Composition Influence on Pulmonary Delivery of Rifampicin Liposomes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Liposomes.
| RFP | P90H | P50 | Chol | OA | |
|---|---|---|---|---|---|
| mg/mL | mg/mL | mg/mL | mg/mL | mg/mL | |
| PC30 | 10 | 15 | 15 | -- | -- |
| PC30 Chol | 10 | 15 | 15 | 10 | -- |
| PC30 Chol OA | 10 | 15 | 15 | 10 | 3 |
| PC60 | 10 | 30 | 30 | -- | -- |
| PC60 Chol | 10 | 30 | 30 | 10 | -- |
| PC60 Chol OA | 10 | 30 | 30 | 10 | 6 |
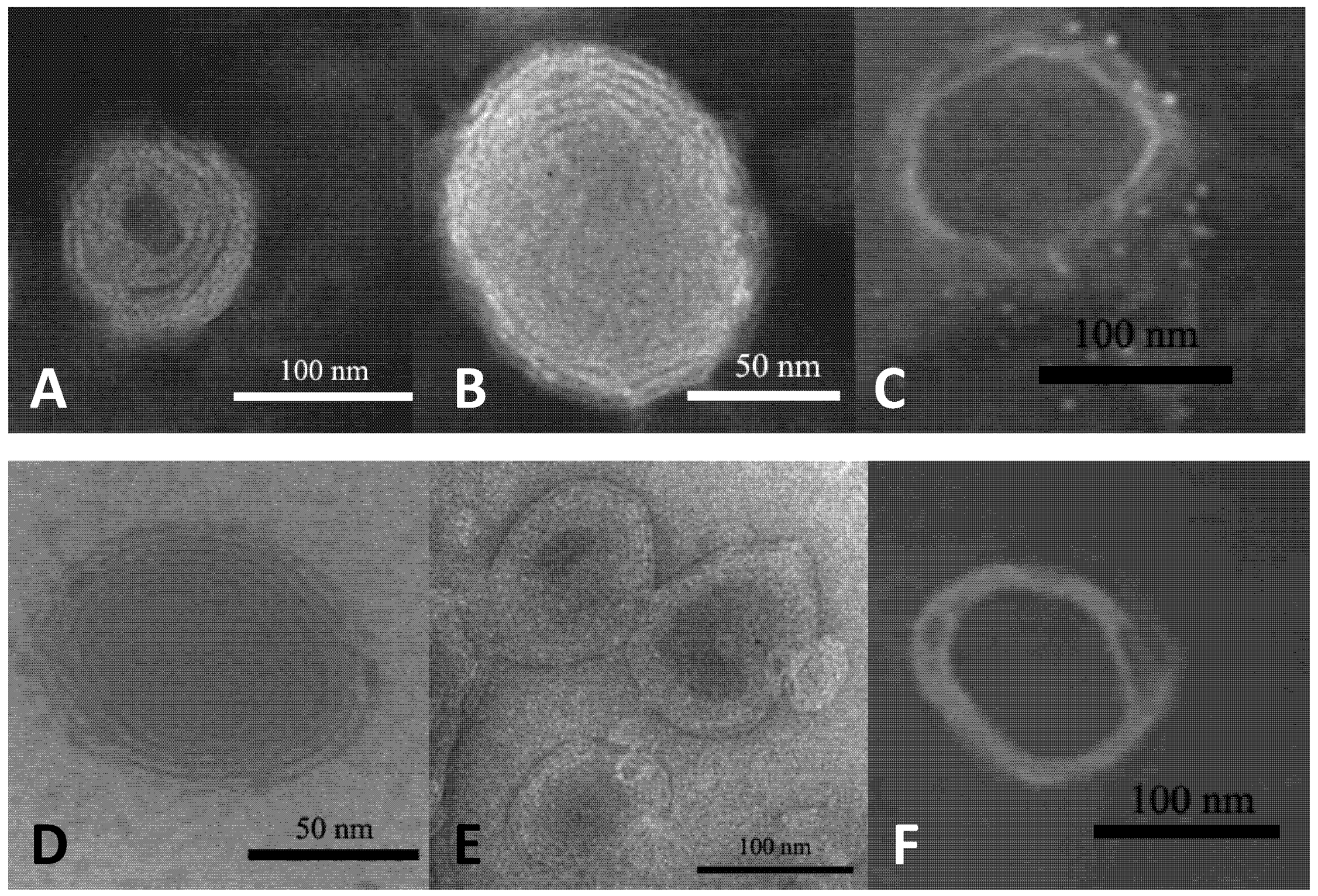

| Before dialysis | After dialysis | ||||||
|---|---|---|---|---|---|---|---|
| Size (nm) ± SD | PI | ZP (mV) ± SD | Size (nm) ± SD | PI | ZP (mV) ± SD | E (%) ± SD | |
| PC30 | 106 ± 11 | 0.21 | −50 ± 2.8 | 101 ± 12 | 0.20 | −58 ± 0.4 | 67 ± 4 |
| PC30 Chol | 128 ± 8 | 0.25 | −57 ± 3.1 | 118 ± 8 | 0.25 | −63 ± 2.9 | 64 ± 6 |
| PC30 Chol OA | 143 ± 15 | 0.23 | −67 ± 3.3 | 153 ± 9 | 0.23 | −67 ± 3.7 | 60 ± 2 |
| PC60 | 104 ± 3 | 0.14 | −54 ± 3.8 | 102±2 | 0.15 | −57 ± 1.3 | 76 ± 4 |
| PC60 Chol | 112 ± 4 | 0.13 | −57 ± 6.6 | 108 ± 3 | 0.17 | −62 ± 1.2 | 74 ± 4 |
| PC60 Chol OA | 79 ± 6 | 0.15 | −63 ± 7.4 | 85 ± 6 | 0.15 | −69 ± 3.3 | 69 ± 2 |
2.2. Rheological Studies
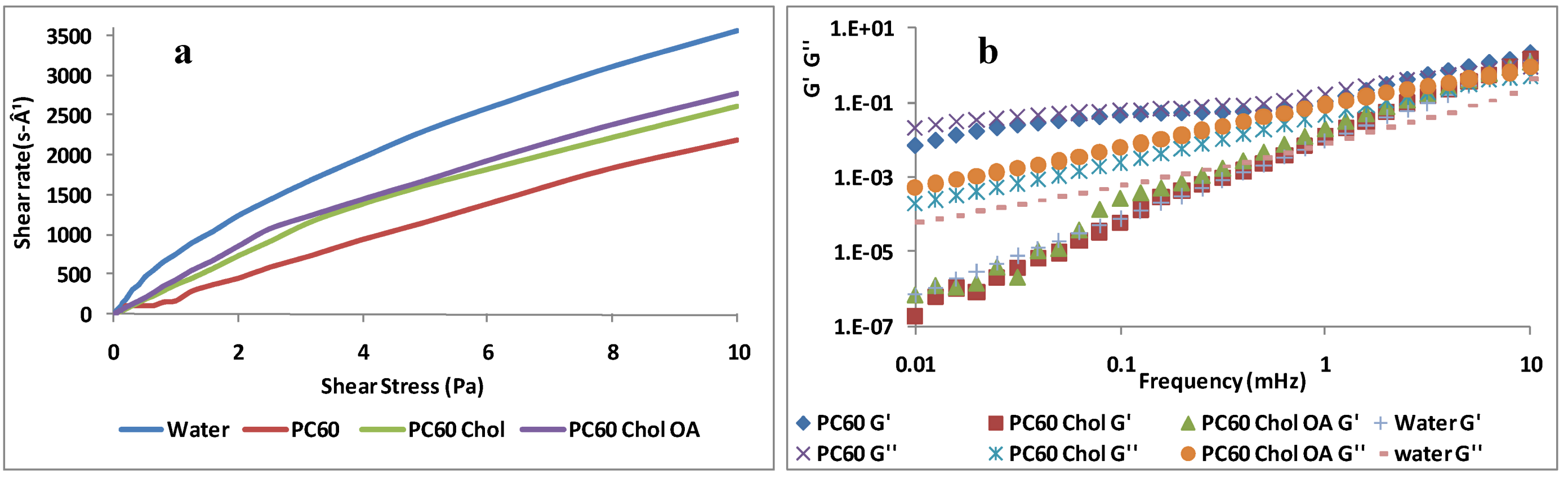
2.3. Nebulization Studies of Liposomes
| NE% | FPD (µg) | FPF (%) | NER% | ||
|---|---|---|---|---|---|
| PC30 | Stage 1 | 35 ± 2.3 | 908 ± 23 | 9 ± 0.7 | 25 ± 0.7 |
| Stage 2 | 5 ± 0.5 | ||||
| Stage 3 | 4 ± 0.1 | ||||
| PC30 Chol | Stage 1 | 48 ± 3.9 | 736 ± 12 | 7 ± 1.3 | 25 ± 1.0 |
| Stage 2 | 5 ± 0.8 | ||||
| Stage 3 | 3 ± 0.2 | ||||
| PC30 Chol OA | Stage 1 | 51 ± 2.9 | 108 ± 32 | 11 ± 0.8 | 49 ± 3.3 |
| Stage 2 | 7 ± 0.2 | ||||
| Stage 3 | 4 ± 0.1 | ||||
| PC60 | Stage 1 | 41 ± 6.8 | 1300 ± 37 | 13 ± 0.9 | 45 ± 3.1 |
| Stage 2 | 10 ± 0.6 | ||||
| Stage 3 | 3 ± 0.1 | ||||
| PC60 Chol | Stage 1 | 54 ± 3.7 | 1260 ± 29 | 13 ± 1.2 | 49 ± 7.9 |
| Stage 2 | 9 ± 0.2 | ||||
| Stage 3 | 3 ± 0.1 | ||||
| PC60 Chol OA | Stage 1 | 64 ± 2.3 | 1685 ± 35 | 17 ± 1.7 | 55 ± 1.4 |
| Stage 2 | 12 ± 0.7 | ||||
| Stage 3 | 5 ± 0.1 |
2.4. Cell Viability and Probe Uptake Studies

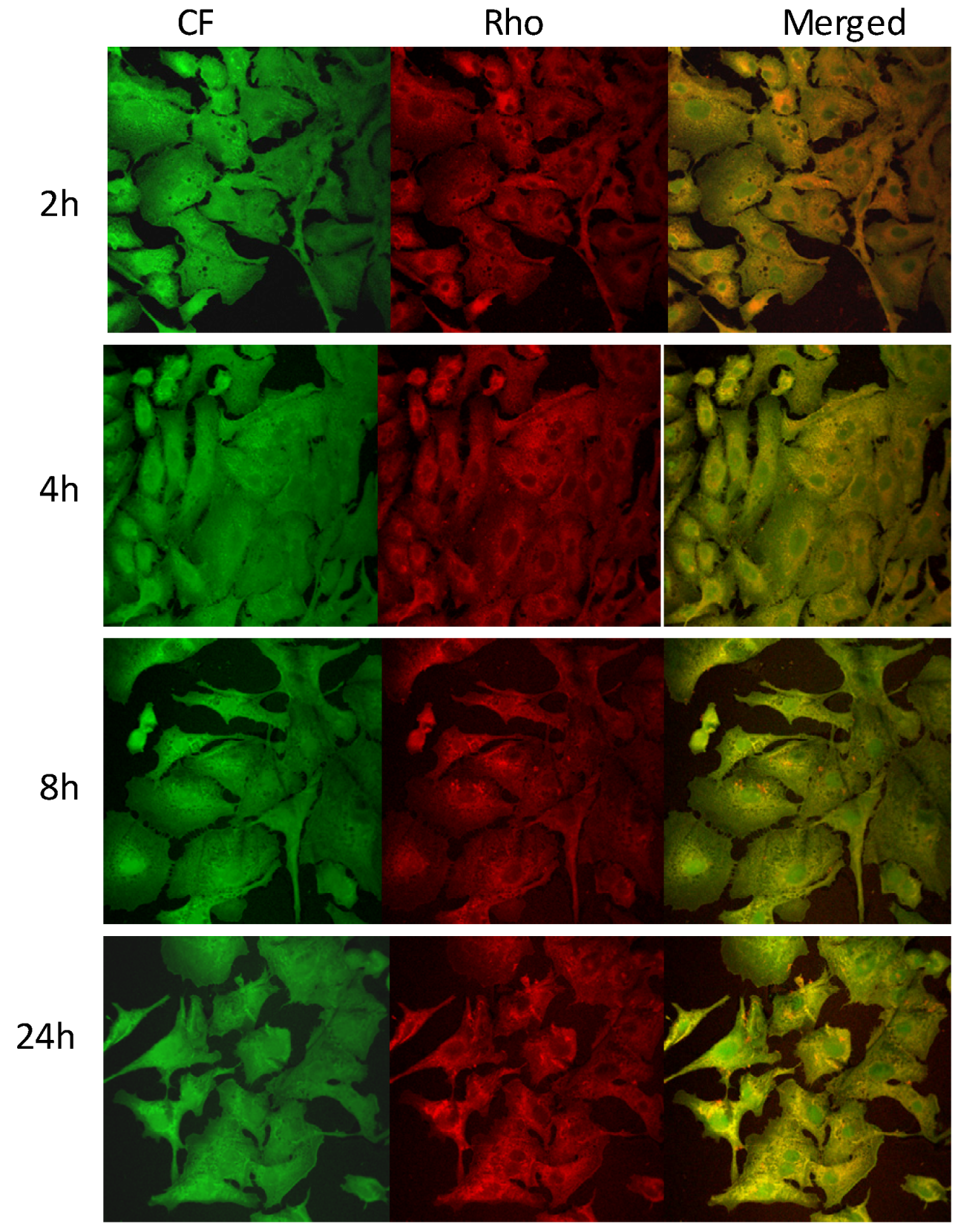
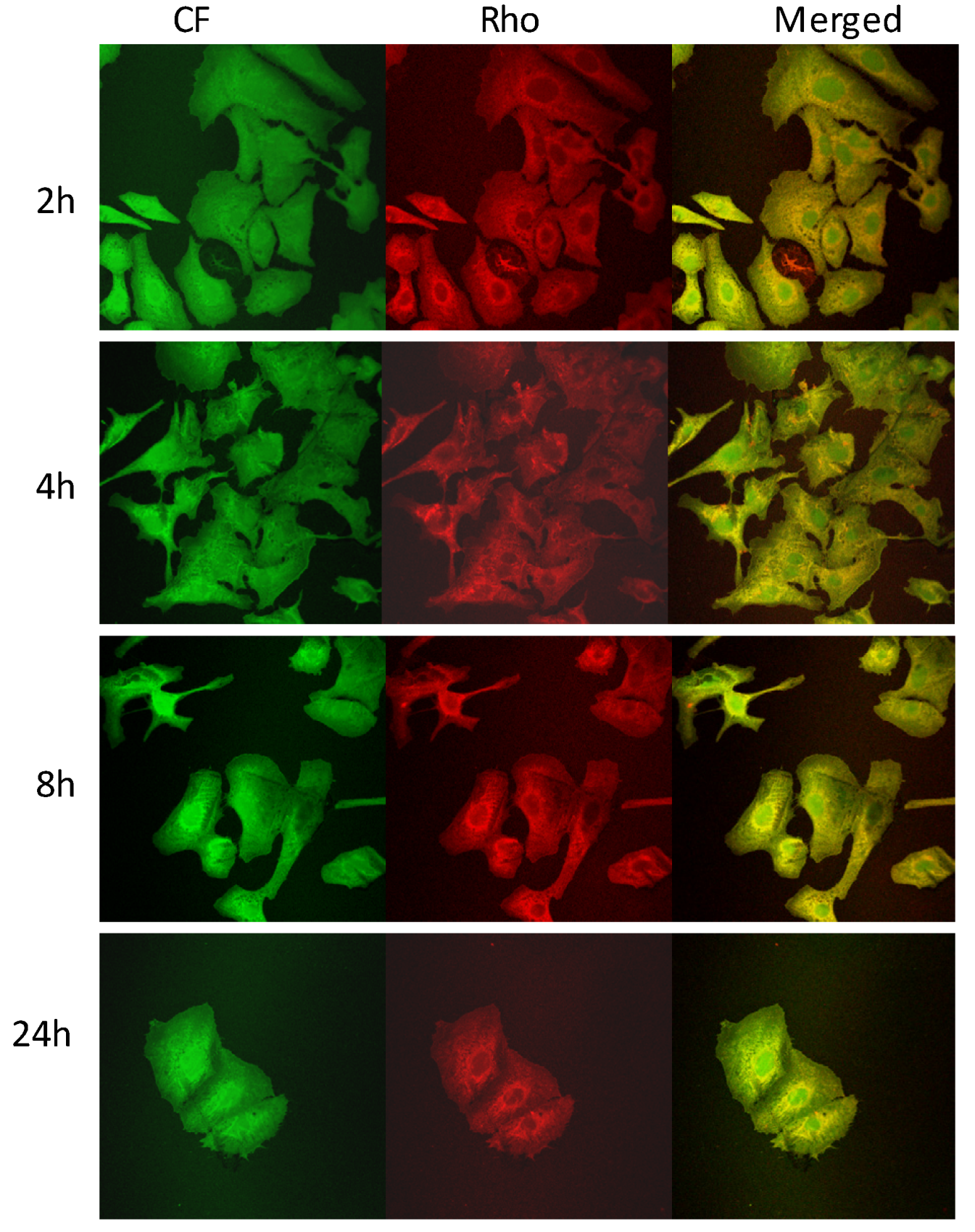
3. Experimental Section
3.1. Materials
3.2. Vesicle Preparation
3.3. Vesicle Characterization
3.4. Rheological Studies
3.5. Nebulization Studies of Liposomes
3.6. Cell Cultures
3.7. Cell Viability Studies (MTT Assay)
3.8. Cellular Uptake of Rho-PE Labeled CF-Loaded Vesicles
3.9. Statistical Analysis of Data
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Mura, C.; Nácher, A.; Merino, V.; Merino-Sanjuan, M.; Carda, C.; Ruiz, A.; Manconi, M.; Diez-Sales, O. N-Succinyl-chitosan systems for 5-aminosalicylic acid colon delivery: In vivo study with TNBS-induced colitis model in rats. Int. J. Pharm. 2011, 416, 145–154. [Google Scholar]
- Delmas, T.; Fraichard, A.; Bayle, P.A.; Texier, I.; Bardet, M.; Baudry, J.; Bibette, J.; Couffin, A.C. Encapsulation and release behavior from lipid nanoparticles: Model study with nile red fluorophore. J. Colloid Sci. Biotechnol. 2012, 1, 16–25. [Google Scholar]
- Gonnet, M.; Bastiat, G.; Ivanova, T.; Boury, F. Interfacial organization of β-carotene loaded lipid nanocapsules modelized by 2D rheological measurements and electrophoretic mobility. J. Colloid Sci. Biotechnol. 2012, 1, 137–146. [Google Scholar]
- Manca, M.L.; Mourtas, S.; Drakopoulos, V.; Fadda, A.M.; Antimisiaris, S. PLGA, chitosan or chitosan-coated PLGA microparticles for alveolar delivery? A comparative study of particle stability during nebulization. Coll. Surf. B Biointerf. 2008, 62, 220–131. [Google Scholar]
- Zaru, M.; Manca, M.L.; Fadda, A.M.; Antimisiaris, S. Chitosan-coated liposomes for delivery to lungs by nebulisation. Coll. Surf. B Biointerf. 2009, 71, 88–95. [Google Scholar] [CrossRef]
- Marianecci, C.; Paolino, P.; Celia, C.; Fresta, M.; Carafa, M.; Alhaique, F. Non-ionic surfactant vesicles in pulmonary glucocorticoid delivery: Characterization and interaction with human lung fibroblasts. J. Contr. Release 2010, 147, 127–135. [Google Scholar] [CrossRef]
- Mura, S.; Hillaireau, H.; Nicolas, J.; Kerdine-Römer, S.; Le Droumaguet, B.; Deloménie, C.; Nicolas, V.; Pallardy, M.; Tsapis, N.; Fattal, E. Biodegradable Nanoparticles Meet the Bronchial Airway Barrier: How Surface Properties Affect Their Interaction with Mucus and Epithelial Cells. Biomacromolecules 2011, 12, 4136–4143. [Google Scholar] [CrossRef]
- Cassano, R.; Trombino, S.; Ferrarelli, T.; Mauro, M.V.; Giraldi, C.; Manconi, M.; Fadda, A.M.; Picci, N. Respirable rifampicin-based microspheres containing isoniazid for tuberculosis treatment. J. Biomed. Mater. Res. Part A 2012, 2, 536–542. [Google Scholar]
- Ungaro, F.; d’Angelo, I.; Miro, A.; La Rotonda, M.I.; Quaglia, F. Engineered PLGA nano- and micro-carriers for pulmonary delivery: Challenges and promises. J. Pharm. Pharmacol. 2012, 64, 1217–1235. [Google Scholar]
- Pinheiro, M.; Lúcio, M.; Lima, J.L.F.C.; Reis, S. Liposomes as drug delivery systems for the treatment of TB. Nanomedicine 2011, 6, 1413–1428. [Google Scholar]
- Kurmi, B.D.; Kayat, J.; Gajbhiye, V.; Tekade, R.K.; JainKurmi, N.K. Micro- and nanocarrier-mediated lung targeting. Expert Opin. Drug Deliv. 2012, 7, 781–794. [Google Scholar]
- Traini, D.; Young, P.M. Delivery of antibiotics to therespiratory tract: an update. Expert Opin. Drug Deliv. 2009, 6, 897–905. [Google Scholar] [CrossRef]
- Jaafar-Maalej, C.; Andrieu, V.; Elaissari, A.; Fessi, H. Beclomethasone-loaded lipidic nanocarriers for pulmonary drug delivery: Preparation, characterization and in vitro drug release. J. Nanosci. Nanotechnol. 2011, 11, 1841–1851. [Google Scholar]
- Jaafar-Maalej, C.; Elaissari, A.; Fessi, H. Lipid-based carriers: Manufacturing and applications for pulmonary route. Expert Opin. Drug Deliv. 2012, 9, 1111–1127. [Google Scholar]
- Geiser, M.; Cruz-Orive, L.M.; Im Hof, V.; Gehr, P. Assessment of particle retention and clearance in the intrapulmonary conducting airways of hamster lungs with the fractionator. J. Microsc. 1990, 160, 75–88. [Google Scholar] [CrossRef]
- Geiser, M.; Baumann, M.; Cruz-Orive, L.M.; Im Hof, V.; Waber, U.; Gehr, P. The effect of particle inhalation on macrophage number and phagocytic activity in the intrapulmonary conducting airways of hamsters. Am. J. Respir. Cell Mol. Biol. 1994, 10, 594–603. [Google Scholar]
- Verma, R.K.; Singh, A.K.; Mohan, M.; Agrawal, A.K.; Misra, A. Inhaled therapies for tuberculosis and the relevance of activation of lung macrophages by particulate drug delivery systems. Therapeutic Delivery 2011, 2, 753–768. [Google Scholar]
- Poletto, F.S.; Fiel, L.A.; Lopes, M.V.; Schaab, G.; Gomes, A.M.O.; Guterres, S.S.; Rossi-Bergmann, B.; Pohlmann, A.R. Fluorescent-Labeled Poly(E-caprolactone) Lipid-Core Nanocapsules: Synthesis, Physicochemical Properties and Macrophage Uptake. J. Colloid Sci. Biotechnol. 2012, 1, 89–98. [Google Scholar]
- Vyas, S.P.; Kannan, M.E.; Jain, S.; Mishra, V.; Singh, P. Design of liposomal aerosols for improved delivery of rifampicin to alveolar macrophages. Int. J. Pharm. 2004, 269, 37–49. [Google Scholar] [CrossRef]
- Chono, S.; Tanino, T.; Seki, T.; Morimoto, K. Efficient drug targeting to rat alveolar macrophages by pulmonary administration of ciprofloxacin incorporated into mannosylated liposomes for treatment of respiratory intracellular parasitic infections. J. Contr. Release 2008, 127, 50–58. [Google Scholar]
- Zaru, M.; Mourtas, S.; Klepetsanis, P.; Fadda, A.M.; Antimisiaris, S.G. Liposomes for drug delivery to the lungs by nebulization. Eur. J. Pharm. Biopharm. 2007, 67, 655–666. [Google Scholar]
- Zaru, M.; Sinico, C.; De Logu, A.; Caddeo, C.; Lai, F.; Manca, M.L.; Fadda, A.M. Rifampicin-loaded liposomes for the passive targeting to alveolar macrophages: in vitro and in vivo evaluation. J. Lip. Res. 2009, 71, 88–95. [Google Scholar]
- Manca, M.L.; Manconi, M.; Valenti, D.; Lai, F.; Loy, G.; Matricardi, P.; Fadda, A.M. Liposomes coated with chitosan-xanthan gum (chitosomes) as potential carriers for pulmonary delivery of rifampicin. J. Pharm. Sci. 2012, 101, 566–575. [Google Scholar] [CrossRef]
- Coderch, L.; Fonollosa, J.; de Pera, M.; Estelrich, J.; de La Maza, A.; Parra, J.L. Influence of cholesterol on liposome fluidity by EPR: Relationship with percutaneous absorption. J. Control. Release 2000, 68, 85–95. [Google Scholar] [CrossRef]
- Calvagno, M.G.; Celia, C.; Paolino, P.; Cosco, D.; Iannone, M.; Castelli, F.; Doldo, P.; Fresta, M. Effects of Lipid Composition and Preparation Conditions on Physical-Chemical Properties, Technological Parameters and in vitro Biological Activity of Gemcitabine-Loaded Liposomes. Curr. Drug Deliv. 2007, 4, 89–101. [Google Scholar] [CrossRef]
- Revilla, J.; Elaissari, A.; Carriere, P.; Pichot, C. Adsorption of Bovine Serum Albumin onto Polystyrene Latex Particles Bearing Saccharidic Moieties. J. Colloid Interf. Sci. 1996, 180, 405–412. [Google Scholar] [CrossRef]
- Manconi, M.; Sinico, C.; Caddeo, C.; Vila, A.O.; Valenti, D.; Fadda, A.M. Penetration enhancer containing vesicles as carriers for dermal delivery of tretinoin. Int. J. Pharm. 2011, 412, 37–46. [Google Scholar] [CrossRef]
- Manconi, M.; Caddeo, C.; Sinico, C.; Valenti, D.; Mostallino, M.C.; Biggio, G.; Fadda, A.M. Ex vivo skin delivery of diclofenac by transcutol containing liposomes and suggested mechanism of vesicle-skin interaction. Eur. J. Pharm. Biopharm. 2011, 78, 27–35. [Google Scholar] [CrossRef]
- Chessa, M.; Caddeo, C.; Valenti, D.; Manconi, M.; Sinico, C.; Fadda, A.M. Effect of Penetration Enhancer Containing Vesicles on the Percutaneous Delivery of Quercetin through New Born Pig Skin. Pharmaceutics 2011, 3, 497–509. [Google Scholar] [CrossRef]
- Gradzielski, M. The rheology of vesicle and disk systems. Relations between macroscopic behaviour and microstructure. Curr. Opin. Colloid. Interface Sci. 2011, 16, 13–17. [Google Scholar] [CrossRef]
- Gaspar, M.M.; Bakowsky, U.; Ehrhardt, C. Inhaled liposomes-Current strategies and future challenges. J. Biomed. Nanotechnol. 2008, 4, 1–13. [Google Scholar]
- Bridges, P.A.; Taylor, K. An investigation of some of the factors influencing the jet nebulisation of liposomes. Int. J. Pharm. 2000, 204, 69–79. [Google Scholar] [CrossRef]
- Taylor, K.M.G.; Taylor, G.; Kellaway, I.W.; Stevens, J. The stability of liposomes to nebulisation. Int. J. Pharm. 1990, 58, 57–61. [Google Scholar] [CrossRef]
- Pilane, C.M.; Labelle, E.F. cPLA2 activator peptide, PLAP, increases arachidonic acid release and apoptosis of vascular smooth muscle cells. J. Cell. Physiol. 2004, 198, 48–52. [Google Scholar] [CrossRef]
- Ulloth, J.E.; Casiano, C.A.; de Leon, M. Palmitic and stearic fatty acids induce caspase-dependent and -independent cell death in nerve growth factor differentiated PC12 cells. J. Neurochem. 2003, 84, 655–668. [Google Scholar] [CrossRef]
- Beeharry, N.; Chambers, J.A.; Green, I.C. Fatty acid protection from palmitic acid-induced apoptosis is lost following PI3-kinase inhibition. Apoptosis 2004, 9, 599–607. [Google Scholar]
- Avivi-Green, C.; Polak-Charcon, S.; Madar, Z.; Schwartz, B. Different molecular events account for butyrate-induced apoptosis in two human colon cancer cell lines. J. Nutr. 2002, 132, 1812–1818. [Google Scholar]
- Lee, R.J.; Wang, S.; Turk, M.J.; Low, P.S. The Effects of pH and Intraliposomal Buffer Strength on the Rate of Liposome Content Release and Intracellular Drug Delivery. Biosci. Rep. 1998, 18, 69–78. [Google Scholar]
- Manconi, M.; Isola, R.; Falchi, A.M.; Sinico, C.; Fadda, A.M. Intracellular distribution of fluorescent probes delivered by vesicles of different lipidic composition. Coll. Surf. B Biointerface 2007, 57, 143–151. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Manca, M.L.; Sinico, C.; Maccioni, A.M.; Diez, O.; Fadda, A.M.; Manconi, M. Composition Influence on Pulmonary Delivery of Rifampicin Liposomes. Pharmaceutics 2012, 4, 590-606. https://doi.org/10.3390/pharmaceutics4040590
Manca ML, Sinico C, Maccioni AM, Diez O, Fadda AM, Manconi M. Composition Influence on Pulmonary Delivery of Rifampicin Liposomes. Pharmaceutics. 2012; 4(4):590-606. https://doi.org/10.3390/pharmaceutics4040590
Chicago/Turabian StyleManca, Maria Letizia, Chiara Sinico, Anna Maria Maccioni, Octavio Diez, Anna Maria Fadda, and Maria Manconi. 2012. "Composition Influence on Pulmonary Delivery of Rifampicin Liposomes" Pharmaceutics 4, no. 4: 590-606. https://doi.org/10.3390/pharmaceutics4040590






