Bioadhesive Mini-Tablets for Vaginal Drug Delivery
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
| Polymer | Substituent Group | Molecular Weight (Dalton) * |
|---|---|---|
 | ||
| Microcrystalline cellulose (MCC) | R = H | <37,500 ** |
| Methyl cellulose (MC) | R = H or CH3 | 70,000 |
| Hydroxyethyl cellulose (HEC) | R = H or CH2CH2OH | 90,000 |
| Hydroxypropyl cellulose (HPC) | R = H or CH2CH(OH)CH3 | 1,150,000 |
| Hydroxypropylmethyl cellulose (HPMC) | R = H or CH3 or CH2CH(OH)CH3 | 1,200,000 |
2.2. Test Media
2.3. Particle Density
2.4. Preparation of Tablets
2.5. Mechanical Strength of the Mini-Tablets
2.6. Ex Vivo Assessment of Bioadhesion
2.6.1. Preparation of Tissue
2.6.2. Rotating Cylinder Method
2.6.3. Detachment Test
2.7. Dissolution Rate of Hexyl Aminolevulinat Hydrochloridum (HAL) from the Mini-Tablets
2.8. Statistics
3. Results and Discussion
3.1. Preparation of Mini-Tablets
| Polymer | Particle Density * [g/cm3] | Tensile Strength ** [N/mm2] |
|---|---|---|
| Microcrystalline cellulose (MCC) | 1.518 ± 0.008 | 3.09 ± 0.55 |
| Methyl cellulose (MC) | 1.319 ± 0.002 | 0.46 ± 0.13 |
| Hydroxyethyl cellulose (HEC) | 1.334 ± 0.001 | 0.13 ± 0.02 |
| Hydroxypropyl cellulose (HPC) | 1.203 ± 0.001 | 1.30 ± 0.37 |
| Hydroxypropylmethyl cellulose (HPMC) | 1.341 ± 0.001 | 1.42 ± 0.45 |
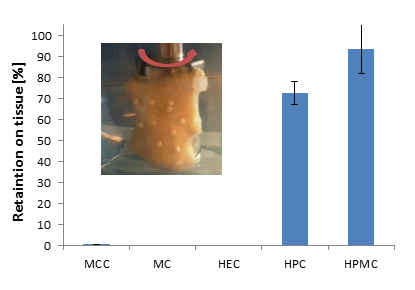
3.2. Bioadhesive Characteristics
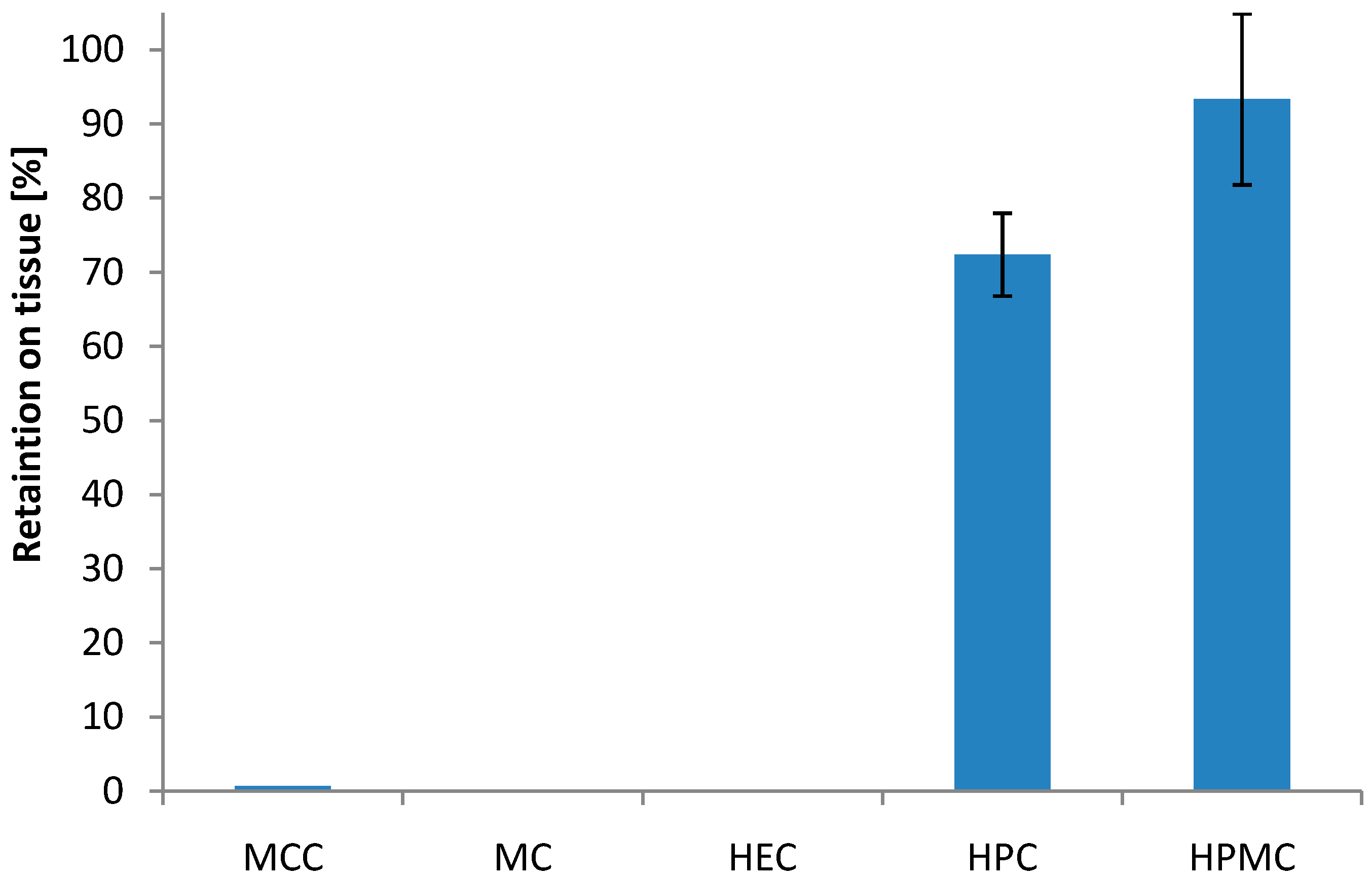
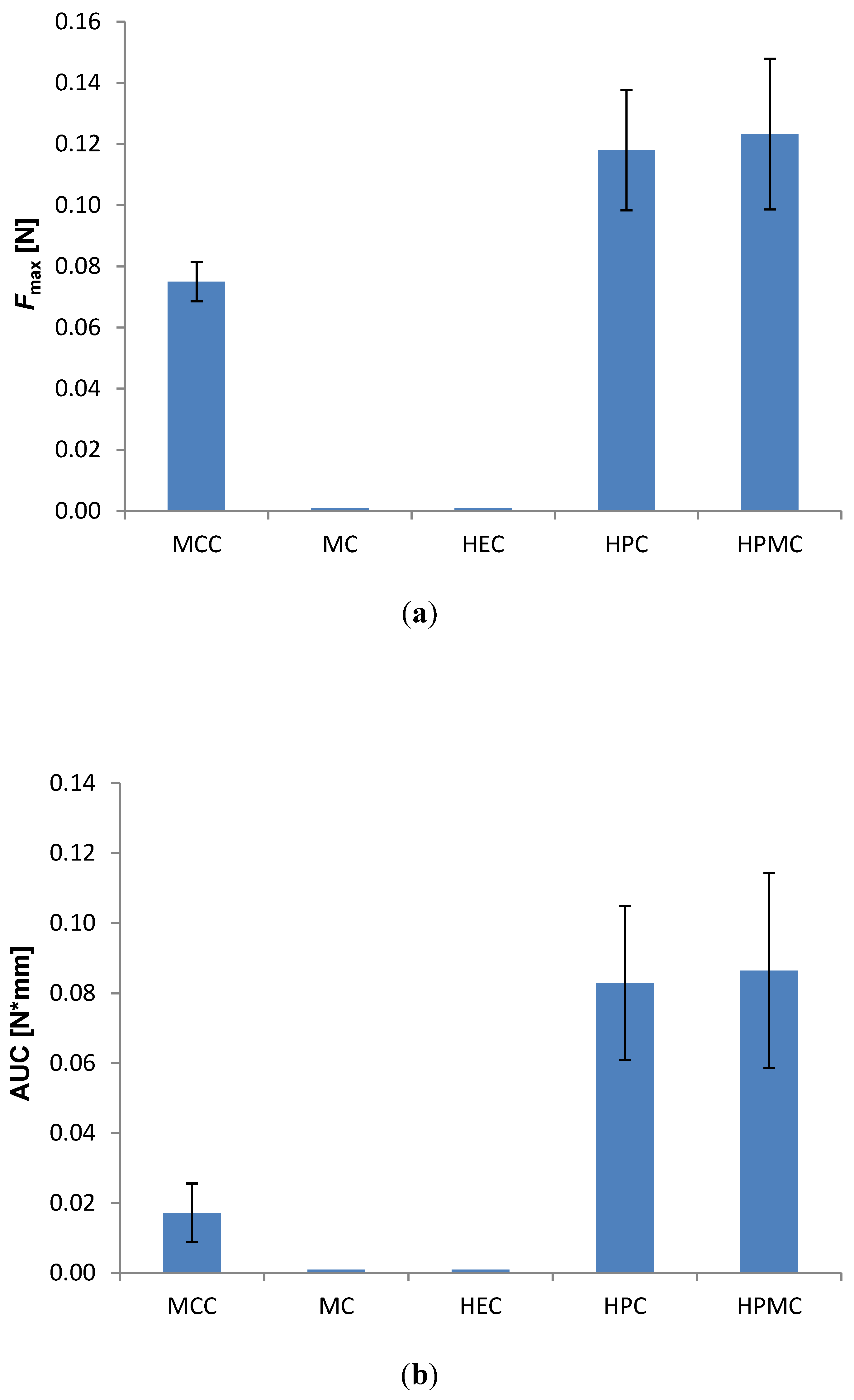
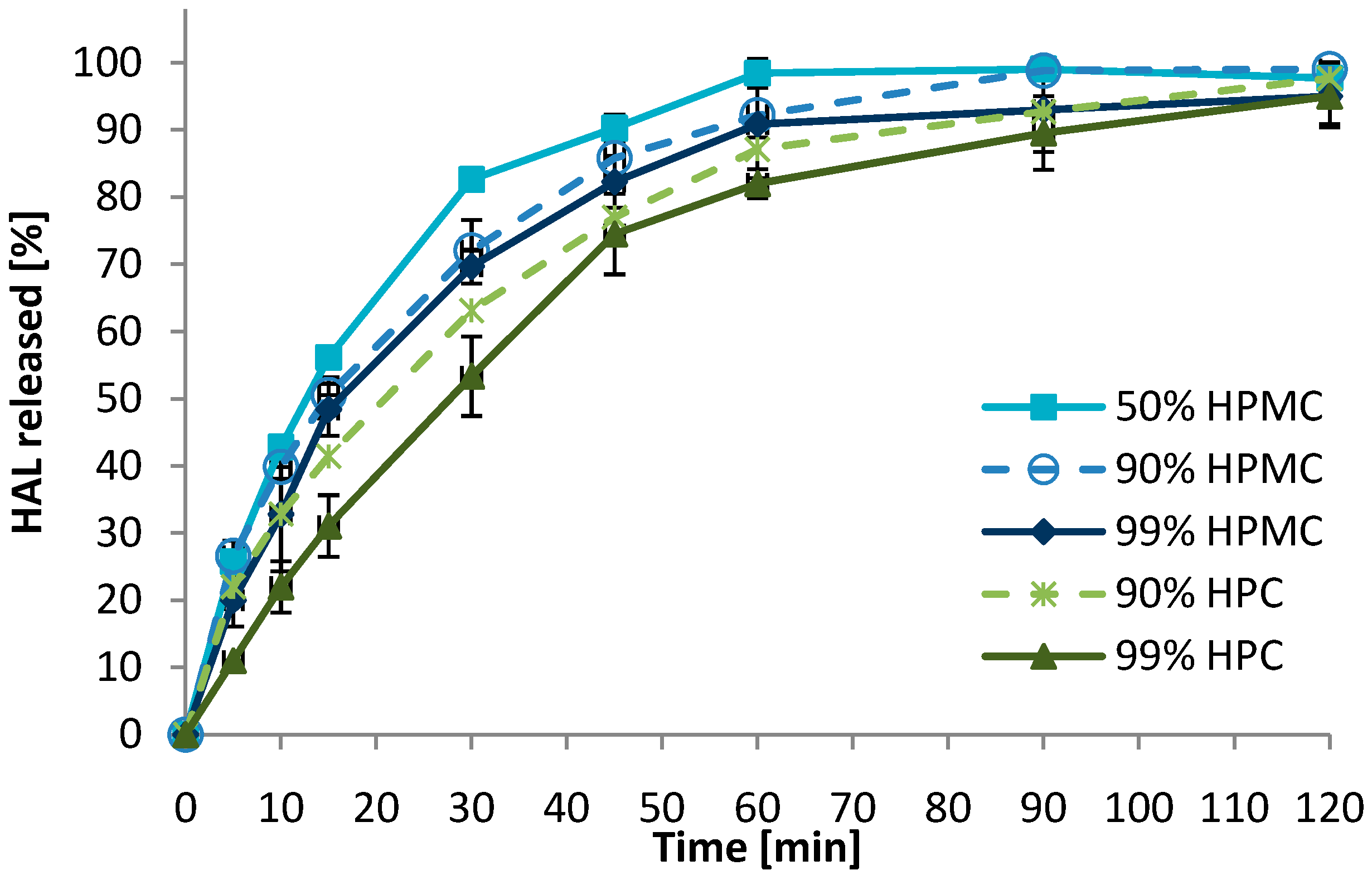
3.3. Dissolution Behavior

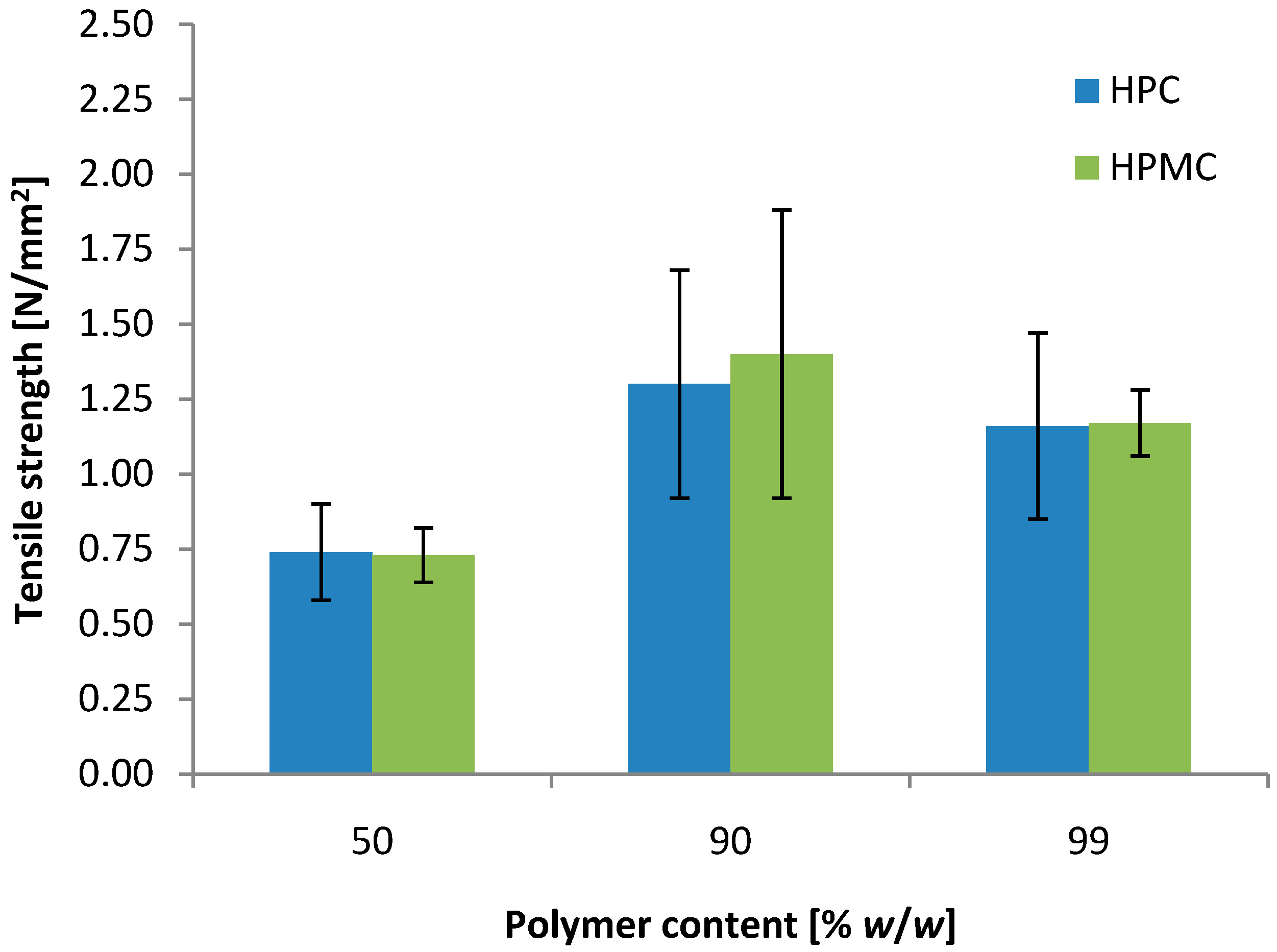
3.4. Further Optimization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pereira, R.R.; Bruschi, M.L. Vaginal mucoadhesive drug delivery systems. Drug Dev. Ind. Pharm. 2012, 38, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Ahsan, F. The vagina as a route for systemic drug delivery. J. Control. Release 2005, 103, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Owen, D.H.; Katz, D.F. A vaginal fluid simulant. Contraception 1999, 59, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Valenta, C. The use of mucoadhesive polymers in vaginal delivery. Adv. Drug Deliv. Rev. 2005, 57, 1692–1712. [Google Scholar] [CrossRef] [PubMed]
- Das Neves, J.; Amiji, M.; Sarmento, B. Mucoadhesive nanosystems for vaginal microbicide development: Friend or foe? Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2011, 3, 389–399. [Google Scholar]
- Smart, J.D. The basics and underlying mechanisms of mucoadhesion. Adv. Drug Deliv. Rev. 2005, 57, 1556–1568. [Google Scholar] [CrossRef] [PubMed]
- Vanić, Ž.; Škalko-Basnet, N. Nanopharmaceuticals for improved topical vaginal therapy: Can they deliver? Eur. J. Pharm. Sci. 2013, 50, 29–41. [Google Scholar]
- Kang, J.-W.; Davaa, E.; Kim, Y.-T.; Park, J.-S. A new vaginal delivery system of amphotericin B: A dispersion of cationic liposomes in a thermosensitive gel. J. Drug Target. 2010, 18, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Berginc, K.; Škalko-Basnet, N.; Basnet, P.; Kristl, A. Development and evaluation of an in vitro vaginal model for assessment of drug’s biopharmaceutical properties: Curcumin. AAPS PharmSciTech 2012, 13, 1045–1053. [Google Scholar]
- Voorspoels, J.; Casteels, M.; Remon, J.P.; Temmerman, M. Local treatment of bacterial vaginosis with a bioadhesive metronidazole tablet. Eur. J. Obstet. Gynecol. Reprod. Biol. 2002, 105, 64–66. [Google Scholar] [CrossRef] [PubMed]
- Baloglu, E.; Ozyazici, M.; Yaprak Hizarcioglu, S.; Senyigit, T.; Ozyurt, D.; Pekcetin, C. Bioadhesive controlled release systems of ornidazole for vaginal delivery. Pharm. Dev. Technol. 2006, 11, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tang, X. A novel ketoconazole bioadhesive effervescent tablet for vaginal delivery: Design, in vitro and in vivo evaluation. Int. J. Pharm. 2008, 350, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Dukić, A.; Mens, R.; Adriaensens, P.; Foreman, P.; Gelan, J.; Remon, J.P.; Vervaet, C. Development of starch-based pellets via extrusion/spheronisation. Eur. J. Pharm. Biopharm. 2007, 66, 83–94. [Google Scholar]
- Dukić-Ott, A.; Remon, J.P.; Foreman, P.; Vervaet, C. Immediate release of poorly soluble drugs from starch-based pellets prepared via extrusion/spheronisation. Eur. J. Pharm. Biopharm. 2007, 67, 715–724. [Google Scholar]
- Dos Santos Santiago, G.L.; Verstraelen, H.; Poelvoorde, N.; de Corte, S.; Claeys, G.; Trog, M.; de Backer, E.; Saeren, B.; Vervaet, C.; de Boeck, F.; et al. A pilot study evaluating the safety of vaginal administration of a multi-particulate pellet formulation. Eur. J. Pharm. Biopharm. 2009, 73, 399–403. [Google Scholar]
- Mehta, S.; Verstraelen, H.; Peremans, K.; Vermeire, S.; de Vos, F.; Mehuys, E.; Remon, J.P.; Vervaet, C. Vaginal distribution and retention of a multiparticulate drug delivery system, assessed by γ scintigraphy and magnetic resonance imaging. Int. J. Pharm. 2012, 426, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.; Verstraelen, H.; Vandaele, L.; Mehuys, E.; Remon, J.P.; Vervaet, C. Vaginal distribution and retention of tablets comprising starch-based multiparticulates: Evaluation by colposcopy. Drug Dev. Ind. Pharm. 2013, 39, 1944–1955. [Google Scholar] [CrossRef] [PubMed]
- Poelvoorde, N.; Verstraelen, H.; Verhelst, R.; Saerens, B.; de Backer, E.; dos Santos Santiago, G.L.; Vervaet, C.; Vaneechoutte, M.; de Boeck, F.; van Bortel, L.; et al. In vivo evaluation of the vaginal distribution and retention of a multi-particulate pellet formulation. Eur. J. Pharm. Biopharm. 2009, 73, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.T.; Clark, M.R.; Shelke, N.B.; Johnson, T.J.; Smith, E.M.; Andreasen, A.K.; Nebeker, J.S.; Fabian, J.; Friend, D.R.; Kiser, P.F. Engineering a segmented dual-reservoir polyurethane intravaginal ring for simultaneous prevention of HIV transmission and unwanted pregnancy. PLoS One 2014, 9, e88509. [Google Scholar] [CrossRef] [PubMed]
- Soergel, P.; Loehr-Schulz, R.; Hillemanns, M.; Landwehr, S.; Makowski, L.; Hillemanns, P. Effects of photodynamic therapy using topical applied hexylaminolevulinate and methylaminolevulinate upon the integrity of cervical epithelium. Lasers Surg. Med. 2010, 42, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Lennartz, P.; Mielck, J.B. Minitabletting: Improving the compactability of paracetamol powder mixtures. Int. J. Pharm. 1998, 173, 75–85. [Google Scholar] [CrossRef]
- Tissen, C.; Woertz, K.; Breitkreutz, J.; Kleinebudde, P. Development of mini-tablets with 1 mm and 2 mm diameter. Int. J. Pharm. 2011, 416, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Soergel, P.; Wang, X.L.; Stepp, H.; Hertel, H.; Hillemanns, P. Photodynamic therapy of cervical intraepithelial neoplasia with hexaminolevulinate. Lasers Surg. Med. 2008, 40, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, D.E.J.G.J.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Hillemanns, P.; Wang, X.; Hertel, H.; Andikyan, V.; Hillemanns, M.; Stepp, H.; Soergel, P. Pharmacokinetics and selectivity of porphyrin synthesis after topical application of hexaminolevulinate in patients with cervical intraepithelial neoplasia. Am. J. Obstet. Gynecol. 2008, 198, 300.e1–300.e7. [Google Scholar]
- Collaud, S.; Peng, Q.; Gurny, R.; Lange, N. Thermosetting gel for the delivery of 5-aminolevulinic acid esters to the cervix. J. Pharm. Sci. 2008, 97, 2680–2690. [Google Scholar] [CrossRef] [PubMed]
- Hiorth, M.; Liereng, L.; Reinertsen, R.; Tho, I. Formulation of bioadhesive hexylaminolevulinate pellets intended for photodynamic therapy in the treatment of cervical cancer. Int. J. Pharm. 2013, 441, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Fell, J.T.; Newton, J.M. Determination of tablet strength by the diametral compression test. J. Pharm. Sci. 1970, 59, 688–691. [Google Scholar] [CrossRef] [PubMed]
- Das Neves, J.; Amaral, M.H.; Bahia, M.F. Performance of an in vitro mucoadhesion testing method for vaginal semisolids: Influence of different testing conditions and instrumental parameters. Eur. J. Pharm. Biopharm. 2008, 69, 622–632. [Google Scholar]
- Bernkop-Schnürch, A.; Steininger, S. Synthesis and characterization of mucoadhesive thiolated polymers. Int. J. Pharm. 2000, 194, 239–247. [Google Scholar]
- Hägerström, H.; Berström, C.A.S.; Edsman, C. The importance of gel properties for mucoadhesion measurements: A multivariate data analysis approach. J. Pharm. Pharmacol. 2004, 56, 161–168. [Google Scholar]
- Handbook of Pharmaceutical Excipients. 2014. Available online: http://www.medicinescomplete.com/mc/excipients/current/ (accessed on 29 June 2014).
- Bottenberg, P.; Cleymaet, R.; de Muynck, C.; Remon, J.P.; Coomans, D.; Michotte, Y.; Slop, D. Development and testing of bioadhesive, fluoride-containing slow-release tablets for oral use. J. Pharm. Pharmacol. 1991, 43, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Woertz, C.; Preis, M.; Breitkreutz, J.; Kleinebudde, P. Assessment of test methods evaluating mucoadhesive polymers and dosage forms: An overview. Eur. J. Pharm. Biopharm. 2013, 85, 843–853. [Google Scholar] [CrossRef] [PubMed]
- European Pharmacopoeia 8.2. 2014. Available online: http://online.pheur.org/EN/entry.htm (accessed on 29 June 2014).
- Das Neves, J.; Bahia, M.F. Gels as vaginal drug delivery systems. Int. J. Pharm. 2006, 318, 1–14. [Google Scholar]
- Stafford, M.K.; Ward, H.; Flanagan, A.; Rosenstein, I.J.; Taylor-Robinson, D.; Smith, J.R.; Weber, J.; Kitchen, V.S. Safety study of nonoxynol-9 as a vaginal microbicide: Evidence of adverse effects. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 1998, 17, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Tien, D.; Schnaare, R.L.; Kang, F.; Cohl, G.; McCormick, T.J.; Moench, T.R.; Doncel, G.; Watson, K.; Buckheit, R.W.; Lewis, M.G.; et al. In vitro and in vivo characterization of a potential universal placebo designed for use in vaginal microbicide clinical trials. Aids Res. Hum. Retrovir. 2005, 21, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.L.; Mauck, C.; Lai, J.-J.; Creinin, M.D.; Brache, V.; Ballagh, S.A.; Weiner, D.H.; Hillier, S.L.; Fichorova, R.N.; Callahan, M. Fourteen-day safety and acceptability study of 6% cellulose sulfate gel: A randomized double-blind Phase I safety study. Contraception 2006, 74, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Adriaens, E.; Remon, J.P. Mucosal irritation potential of personal lubricants relates to product osmolality as detected by the slug mucosal irritation assay. Sex. Transm. Dis. 2008, 35, 512–516. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hiorth, M.; Nilsen, S.; Tho, I. Bioadhesive Mini-Tablets for Vaginal Drug Delivery. Pharmaceutics 2014, 6, 494-511. https://doi.org/10.3390/pharmaceutics6030494
Hiorth M, Nilsen S, Tho I. Bioadhesive Mini-Tablets for Vaginal Drug Delivery. Pharmaceutics. 2014; 6(3):494-511. https://doi.org/10.3390/pharmaceutics6030494
Chicago/Turabian StyleHiorth, Marianne, Susanne Nilsen, and Ingunn Tho. 2014. "Bioadhesive Mini-Tablets for Vaginal Drug Delivery" Pharmaceutics 6, no. 3: 494-511. https://doi.org/10.3390/pharmaceutics6030494