dispos. 2006, 34, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Bentz, J.; O'Connor, M.P.; Bednarczyk, D.; Coleman, J.; Lee, C.; Palm, J.; Pak, Y.A.; Perloff, E.S.; Reyner, E.; Balimane, P.; et al. Variability in P-glycoprotein inhibitory potency (IC50) using various in vitro experimental systems: Implications for universal digoxin drug-drug interaction risk assessment decision criteria. Drug Metab. Dispos. 2013, 41, 1347–1366. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.A.; Kalvass, J.C.; Galetin, A.; Zamek-Gliszczynski, M.J. ITC commentary on the prediction of digoxin clinical drug-drug interactions from in vitro transporter assays. Clin. Pharmacol. Ther. 2014, 96, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Lumen, A.A.; Li, L.; Li, J.; Ahmed, Z.; Meng, Z.; Owen, A.; Ellens, H.; Hidalgo, I.J.; Bentz, J. Transport inhibition of digoxin using several common P-gp expressing cell lines is not necessarily reporting only on inhibitor binding to P-gp. PLoS ONE 2013, 8, e69394. [Google Scholar] [CrossRef] [PubMed]
- Fardel, O.; Le Vee, M.; Jouan, E.; Denizot, C.; Parmentier, Y. Nature and uses of fluorescent dyes for drug transporter studies. Expert Opin. Drug Metab. Toxicol. 2015, 11, 1233–1251. [Google Scholar] [CrossRef] [PubMed]
- Fortuna, A.; Alves, G.; Falcao, A. In vitro and in vivo relevance of the P-glycoprotein probe substrates in drug discovery and development: Focus on rhodamine 123, digoxin and talinolol. J. Bioequiv. Availab. 2011, S2. [Google Scholar] [CrossRef]
- Gattringer, C.L.; Drach, J.; Hofmann, J.; Grunicke, H. Rapid functional assay for the detection of multidrug-resistant cells using the fluorescent dye rhodamine 123. Blood 1991, 78, 1385–1387. [Google Scholar]
- Drenou, B.; Fardel, O.; Amiot, L.; Fauchet, R. Detection of P-glycoprotein activity on normal and leukemic CD34+ cells. Leuk. Res. 1993, 17, 1031–1035. [Google Scholar] [CrossRef]
- Lizard, G.; Roignot, P.; Maynadie, M.; Lizard-Nacol, S.; Poupon, M.F.; Bordes, M. Flow cytometry evaluation of the multidrug-resistant phenotype with functional tests involving uptake of daunorubicin, Hoechst 33342, or rhodamine 123: A comparative study. Cancer Detect. Prev. 1995, 19, 527–534. [Google Scholar] [PubMed]
- Sarver, J.G.; Klis, W.A.; Byers, J.P.; Erhardt, P.W. Microplate screening of the differential effects of test agents on Hoechst 33342, rhodamine 123, and rhodamine 6G accumulation in breast cancer cells that overexpress P-glycoprotein. J. Biomol. Screen. 2002, 7, 29–34. [Google Scholar] [PubMed]
- Morjani, H.; Aouali, N.; Belhoussine, R.; Veldman, R.J.; Levade, T.; Manfait, M. Elevation of glucosylceramide in multidrug-resistant cancer cells and accumulation in cytoplasmic droplets. Int. J. Cancer 2001, 94, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Batist, G.; Tulpule, A.; Sinha, B.K.; Katki, A.G.; Myers, C.E.; Cowan, K.H. Overexpression of a novel anionic glutathione transferase in multidrug-resistant human breast cancer cells. J. Biol. Chem. 1986, 261, 15544–15549. [Google Scholar] [PubMed]
- Le Vee, M.; Lecureur, V.; Stieger, B.; Fardel, O. Regulation of drug transporter expression in human hepatocytes exposed to the proinflammatory cytokines tumor necrosis factor-alpha or interleukin-6. Drug Metab. Dispos. 2009, 37, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Moreau, A.; Le Vee, M.; Jouan, E.; Parmentier, Y.; Fardel, O. Drug transporter expression in human macrophages. Fundam. Clin. Pharmacol. 2011, 25, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Le Vee, M.; Jouan, E.; Noel, G.; Stieger, B.; Fardel, O. Polarized location of SLC and ABC drug transporters in monolayer-cultured human hepatocytes. Toxicol. In Vitro 2015, 29, 938–946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Grevenynghe, J.; Sparfel, L.; Le Vee, M.; Gilot, D.; Drenou, B.; Fauchet, R.; Fardel, O. Cytochrome P450-dependent toxicity of environmental polycyclic aromatic hydrocarbons towards human macrophages. Biochem. Biophys. Res. Commun. 2004, 317, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Le Vee, M.; Jouan, E.; Stieger, B.; Lecureur, V.; Fardel, O. Regulation of human hepatic drug transporter activity and expression by diesel exhaust particle extract. PLoS ONE 2015, 10, e0121232. [Google Scholar]
- Cook, J.A.; Feng, B.; Fenner, K.S.; Kempshall, S.; Liu, R.; Rotter, C.; Smith, D.A.; Troutman, M.D.; Ullah, M.; Lee, C.A. Refining the in vitro and in vivo critical parameters for P-glycoprotein, [I]/IC50 and [I2]/IC50, that allow for the exclusion of drug candidates from clinical digoxin interaction studies. Mol. Pharm. 2010, 7, 398–411. [Google Scholar] [CrossRef] [PubMed]
- Honjo, Y.; Hrycyna, C.A.; Yan, Q.W.; Medina-Perez, W.Y.; Robey, R.W.; van de Laar, A.; Litman, T.; Dean, M.; Bates, S.E. Acquired mutations in the MXR/BCRP/ABCP gene alter substrate specificity in MXR/BCRP/ABCP-overexpressing cells. Cancer Res. 2001, 61, 6635–6639. [Google Scholar] [PubMed]
- Jouan, E.; Le Vee, M.; Denizot, C.; Da Violante, G.; Fardel, O. The mitochondrial fluorescent dye rhodamine 123 is a high-affinity substrate for organic cation transporters (OCTs) 1 and 2. Fundam. Clin. Pharmacol. 2014, 28, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Forster, S.; Thumser, A.E.; Hood, S.R.; Plant, N. Characterization of rhodamine-123 as a tracer dye for use in in vitro drug transport assays. PLoS ONE 2012, 7, e33253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durie, B.G.; Dalton, W.S. Reversal of drug-resistance in multiple myeloma with verapamil. Br. J. Haematol. 1988, 68, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Twentyman, P.R. Cyclosporins as drug resistance modifiers. Biochem. Pharmacol. 1992, 43, 109–117. [Google Scholar] [CrossRef]
- Hyafil, F.; Vergely, C.; Du Vignaud, P.; Grand-Perret, T. In vitro and in vivo reversal of multidrug resistance by GF120918, an acridonecarboxamide derivative. Cancer Res. 1993, 53, 4595–4602. [Google Scholar] [PubMed]
- Dantzig, A.H.; Shepard, R.L.; Cao, J.; Law, K.L.; Ehlhardt, W.J.; Baughman, T.M.; Bumol, T.F.; Starling, J.J. Reversal of P-glycoprotein-mediated multidrug resistance by a potent cyclopropyldibenzosuberane modulator, LY335979. Cancer Res. 1996, 56, 4171–4179. [Google Scholar] [PubMed]
- Versantvoort, C.H.; Bagrij, T.; Wright, K.A.; Twentyman, P.R. On the relationship between the probenecid-sensitive transport of daunorubicin or calcein and the glutathione status of cells overexpressing the multidrug resistance-associated protein (MRP). Int. J. Cancer 1995, 63, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Rabindran, S.K.; Ross, D.D.; Doyle, L.A.; Yang, W.; Greenberger, L.M. Fumitremorgin C reverses multidrug resistance in cells transfected with the breast cancer resistance protein. Cancer Res. 2000, 60, 47–50. [Google Scholar] [PubMed]
- Yumoto, R.; Murakami, T.; Sanemasa, M.; Nasu, R.; Nagai, J.; Takano, M. Pharmacokinetic interaction of cytochrome P450 3A-related compounds with rhodamine 123, a P-glycoprotein substrate, in rats pretreated with dexamethasone. Drug Metab. Dispos. 2001, 29, 145–151. [Google Scholar] [PubMed]
- Wacher, V.J.; Wu, C.Y.; Benet, L.Z. Overlapping substrate specificities and tissue distribution of cytochrome P450 3A and P-glycoprotein: Implications for drug delivery and activity in cancer chemotherapy. Mol. Carcinog. 1995, 13, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Sweatman, T.W.; Seshadri, R.; Israel, M. Metabolism and elimination of rhodamine 123 in the rat. Cancer Chemother. Pharmacol. 1990, 27, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Cooper, D.D.; Hayes, S.F.; Spangrude, G.J. Rhodamine-123 staining in hematopoietic stem cells of young mice indicates mitochondrial activation rather than dye efflux. Blood 1998, 91, 4106–4117. [Google Scholar] [PubMed]
- Varga, Z.V.; Ferdinandy, P.; Liaudet, L.; Pacher, P. Drug-induced mitochondrial dysfunction and cardiotoxicity. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H1453–H1467. [Google Scholar] [CrossRef] [PubMed]
- Poirier, A.; Cascais, A.C.; Bader, U.; Portmann, R.; Brun, M.E.; Walter, I.; Hillebrecht, A.; Ullah, M.; Funk, C. Calibration of in vitro multidrug resistance protein 1 substrate and inhibition assays as a basis to support the prediction of clinically relevant interactions in vivo. Drug Metab. Dispos. 2014, 42, 1411–1422. [Google Scholar] [CrossRef] [PubMed]
- Ellens, H.; Deng, S.; Coleman, J.; Bentz, J.; Taub, M.E.; Ragueneau-Majlessi, I.; Chung, S.P.; Heredi-Szabo, K.; Neuhoff, S.; Palm, J.; et al. Application of receiver operating characteristic analysis to refine the prediction of potential digoxin drug interactions. Drug Metab. Dispos. 2013, 41, 1367–1374. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Uno, T.; Sugawara, K.; Tateishi, T. Effects of single and multiple doses of itraconazole on the pharmacokinetics of fexofenadine, a substrate of P-glycoprotein. Br. J. Clin. Pharmacol. 2006, 62, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Jalava, K.M.; Partanen, J.; Neuvonen, P.J. Itraconazole decreases renal clearance of digoxin. Ther. Drug Monit. 1997, 19, 609–613. [Google Scholar] [CrossRef] [PubMed]
- Juurlink, D.N.; Mamdani, M.M.; Kopp, A.; Herrmann, N.; Laupacis, A. A population-based assessment of the potential interaction between serotonin-specific reuptake inhibitors and digoxin. Br. J. Clin. Pharmacol. 2005, 59, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.J.; Lew, K.; Casciano, C.N.; Clement, R.P.; Johnson, W.W. Interaction of common azole antifungals with P-glycoprotein. Antimicrob. Agents Chemother. 2002, 46, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Schwab, D.; Fischer, H.; Tabatabaei, A.; Poli, S.; Huwyler, J. Comparison of in vitro P-glycoprotein screening assays: Recommendations for their use in drug discovery. J. Med. Chem. 2003, 46, 1716–1725. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, A.B.; Ling, V. Positively cooperative sites for drug transport by P-glycoprotein with distinct drug specificities. Eur. J. Biochem. 1997, 250, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, A.B.; Fox, K.; Lam, P.; Ling, V. Stimulation of P-glycoprotein-mediated drug transport by prazosin and progesterone. Evidence for a third drug-binding site. Eur. J. Biochem. 1999, 259, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Zolnerciks, J.K.; Booth-Genthe, C.L.; Gupta, A.; Harris, J.; Unadkat, J.D. Substrate- and species-dependent inhibition of P-glycoprotein-mediated transport: Implications for predicting in vivo drug interactions. J. Pharm. Sci. 2011, 100, 3055–3061. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Berridge, G.; Higgins, C.F.; Mistry, P.; Charlton, P.; Callaghan, R. Communication between multiple drug binding sites on P-glycoprotein. Mol. Pharmacol. 2000, 58, 624–632. [Google Scholar] [PubMed]
- Sugimoto, H.; Matsumoto, S.; Tachibana, M.; Niwa, S.; Hirabayashi, H.; Amano, N.; Moriwaki, T. Establishment of in vitro P-glycoprotein inhibition assay and its exclusion criteria to assess the risk of drug-drug interaction at the drug discovery stage. J. Pharm. Sci. 2011, 100, 4013–4023. [Google Scholar] [CrossRef] [PubMed]
- Taub, M.E.; Mease, K.; Sane, R.S.; Watson, C.A.; Chen, L.; Ellens, H.; Hirakawa, B.; Reyner, E.L.; Jani, M.; Lee, C.A. Digoxin is not a substrate for organic anion-transporting polypeptide transporters oatp1a2, oatp1b1, oatp1b3, and oatp2b1 but is a substrate for a sodium-dependent transporter expressed in HEK293 cells. Drug Metab. Dispos. 2011, 39, 2093–2102. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Y.; Hidalgo, I.J. Kinetic analysis of human and canine P-glycoprotein-mediated drug transport in MDR1-MDCK cell model: Approaches to reduce false-negative substrate classification. J. Pharm. Sci. 2013, 102, 3436–3446. [Google Scholar] [CrossRef] [PubMed]
| Drug | IC50 (µM) (Rhodamine 123) 1 | IC50 (µM) (Digoxin) 2 | Ratio IC50 Rhodamine 123/IC50 Digoxin | IC50 Range for P-gp Inhibition (-fold) 3 |
|---|---|---|---|---|
| Amiodarone | 22.9 ± 2.0 | 9.6 | 2.4 | 27 |
| Carvedilol | 3.4 ± 1.2 | 1.3 | 2.7 | 168 |
| Cyclosporin A | 4.8 ± 1.1 | 1.1 | 4.4 | NR 4 |
| Diltiazem | 11.7 ± 2.2 | 4.6 | 2.6 | 76 |
| Elacridar | 0.05 ± 0.01 | 0.03 | 1.9 | NR |
| Felodipine | 127.3 ± 6.0 | 4.2 | 30.3 | 188 |
| Isradipine | 83.1 ± 10.8 | 7.7 | 10.8 | 24 |
| Itraconazole | No inhibition 5 | 1.8 | NA 6 | NR |
| Mibefradil | 4.7 ± 1.8 | 1.9 | 2.5 | 48 |
| Nicardipine | 19.4 ± 1.5 | 3.8 | 5.1 | 78 |
| Nitrendipine | 250.5 ± 43.8 | 18.2 | 13.8 | 72 |
| Quinidine | 51.3 ± 1.5 | 4.9 | 10.5 | 98 |
| Sertraline | No inhibition 5 | 12.9 | NA | 18 |
| Troglitazone | 44.1 ± 7.7 | 15.9 | 2.8 | 36 |
| Verapamil | 14.3 ± 1.4 | 2.8 | 5.1 | 796 |
| Zosuquidar | 0.18 ± 0.12 | 0.02 | 9.7 | NR |
| Perpetrator | [I1]; [I2] (µM) 1,2 | AUCi/AUC 2,3 | Cmaxi/Cmax 2,4 | Clinical Relevance 5 | P-gp Inhibition Prediction (FDA Criteria 6) | |
|---|---|---|---|---|---|---|
| Rhodamine 123 IC50 | Digoxin IC50 2 | |||||
| Carvelidol | 0.13; 62 | 1.24 | 1.00 | − | + (FP) 7 | + (FP) |
| Diltiazem | 0.7; 868 | 1.24 | 1.24 | − | + (FP) | + (FP) |
| Mibefradil | 0.8; 404 | 1.08 | 1.22 | − | + (FP) | + (FP) |
| Nicardipine | 0.18; 250 | 1.06 | 1.06 | − | + (FP) | + (FP) |
| Nitrendipine | 0.015; 111 | 1.09 | 1.22 | − | − (TN) 8 | − (TN) |
| Troglitazone | 3.62; 3,624 | 1.04 | 1.05 | − | + (FP) | + (FP) |
| Amiodarone | 2.49; 2479 | 1.63 | 1.72 | + | + (TP) 9 | + (TP) |
| Amiodarone | 2.20; 4959 | 1.68 | 1.84 | + | + (TP) | + (TP) |
| Carvelidol | 0.52; 246 | 1.2 | 1.60 | + | + (TP) | + (TP) |
| Carvelidol | 0.13; 62 | 1.56 | 1.38 | + | +(TP) | +(TP) |
| Cyclosporin A | 2.8; 1167 | NR 10 | 1.44 | + | + (TP) | + (TP) |
| Diltiazem | 0.7; 579 | 1.44 | 1.38 | + | + (TP) | + (TP) |
| Diltiazem | 0.17; 579 | 1.51 | 1.37 | + | +(TP) | +(TP) |
| Felodipine | 0.03; 104 | 1.18 | 1.34 | + | − (FN)11 | + (TP) |
| Isradipine | 0.02; 54 | 1.11 | 1.26 | + | − (FN) | − (FN) |
| Itraconazole | 0.95; 1134 | 1.68 | 1.34 | + | − (FN) | + (TP) |
| Mibefradil | 2.42; 1211 | 1.31 | 1.41 | + | + (TP) | + (TP) |
| Mibefradil | 1.61; 807 | 1.07 | 1.25 | + | + (TP) | + (TP) |
| Nitrendipine | 0.03; 222 | 1.15 | 1.57 | + | − (FN) | + (TP) |
| Quinidine | 3.54; 3397 | 1.76 | 1.75 | + | + (TP) | + (TP) |
| Quinidine | 5.10; 2466 | 2.65 | NR | + | + (TP) | + (TP) |
| Quinidine | 4.5; 2466 | NR | 1.44 | + | + (TP) | + (TP) |
| Verapamil | 1.2; 704 | 1.51 | 1.44 | + | + (TP) | + (TP) |
| Verapamil | 0.026; 704 | NR | 1.53 | + | + (TP) | + (TP) |
| Verapamil | 0.058; 1056 | NR | 1.61 | + | + (TP) | + (TP) |
| Verapamil | 0.033; 704 | NR | 1.77 | + | + (TP) | + (TP) |
| Performance Metrics | Prediction from Rhodamine 123 Accumulation Assay | Prediction from Digoxin Transport Assay |
|---|---|---|
| Accuracy (%) 2 | 65.4 | 76.9 |
| Sensitivity (%) 2 | 80.0 | 95.0 |
| Specificity (%) 2 | 16.7 | 16.7 |
| Performance Metrics | Prediction with “P-gp IC50 Working Group” Criteria | Prediction with Poirier et al. Criteria |
|---|---|---|
| Accuracy (%) 1 | 73.1 | 65.4 |
| Sensitivity (%) 1 | 80.0 | 80.0 |
| Specificity (%) 1 | 50.0 | 16.7 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jouan, E.; Le Vée, M.; Mayati, A.; Denizot, C.; Parmentier, Y.; Fardel, O. Evaluation of P-Glycoprotein Inhibitory Potential Using a Rhodamine 123 Accumulation Assay. Pharmaceutics 2016, 8, 12. https://doi.org/10.3390/pharmaceutics8020012
Jouan E, Le Vée M, Mayati A, Denizot C, Parmentier Y, Fardel O. Evaluation of P-Glycoprotein Inhibitory Potential Using a Rhodamine 123 Accumulation Assay. Pharmaceutics. 2016; 8(2):12. https://doi.org/10.3390/pharmaceutics8020012
Chicago/Turabian StyleJouan, Elodie, Marc Le Vée, Abdullah Mayati, Claire Denizot, Yannick Parmentier, and Olivier Fardel. 2016. "Evaluation of P-Glycoprotein Inhibitory Potential Using a Rhodamine 123 Accumulation Assay" Pharmaceutics 8, no. 2: 12. https://doi.org/10.3390/pharmaceutics8020012