Performance of Dry Powder Inhalers with Single Dosed Capsules in Preschool Children and Adults Using Improved Upper Airway Models
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.1.1. Dry Powder Inhalers
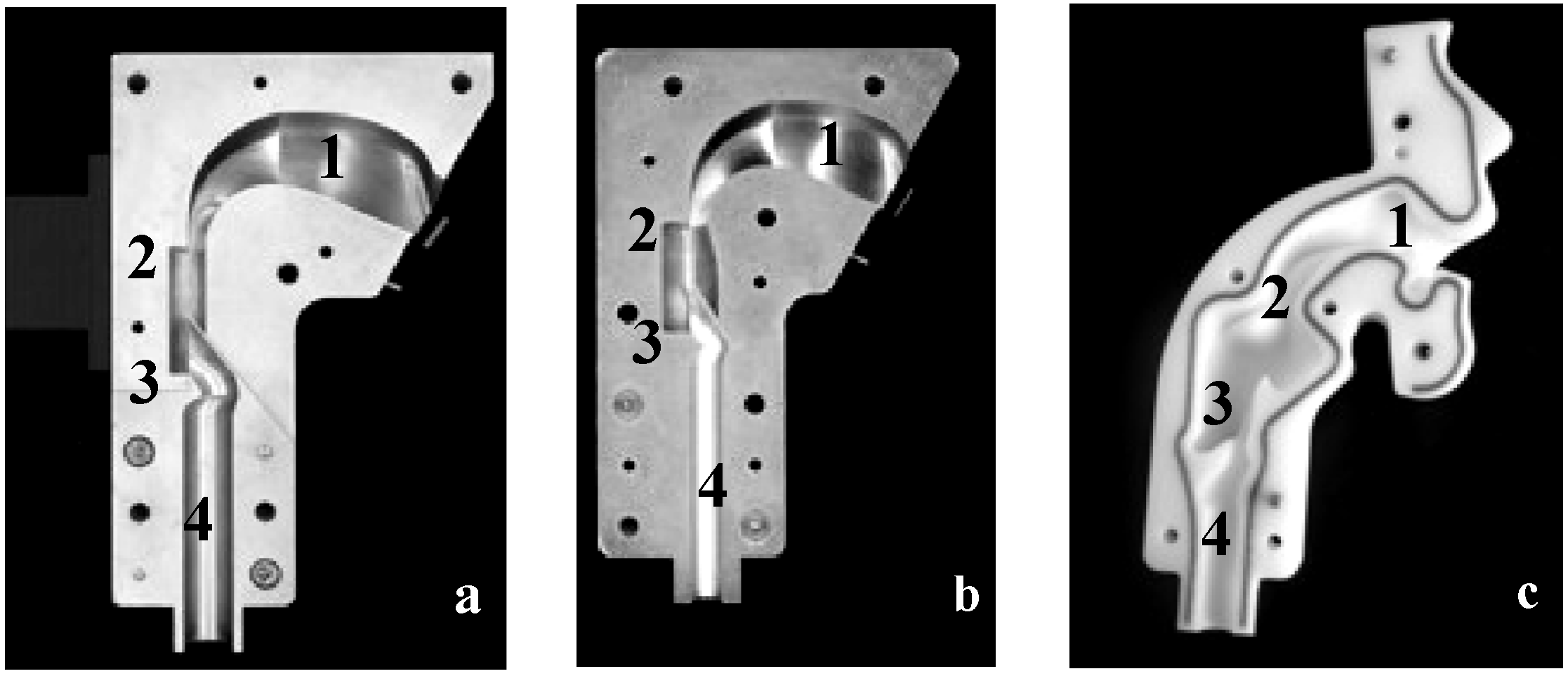
2.1.2. Upper Airway Models
2.2. Methods
2.2.1. Time-Varying Inhalation Profile

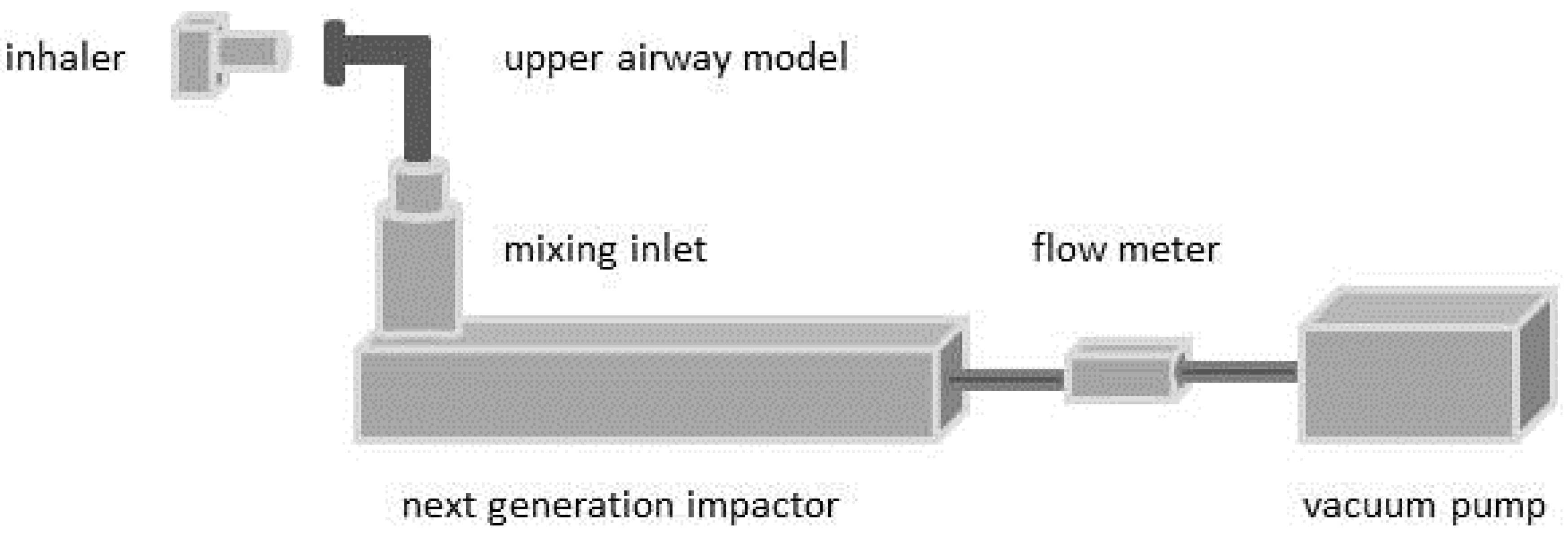
2.2.2. Deposition Measurements—Steady Flow Rates
| Flow rate [L/min] | Pressure drop [kPa] ± s Cyclohaler® | Pressure drop [kPa] ± s Handihaler® | Pressure drop [kPa] ± s Spinhaler® |
|---|---|---|---|
| 30 | 0.22 ± 0.01 | 1.59 ± 0.05 | 0.14 ± 0.01 |
| 60 | 0.94 ± 0.03 | 6.14 ± 0.02 | 1.04 ± 0.01 |
| 75 | 1.17 ± 0.02 | - | - |
2.2.3. Deposition Measurements—Time-Varying Inhalation Profile
2.2.4. High Performance Liquid Chromatography
3. Results and Discussion
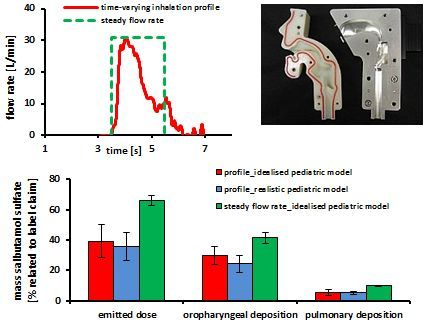
3.1. Time-Varying Inhalation Profile—Experimental Plan

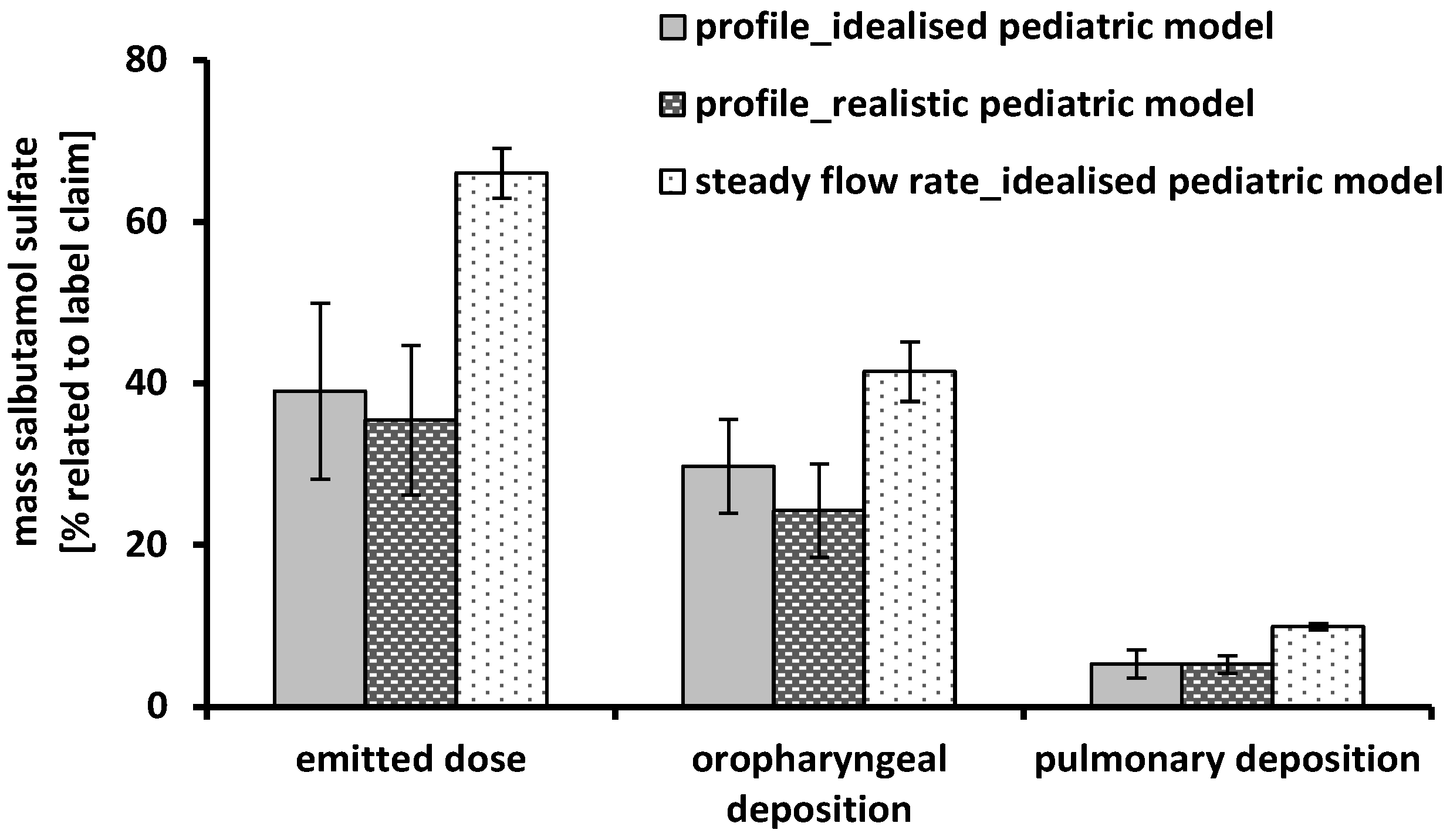
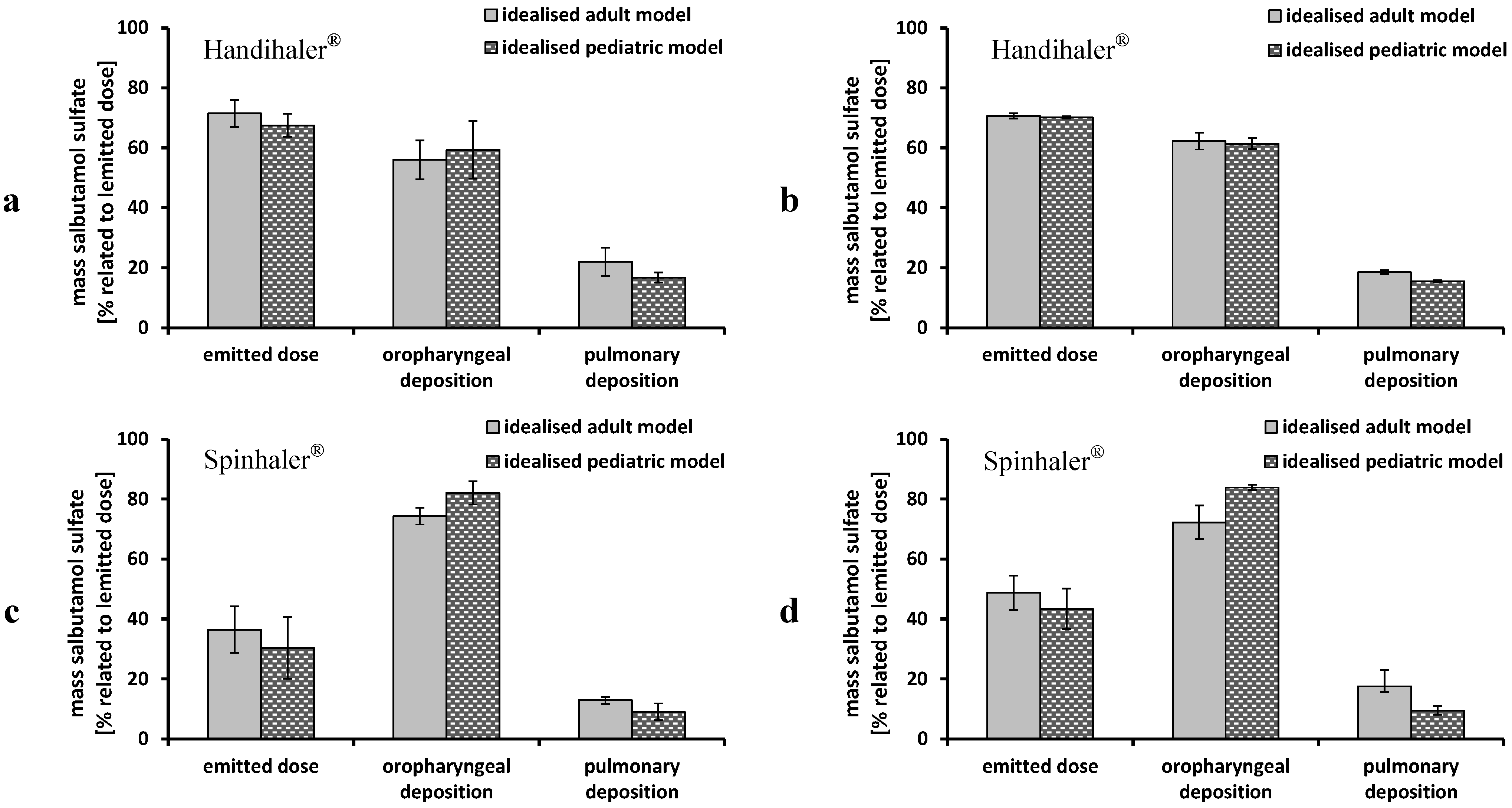
3.2. Deposition Measurements—Steady Flow Rates

3.3. Deposition Measurements—Time-Varying Inhalation Profile
3.4. Transfer to Further DPIs
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bacharier, L.B.; Boner, A.; Carlsen, K.-H.; Eigenmann, P.A.; Frischer, T.; Götz, M.; Helms, P.J.; Hunt, J.; Liu, A.; Papadopoulos, N.; et al. Diagnosis and treatment of asthma in childhood: A PRACTALL consensus report. Allergy 2008, 63, 5–34. [Google Scholar] [CrossRef]
- Bisgaard, H.; Szefler, S. Prevalence of asthma-like symptoms in young children. Pediatr. Pulmonol. 2007, 42, 723–728. [Google Scholar] [CrossRef]
- Walsh, J.; Bickmann, D.; Breitkreutz, J.; Chariot-Goulet, M.; EuPFI. Delivery devices for the administration of paediatric formulations: Overview of current practice, challenges and recent developments. Int. J. Pharm. 2011, 415, 221–231. [Google Scholar] [CrossRef]
- Bressan, S.; Balzani, M.; Krauss, B.; Pettenazzo, A.; Zanconato, S.; Baraldi, E. High-flow nasal cannula oxygen for bronchiolitis in a pediatric ward: A pilot study. Eur. J. Pediatr. 2013, 172, 1649–1656. [Google Scholar] [CrossRef]
- Reflection paper: Formulations of choice for the paediatric formulation. Presented at Committee for Medicinal Products for Human Use (CHMP). Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003782.pdf (accessed on 16 January 2014).
- Laube, B.L.; Janssens, H.M.; de Jongh, F.H.; Devadason, S.G.; Dhand, R.; Diot, P.; Everard, M.L.; Horvath, I.; Navalesi, P.; Voshaar, T.; et al. What the pulmonary specialist should know about the new inhalation therapies. Eur. Respir. J. 2011, 37, 1308–1331. [Google Scholar] [CrossRef]
- Timsina, M.P.; Martin, G.P.; Marriott, C.; Ganderton, D.; Yianneskis, M. Drug delivery to the respiratory tract using dry powder inhalers. Int. J. Pharm. 1994, 101, 1–13. [Google Scholar] [CrossRef]
- Grgic, B.; Finlay, W.H.; Burnell, P.K.; Heenan, A.F. In vitro intersubject and intrasubject deposition measurements in realistic mouth-throat geometries. J. Aerosol Sci. 2004, 35, 1025–1040. [Google Scholar] [CrossRef]
- Amirav, I.; Newhouse, M.T. Deposition of small particles in the developing lung. Paediatr. Respir. Rev. 2012, 13, 73–78. [Google Scholar] [CrossRef]
- Bickmann, D.; Wachtel, H.; Kröger, R.; Langguth, P. Examining Inhaler Performance Using A Child’s Throat Model. Available online: http://www.rddonline.com/publications/articles/article.php?ArticleID=1189&return=1 (accessed on 15 December 2013).
- Amirav, I.; Newhouse, M.T.; Mansour, Y. Measurement of peak inspiratory flow with in-check dial device to simulate low-resistance (Diskus) and high-resistance (Turbohaler) dry powder inhalers in children with asthma. Pediatr. Pulmonol. 2005, 39, 447–451. [Google Scholar] [CrossRef]
- Kamps, A.W.; Brand, P.L.; Jan Roorda, R. Variation of peak inspiratory flow through dry powder inhalers in children with stable and unstable asthma. Pediatr. Pulmonol. 2004, 37, 65–70. [Google Scholar] [CrossRef]
- Adachi, Y.S.; Adachi, Y.; Itazawa, T.; Yamamoto, J.; Murakami, G.; Miyawaki, T. Ability of preschool children to use dry powder inhalers as evaluated by in-check meter. Pediatr. Int. 2006, 48, 62–65. [Google Scholar] [CrossRef]
- Bentur, L.; Mansour, Y.; Hamzani, Y.; Beck, R.; Elias, N.; Amirav, I. Measurement of inspiratory flow in children with acute asthma. Pediatr. Pulmonol. 2004, 38, 304–307. [Google Scholar] [CrossRef]
- Vidgren, M.; Kärkkäinen, A.; Karjalainen, P.; Paronen, P.; Nuutinen, J. Effect of powder inhaler design on drug deposition in the respiratory tract. Int. J. Pharm. 1988, 42, 211–216. [Google Scholar] [CrossRef]
- Clark, A.R.; Hollingworth, A.M. The relationship between powder inhaler resistance and peak inspiratory conditions in healthy volunteers—Implications for in vitro testing. J. Aerosol Med. 1993, 6, 99–110. [Google Scholar] [CrossRef]
- Borgström, L.; Nilsson, M. A method for determination of the absolute pulmonary bioavailability of inhaled drugs: Terbutaline. Pharm. Res. 1990, 7, 1068–1070. [Google Scholar] [CrossRef]
- Isaacs, K.K.; Martonen, T.B. Particle deposition in children’s lungs: Theory and experiment. J. Aerosol Med. 2005, 18, 337–353. [Google Scholar] [CrossRef]
- Cheng, Y.S.; Yamada, Y.; Yeh, H.C.; Swift, D.L. Deposition of ultrafine aerosols in a human oral cast. Aerosol Sci. Technol. 1990, 12, 1075–1081. [Google Scholar] [CrossRef]
- Olsson, B. Aerosol particle generation from dry powder inhalers: Can they equal pressurized metered dose inhaler? J. Aerosol Med. 1995, 8, 13–20. [Google Scholar] [CrossRef]
- Stapleton, K.W.; Guentsch, E.; Hoskinson, M.K.; Finlay, W.H. On the suitability of κ–ε turbulence modeling for aerosol deposition in the mouth and throat: A comparison experiment. J. Aerosol Sci. 2000, 31, 739–749. [Google Scholar]
- Minocchieri, S.; Burren, J.M.; Bachmann, M.A.; Stern, G.; Wildhaber, J.; Buob, S.; Schindel, R.; Kraemer, R.; Frey, U.P.; Nelle, M. Development of the premature infant nose throat-model (PrINT-Model)—An upper airway replica of a premature neonate for the study of aerosol delivery. Pediatr. Res. 2008, 64, 141–146. [Google Scholar] [CrossRef]
- Janssens, H.M.; de Jongste, J.C.; Fokkens, W.F.; Robben, S.G.F.; Wouters, K.; Tiddens, H.A. The Sophia anatomical infant nose-throat (Saint) model: A valuable tool to study aerosol deposition in infants. J. Aerosol Med. 2001, 14, 433–441. [Google Scholar] [CrossRef]
- Golshahi, L.; Finlay, W.H. An idealized child throat that mimics average pediatric oropharyngeal deposition. Aerosol Sci. Technol. 2012, 46, I–IV. [Google Scholar] [CrossRef]
- Wachtel, H.; Bickmann, D.; Breitkreutz, J.; Langguth, P. Can pediatric throat models and air flow profiles improve our dose finding strategy? Available online: http://www.rddonline.com/publications/articles/article.php?ArticleID=1435 (accessed on 15 December 2013).
- Corcoran, T.E.; Shortall, B.P.; Kim, I.K.; Meza, M.P.; Chigier, N. Aerosol drug delivery using heliox and nebulizer reservoirs: Results from an MRI-based pediatric model. J. Aerosol Med. 2003, 16, 263–271. [Google Scholar] [CrossRef]
- Below, A.; Bickmann, D.; Breitkreutz, J. Assessing the performance of two dry powder inhalers in preschool children using an idealized pediatric upper airway model. Int. J. Pharm. 2013, 444, 169–174. [Google Scholar] [CrossRef]
- Bronsky, E.A.; Grossmann, J.; Henis, M.J.; Gallo, P.P.; Yegen, Ü.; Della Cioppa, G.; Kottakis, J.; Mehra, S. Inspiratory flow rates and volumes with the Aerolizer dry powder inhaler in asthmatic children and adults. Curr. Med. Res. Opin. 2004, 20, 131–137. [Google Scholar] [CrossRef]
- Al-Showair, R.A.; Tarsin, W.Y.; Assi, K.H.; Pearson, S.B.; Chrystyn, H. Can all patients with COPD use the correct inhalation flow with all inhalers and does training help? Respir. Med. 2007, 101, 2395–2401. [Google Scholar] [CrossRef]
- European Pharmacopeia Commission. Preparations for Inhalation: Aerodynamic Assessment of Fine Particles (2.9.18). In European Pharmacopeia 7.8; European Directorate for the Quality of Medicines (EDQM): Strasbourg, France, 2013; pp. 274–285. [Google Scholar]
- Lohrmann, M.; Kappl, M.; Butt, H.-J.; Urbanetz, N.A.; Lippold, B.C. Adhesion forces in interactive mixtures for dry powder inhalers—Evaluation of a new measuring method. Eur. J. Pharm. Biopharm. 2007, 67, 579–586. [Google Scholar] [CrossRef]
- Tiddens, H.A.; Geller, D.E.; Challoner, P.; Speirs, R.J.; Kesser, K.C.; Overbeek, S.E.; Humble, D.; Shrewsbury, S.B.; Standaert, T.A. Effect of dry powder inhaler resistance on the inspiratory flow rates and volumes of cystic fibrosis patients of six years and older. J. Aerosol Med. 2006, 19, 456–465. [Google Scholar] [CrossRef]
- Nielsen, K.G.; Skov, M.; Klug, B.; Ifversen, M.; Bisgaard, H. Flow-dependent effect of formoterol dry-powder inhaled from the Aerolizer®. Eur. Respir. J. 1997, 10, 2105–2109. [Google Scholar] [CrossRef]
- Chew, N.Y.; Chan, H.-K. In vitro aerosol performance and dose uniformity between the Foradile® Aerolizer® and the Oxis® Turbohaler®. J. Aerosol Med. 2001, 14, 495–501. [Google Scholar] [CrossRef]
- De Boer, A.H.; Winter, H.M.; Lerk, C.F. Inhalation characteristics and their effects on in vitro drug delivery from dry powder inhalers. Part 1. Inhalation characteristics, work of breathing and volunteer’s preference in dependence of the inhaler resistance. Int. J. Pharm. 1996, 130, 231–244. [Google Scholar] [CrossRef]
- Behara, S.R.; Farkas, D.; Hindle, M.; Longest, P.W. Development of a high efficiency dry powder inhaler: Effects of capsule chamber design and inhaler surface modifications. Pharm. Res. 2013. [Google Scholar] [CrossRef]
- Behara, S.R.; Longest, P.W.; Farkas, D.; Hindle, M. Development and comparison of new high-efficiency dry powder inhalers for carrier-free formulations. J. Pharm. Sci. 2013. [Google Scholar] [CrossRef]
- Son, Y.-J.; Longest, P.W.; Tian, G.; Hindel, M. Evaluation and modification of commercial dry powder inhalers for the aerolization of a submicrometer excipient enhanced growth (EEG) formulation. Eur. J. Pharm. Sci. 2013, 49, 390–399. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lindert, S.; Below, A.; Breitkreutz, J. Performance of Dry Powder Inhalers with Single Dosed Capsules in Preschool Children and Adults Using Improved Upper Airway Models. Pharmaceutics 2014, 6, 36-51. https://doi.org/10.3390/pharmaceutics6010036
Lindert S, Below A, Breitkreutz J. Performance of Dry Powder Inhalers with Single Dosed Capsules in Preschool Children and Adults Using Improved Upper Airway Models. Pharmaceutics. 2014; 6(1):36-51. https://doi.org/10.3390/pharmaceutics6010036
Chicago/Turabian StyleLindert, Sandra, Antje Below, and Joerg Breitkreutz. 2014. "Performance of Dry Powder Inhalers with Single Dosed Capsules in Preschool Children and Adults Using Improved Upper Airway Models" Pharmaceutics 6, no. 1: 36-51. https://doi.org/10.3390/pharmaceutics6010036