Release of Tenofovir from Carrageenan-Based Vaginal Suppositories
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
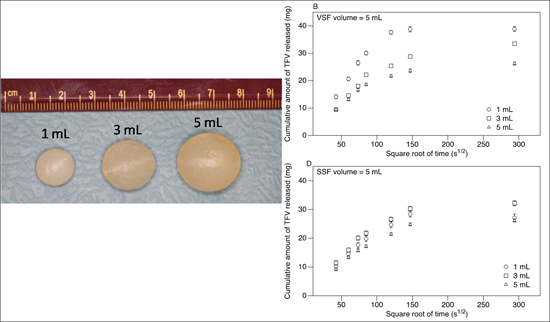
2.2. Suppository Preparation
2.3. Rheological Characterization of Gel
2.4. Characterization of Drug Release

2.5. Statistical Analysis

3. Results
3.1. Release of Tenofovir (TFV) in Water
3.2. Release of TFV into Vaginal Simulant Fluid (VSF) and Semen Simulant Fluid (SSF)

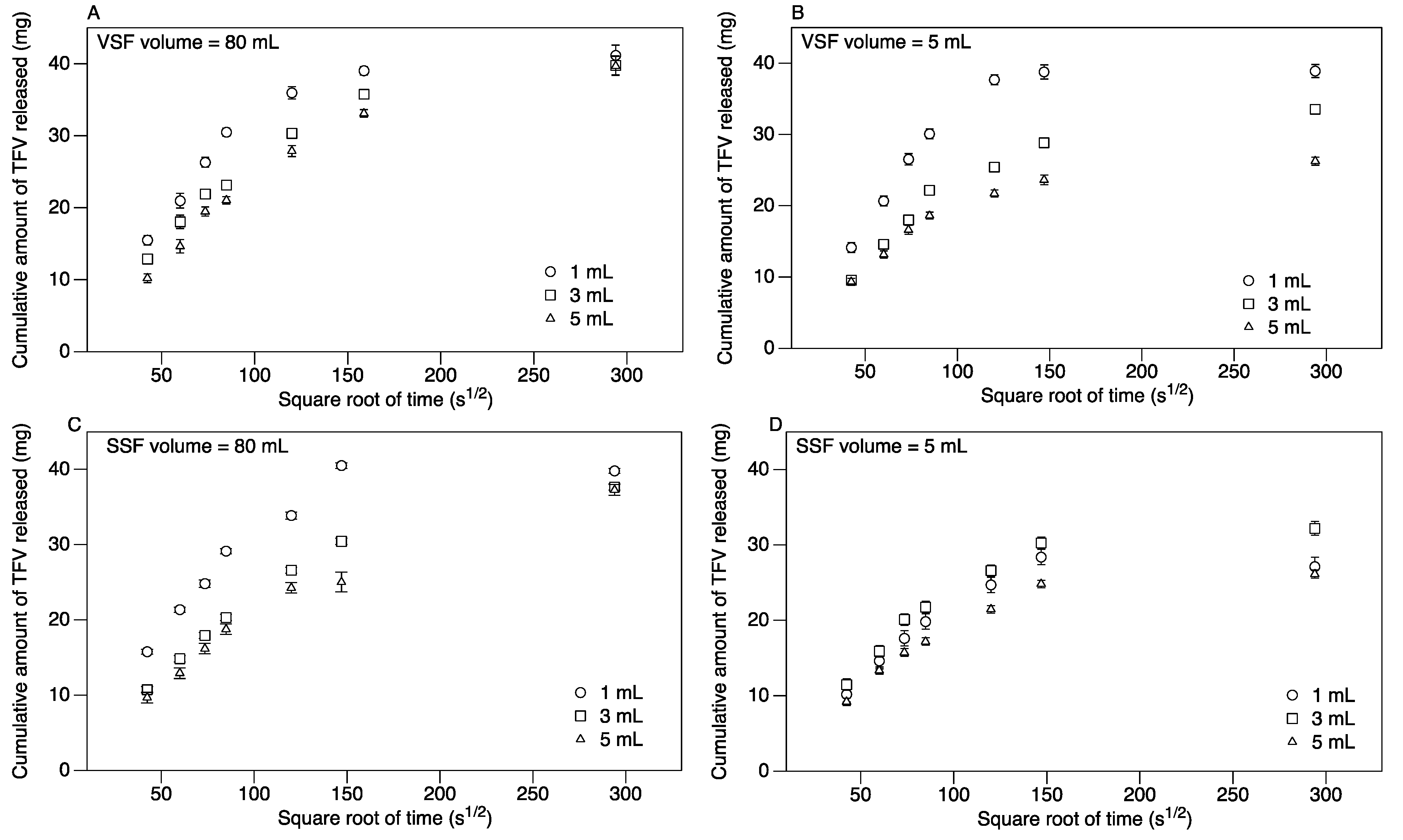
| Suppository Size | LSM release rate in mg/(s½) | |||
|---|---|---|---|---|
| 80 mL VSF | 5 mL VSF | 80 mL SSF | 5 mL SSF | |
| 1 mL | 0.345 a,A,1 ± 0.002 | 0.356 x,A,6 ± 0.005 | 0.341 d,F,1 ± 0.002 | 0.238 g,G,7 ± 0.003 |
| 3 mL | 0.282 b,B,2 ± 0.002 | 0.257 y,C,8 ± 0.005 | 0.241 e,H,3 ± 0.002 | 0.256 h,I,8 ± 0.003 |
| 5 mL | 0.251 c,D,4 ± 0.002 | 0.217 z,E,9 ±0.005 | 0.219 f,J,5 ± 0.002 | 0.210 k,J,9 ± 0.003 |
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- 2012 UNAIDS World AIDS Day Report-Result. Available online: http://www.unaids.org/en/resources/publications/2012/name,76120,en.asp (accessed on 23 January 2014).
- Davis, K.R.; Weller, S.C. The effectiveness of condoms in reducing heterosexual transmission of HIV. Fam. Plan. Perspect. 1999, 31, 272–279. [Google Scholar] [CrossRef]
- Alfonsi, G.A.; Shlay, J.C. The effectiveness of condoms for the prevention of sexually transmitted diseases. Curr. Womens Health Rev. 2005, 1, 151–159. [Google Scholar] [CrossRef]
- Steiner, M.J.; Cates, W.; Warner, L. The real problem with male condoms is nonuse. Sex. Transm. Dis. 1999, 26, 459–462. [Google Scholar] [CrossRef]
- Turmen, T. Gender and HIV/AIDS. Int. J. Gynecol. Obstet. 2003, 82, 411–418. [Google Scholar] [CrossRef]
- Stone, A. Microbicides: A new approach to preventing HIV and other sexually transmitted infections. Nat. Rev. Drug Discov. 2002, 1, 977–985. [Google Scholar] [CrossRef]
- Prejean, J.; Song, R.; Hernandez, A.; Ziebell, R.; Green, T.; Walker, F.; Lin, L.S.; An, Q.; Mermin, J.; Lansky, A.; et al. Estimated HIV incidence in the united states, 2006–2009. PLoS One 2011, 6, e17502. [Google Scholar]
- AVAC, Ongoing Clinical Trials of Topical Microbicide Candidates. Available online: https://www.k4health.org/toolkits/microbicides/ongoing-clinical-trials-topical-microbicide-candidates (accessed on 22 January 2014).
- Abdool Karim, Q.; Abdool Karim, S.S.; Frohlich, J.A.; Grobler, A.C.; Baxter, C.; Mansoor, L.E.; Kharsany, A.B.; Sibeko, S.; Mlisana, K.P.; Omar, Z.; et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 2010, 329, 1168–1174. [Google Scholar] [CrossRef]
- Bentley, M.E.; Fullem, A.M.; Tolley, E.E.; Kelly, C.W.; Jogelkar, N.; Srirak, N.; Mwafulirwa, L.; Khumalo-Sakutukwa, G.; Celentano, D.D. Acceptability of a microbicide among women and their partners in a 4-country phase I trial. Am. J. Public Health 2004, 94, 1159–1164. [Google Scholar] [CrossRef]
- Morrow, K.; Rosen, R.; Richter, L.; Emans, A.; Forbes, A.; Day, J.; Morar, N.; Maslankowski, L.; Profy, A.T.; Kelly, C.; et al. The acceptability of an investigational vaginal microbicide, pro 2000 gel, among women in a phase I clinical trial. J. Womens Health 2003, 12, 655–666. [Google Scholar] [CrossRef]
- Van den Berg, J.J.; Rosen, R.K.; Bregman, D.E.; Thompson, L.A.; Jensen, K.M.; Kiser, P.F.; Katz, D.F.; Buckheit, K.; Buckheit, R.W., Jr.; Morrow, K.M. “Set it and forget it”: Women’s perceptions and opinions of long-acting topical vaginal gels. AIDS Behav. 2014, 18, 862–870. [Google Scholar] [CrossRef]
- Zaveri, T.; Powell, K.A.; Li, B.; Ziegler, G.R.; Hayes, J.E. Improving Acceptability of Vaginal Drug Delivery Systems by Using Sensory Methods. In Proceedings of the Society of Sensory Professionals 3rd Technical and Professional Conference, Jersey City, NJ, USA, 10–12 October 2012.
- Morrow, K.; Underhill, K.; Berg, J.; Vargas, S.; Rosen, R.; Katz, D. User-identified gel characteristics: A qualitative exploration of perceived product efficacy of topical vaginal microbicides. Arch. Sex. Behav. 2014, 1–9. [Google Scholar]
- Nel, A.M.; Mitchnick, L.B.; Risha, P.; Muungo, L.T.; Norick, P.M. Acceptability of vaginal film, soft-gel capsule, and tablet as potential microbicide delivery methods among african women. J. Womens Health 2011, 20, 1207–1214. [Google Scholar] [CrossRef]
- Devlin, B.; Nuttall, J.; Wilder, S.; Woodsong, C.; Rosenberg, Z. Development of dapivirine vaginal ring for HIV prevention. Antivir. Res. 2013, 100, S3–S8. [Google Scholar] [CrossRef]
- Fetherston, S.M.; Boyd, P.; McCoy, C.F.; McBride, M.C.; Edwards, K.-L.; Ampofo, S.; Malcolm, R.K. A silicone elastomer vaginal ring for HIV prevention containing two microbicides with different mechanisms of action. Eur. J. Pharm. Sci. 2013, 48, 406–415. [Google Scholar] [CrossRef] [Green Version]
- Pereira, L.E.; Clark, M.R.; Friend, D.R.; Garber, D.A.; McNicholl, J.M.; Hendry, R.M.; Doncel, G.F.; Smith, J.M. Pharmacokinetic and safety analyses of tenofovir and tenofovir-emtricitabine vaginal tablets in pigtailed macaques. Antimicrob. Agents Chemother. 2014, 58, 2665–2674. [Google Scholar] [CrossRef]
- Cole, A.M.; Patton, D.L.; Rohan, L.C.; Cole, A.L.; Cosgrove-Sweeney, Y.; Rogers, N.A.; Ratner, D.; Sassi, A.B.; Lackman-Smith, C.; Tarwater, P.; et al. The formulated microbicide rc-101 was safe and antivirally active following intravaginal application in pigtailed macaques. PLoS One 2010, 5, e15111. [Google Scholar] [CrossRef] [Green Version]
- Akil, A.; Parniak, M.A.; Dezzuitti, C.S.; Moncla, B.J.; Cost, M.R.; Li, M.; Rohan, L.C. Development and characterization of a vaginal film containing dapivirine, a non-nucleoside reverse transcriptase inhibitor (NNRTI), for prevention of HIV-1 sexual transmission. Drug Deliv. Transl. Res. 2011, 1, 209–222. [Google Scholar] [CrossRef]
- Giguere, R.; Carballo-Dieguez, A.; Ventuneac, A.; Mabragana, M.; Dolezal, C.; Chen, B.A.; Kahn, J.A.; Zimet, G.D.; McGowan, I. Variations in microbicide gel acceptability among young women in the USA and Puerto Rico. Cult. Health Sex. 2012, 14, 151–166. [Google Scholar] [CrossRef]
- Grenha, A.; Gomes, M.E.; Rodrigues, M.; Santo, V.E.; Mano, J.F.; Neves, N.M.; Reis, R.L. Development of new chitosan/carrageenan nanoparticles for drug delivery applications. J. Biomed. Mater. Res. A 2010, 92A, 1265–1272. [Google Scholar]
- en, M.; Avcı, E.N. Radiation synthesis of poly(n-vinyl-2-pyrrolidone)-κ-carrageenan hydrogels and their use in wound dressing applications. I. Preliminary laboratory tests. J. Biomed. Mater. Res. A 2005, 74A, 187–196. [Google Scholar] [CrossRef]
- Zacharopoulos, V.R.; Phillips, D.M. Vaginal formulations of carrageenan protect mice from herpes simplex virus infection. Clin. Diagn. Lab. Immunol. 1997, 4, 465–468. [Google Scholar]
- Buck, C.B.; Thompson, C.D.; Roberts, J.N.; Müller, M.; Lowy, D.R.; Schiller, J.T. Carrageenan is a potent inhibitor of papillomavirus infection. PLoS Pathog. 2006, 2, e69. [Google Scholar] [CrossRef]
- Skoler-Karpoff, S.; Ramjee, G.; Ahmed, K.; Altini, L.; Plagianos, M.G.; Friedland, B.; Govender, S.; de Kock, A.; Cassim, N.; Palanee, T.; et al. Efficacy of carraguard for prevention of HIV infection in women in South Africa: A randomised, double-blind, placebo-controlled trial. Lancet 2008, 372, 1977–1987. [Google Scholar] [CrossRef]
- González, M.E.; Alarcón, B.; Carrasco, L. Polysaccharides as antiviral agents: Antiviral activity of carrageenan. Antimicrob. Agents Chemother. 1987, 31, 1388–1393. [Google Scholar] [CrossRef]
- Li, B.; Zaveri, T.; Ziegler, G.R.; Hayes, J.E. User preferences in a carrageenan-based vaginal drug delivery system. PLoS One 2013, 8, e54975. [Google Scholar]
- Li, B.; Zaveri, T.; Ziegler, G.R.; Hayes, J.E. Shape of vaginal suppositories affects willingness-to-try and preference. Antivir. Res. 2012, 97, 280–284. [Google Scholar]
- Rohan, L.; Sassi, A. Vaginal drug delivery systems for HIV prevention. AAPS J. 2009, 11, 78–87. [Google Scholar] [CrossRef]
- Godley, M.J. Quantitation of vaginal discharge in healthy volunteers. Br. J. Obstet. Gynaecol. 1985, 92, 739–742. [Google Scholar] [CrossRef]
- Stone, A.; Gamble, C.J. The quantity of vaginal fluid. Am. J. Obstet. Gynecol. 1959, 78, 279–281. [Google Scholar]
- Wagner, G.; Levin, R.J. Electrolytes in vaginal fluid during the menstrual cycle of coitally active and inactive women. J. Reprod. Fertil. 1980, 60, 17–27. [Google Scholar]
- Stone, B.A.; Alex, A.; Werlin, L.B.; Marrs, R.P. Age thresholds for changes in semen parameters in men. Fertil. Steril. 2013, 100, 952–958. [Google Scholar] [CrossRef]
- Lotti, F.; Corona, G.; Maseroli, E.; Rossi, M.; Silverii, A.; Degl’innocenti, S.; Rastrelli, G.; Forti, G.; Maggi, M. Clinical implications of measuring prolactin levels in males of infertile couples. Andrology 2013, 1, 764–771. [Google Scholar] [CrossRef]
- Owen, D.H.; Katz, D.F. A vaginal fluid simulant. Contraception 1999, 59, 91–95. [Google Scholar] [CrossRef]
- Owen, D.H.; Katz, D.F. A review of the physical and chemical properties of human semen and the formulation of a semen simulant. J. Androl. 2005, 26, 459–469. [Google Scholar] [CrossRef]
- Wen, H.; Park, K. Oral Controlled Release Formulation Design and Drug Delivery: Theory to Practice; Wiley: Hoboken, NJ, USA, 2011. [Google Scholar]
- Mitchell, C.; Paul, K.; Agnew, K.; Gaussman, R.; Coombs, R.W.; Hitti, J. Estimating volume of cervicovaginal secretions in cervicovaginal lavage fluid collected for measurement of genital HIV-1 RNA levels in women. J. Clin. Microbiol. 2011, 49, 735–736. [Google Scholar] [CrossRef]
- Dusitsin, N.; Gregoire, A.T.; Johnson, W.D.; Rakoff, A.E. Histidine in human vaginal fluid. Obstet. Gynecol. 1967, 29, 125–129. [Google Scholar]
- Preti, G.; Huggins, G.R.; Silverberg, G.D. Alterations in the organic-compounds of vaginal secretions caused by sexual arousal. Fertil. Steril. 1979, 32, 47–54. [Google Scholar]
- Huggins, G.R.; Preti, G. Vaginal odors and secretions. Clin. Obstet. Gynecol. 1981, 24, 355–377. [Google Scholar] [CrossRef]
- Schwartz, J.L.; Rountree, W.; Kashuba, A.D.M.; Brache, V.; Creinin, M.D.; Poindexter, A.; Kearney, B.P. A multi-compartment, single and multiple dose pharmacokinetic study of the vaginal candidate microbicide 1% tenofovir gel. PLoS One 2011, 6, e25974. [Google Scholar] [CrossRef] [Green Version]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zaveri, T.; Hayes, J.E.; Ziegler, G.R. Release of Tenofovir from Carrageenan-Based Vaginal Suppositories. Pharmaceutics 2014, 6, 366-377. https://doi.org/10.3390/pharmaceutics6030366
Zaveri T, Hayes JE, Ziegler GR. Release of Tenofovir from Carrageenan-Based Vaginal Suppositories. Pharmaceutics. 2014; 6(3):366-377. https://doi.org/10.3390/pharmaceutics6030366
Chicago/Turabian StyleZaveri, Toral, John E. Hayes, and Gregory R. Ziegler. 2014. "Release of Tenofovir from Carrageenan-Based Vaginal Suppositories" Pharmaceutics 6, no. 3: 366-377. https://doi.org/10.3390/pharmaceutics6030366