Pediatric Dosing and Body Size in Biotherapeutics
Abstract
:1. Introduction
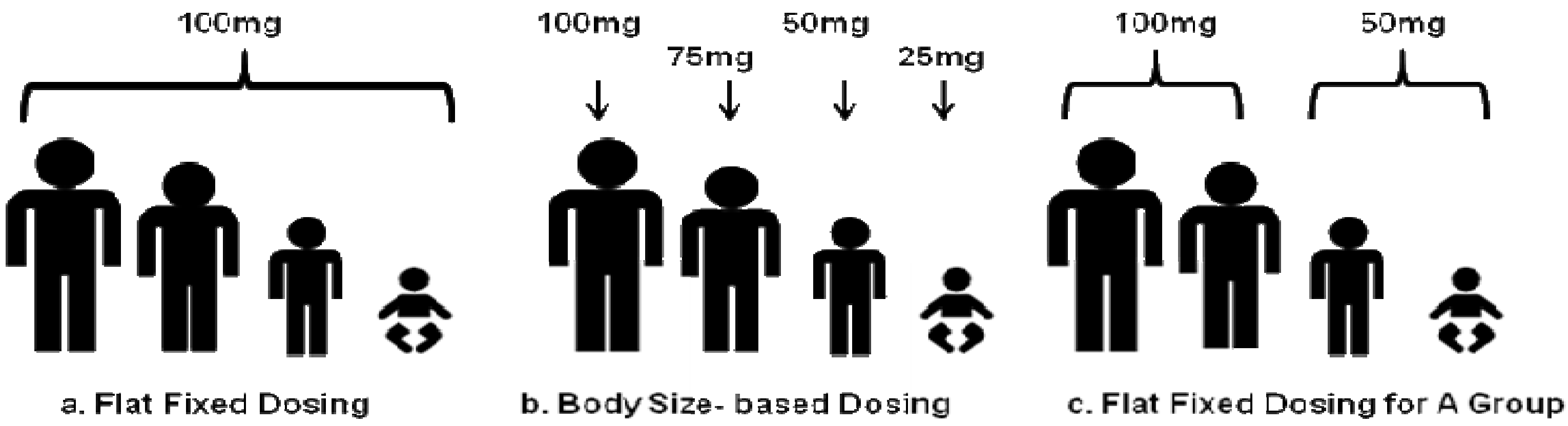
1.1. General Pharmacokinetics in Pediatrics
- Premature: gestational age < 36 weeks
- Full-term: gestational age ≥ 36
- Neonates: 0–1 months
- Infants: 1–12 months
- Children: 1–12 years
- Adolescent: 12–16 years
| Age | Total body water (%) | Extracellular fluid (%) | Intracellular fluid (%) |
|---|---|---|---|
| Fetus (<3 months) | 90 | 65 | 25 |
| Neonate (Premature) | 85 | 50 | 35 |
| Neonate (Full-term) | 75 | 40 | 35 |
| Infant (4–6 months) | 60 | 23 | 37 |
| Adolescent | 60 | 20 | 40 |
| Adult | 60 | 20 | 40 |
| Organ | Newborn | Adults |
|---|---|---|
| Muscle | 25 | 40 |
| Skin | 4 | 6 |
| Heart | 0.5 | 0.4 |
| Liver | 5 | 2 |
| Kidney | 1 | 0.5 |
| Brain | 2 | 2 |
| Age | GFR (mL/min) | RPF (mL/min) |
|---|---|---|
| 1–10 days | 15–45 | 20–125 |
| 1 month | 30–60 | 100–400 |
| 6 months | 50–100 | 400–500 |
| 1 years | 80–120 | 500–600 |
| 1–70 years | 80–140 | 500–700 |
| 70–80 years | 70–110 | 250–450 |
| 80–90 years | 45–85 | 200–400 |
1.2. Pharmacokinetics of Proteins and Peptides
1.2.1. Distribution
1.2.2. Elimination
2. Results and Discussion
2.1. Monoclonal Antibodies (mAbs)
2.1.1. Basiliximab
| Generic Name | Class | Route | Pharmacokinetics |
|---|---|---|---|
| Alemtuzumab | mAbs | i.v. | More rapid clearance in children than in adults. |
| Basiliximab | mAbs | i.v. | CL (mL/h) in infants and children is about half that of adults. Use 35 kg as a cut-off weight for 10 or 20 mg in pediatrics. |
| Bevacizumab | mAbs | i.v. | Body weight (BW) based dose exhibits similar PK parameters in children and adults, and large variability in both populations. |
| Cetuximab | mAbs | i.v. | Dose-dependent nonlinear elimination. BSA based dose provides similar exposure in children and adults, and age has no effect on PK. |
| Daclizumab | mAbs | i.v. | The 4.2-fold range in CL, 7.4-fold range in V are less proportional than a 12-fold range in body weight |
| Darbepoetin Alfa | Growth factor | i.v., s.c. | The lack of dose-proportionality is likely due to pediatric population rather than nonlinear PK; neonates have a shorter half-life, larger V and CL than children. |
| Drotrecogin alfa | Blood factor | i.v. | Weight-normalized clearance decreases significantly with age in patients <18 years old. |
| Enfuvirtide | Peptide | s.c. | One study justified body weight (BW) based pediatric dosing. |
| Epoetin Alfa | Growth factor | i.v., s.c. | CL (mL/h/kg) and bioavailability in pediatrics were two-fold of that in adults. |
| Epoetin Delta | Growth factor | i.v., s.c. | BW adjusted PK parameters are similar in children and in adults. |
| Etanercept | Fusion protein | s.c. | The analysis justified the body weight based dose adjustment for etanercept in JRA patients; gender difference was reported both in children and adults. |
| Exenatide | Incretin | s.c. | The max recommended adult dose instead of half of the max dose was suggested to be explored in adolescent patients. |
| Factor VII | Blood factor | i.v. | Total body weight normalized clearance was significantly faster in children than in adults. |
| Factor VIII | Blood factor | i.v. | BW adjusted clearance in mL/h/ kg and Vss in L/ kg seems to decrease with age. |
| Factor VIX | Blood factor | i.v. | Higher weight-adjusted CL in children than adults. |
| Filgrastim | Growth factor | s.c. | ANC-adjusted G-CSF dosing adjustment might improve PBPC mobilization in pediatric patients. |
| Gemtuzumab | mAbs | i.v. | Both faster CL (L/h) and CL (L/h/m2) in adults than children and infants. |
| Humatrope | Growth hormone | s.c. | No significant effect of weight and age on humatrope pharmacokinetic parameters. |
| Infliximab | mAbs | i.v. | BW based dose provides similar exposure in children and adults; PK of infliximab does not differ as age increases. |
| Insulin aspart | Insulin | s.c. | In pediatrics, insulin aspart had a quicker onset than human insulin; aspart has a higher exposure in adolescents than in children. |
| Insulin detemir | Insulin | i.v. | Less PK variability in insulin determir than glargine. |
| Insulin glulisin | Insulin | i.v. | The profile of insulin glulisine is similar for children and adolescents, whereas human insulin exhibits higher level in adolescents. |
| Interferon-α2a | Interferon | s.c. | Higher drug exposure in pediatrics; wide intersubject variability suggests further individualized dosing. |
| Interferon-α2b | Interferon | i.v. | BSA based PK parameters in pediatrics is about twice that in adults. |
| Interferon-αnl | Interferon | i.v., i.m. | No BW/BSA or age affect was discussed. Slightly lower exposure in pediatrics than in adults. |
| Interleukin | Cytokines | i.v., s.c. | Higher rhIL-11 clearance in pediatrics than adults |
| Asparaginase | Enzyme | i.m, i.p. | After adjusting dose by BSA, neither age nor the BSA had any influence on the distribution. |
| LB03002 | Growth hormone | s.c. | Body weight adjusted dosing gives comparable exposure in pediatrics to in adults. |
| Natalizumab | mAbs | i.v. | BW base dose tends to underdose adolescents. |
| Nutropin | Growth hormone | s.c. | Drug exposure was approximately proportional to the dose. |
| Palivizumab | mAbs | i.v., i.m. | BW based dose for palivizumab, but body weight effect not discussed; no significant clinical outcome between placebo, 5 and 15 mg/kg were observed. |
| Somatropin | Growth hormone | Inhaled | No significant effect of weight and age on somatropin pharmacokinetic parameters. |
| Zomacton | Growth hormone | s.c. | No BW/BSA or age correlation was analyzed for its pharmacokinetic parameters. |
2.1.2. Daclizumab
2.1.3. Palivizumab
2.1.4. Infliximab
2.1.5. Gemtuzumab
2.1.6. Alemtuzumab (Campath-1H)
2.1.7. Cetuximab
2.1.8. Bevacizumab
2.1.9. Natalizumab
2.2. Growth Factors
2.2.1. Epoetin Alfa and Delta
2.2.2. Darbepoetin Alfa
2.2.3. Filgrastim
2.3. Interferon
2.4. Blood Factors
2.4.1. Factor VII
2.4.2. Factor VIII
2.4.3. Factor IX
2.4.4. Drotrecogin Alfa
2.5. Hormones
2.5.1. Insulin Hormones
2.5.2. Growth Hormone
2.6. Other Proteins and Peptides
2.6.1. Interleukin
2.6.2. Etanercept
2.6.3. Enfuvirtide
2.6.4. L-Asparaginase
3. Conclusions
References and Notes
- Roberts, R.; Rodriguez, W.; Murphy, D.; Crescenzi, T. Pediatric drug labeling: improving the safety and efficacy of pediatric therapies. JAMA 2003, 290, 905–911. [Google Scholar] [CrossRef]
- Alcorn, J.; McNamara, P.J. Pharmacokinetics in the newborn. Adv. Drug Deliv. Rev. 2003, 55, 667–686. [Google Scholar] [CrossRef]
- Rodriguez, W.; Selen, A.; Avant, D.; Chaurasia, C.; Crescenzi, T.; Gieser, G.; Di Giacinto, J.; Huang, S.M.; Lee, P.; Mathis, L.; Murphy, D.; Murphy, S.; Roberts, R.; Sachs, H.C.; Suarez, S.; Tandon, V.; Uppoor, R.S. Improving pediatric dosing through pediatric initiatives: What we have learned. Pediatrics 2008, 121, 530–539. [Google Scholar]
- Derendorf, H. Pediatrics. Available online: http://www.cop.ufl.edu/safezone/pat/pha5128/index.htm (accessed on 30 September 2010).
- Schoeller, D.A. Changes in total body water with age. Am. J. Clin. Nutr. 1989, 50, 1176–1181. [Google Scholar]
- Peters, A.M.; Henderson, B.L.; Lui, D. Indexed glomerular filtration rate as a function of age and body size. Clin. Sci. (Lond) 2000, 98, 439–444. [Google Scholar] [CrossRef]
- Hines, R.N. Ontogeny of human hepatic cytochromes P450. J. Biochem. Mol. Toxicol. 2007, 21, 169–175. [Google Scholar] [CrossRef]
- Koukouritaki, S.B.; Manro, J.R.; Marsh, S.A.; Stevens, J.C.; Rettie, A.E.; McCarver, D.G.; Hines, R.N. Developmental expression of human hepatic CYP2C9 and CYP2C19. J. Pharmacol. Exp. Ther. 2004, 308, 965–974. [Google Scholar]
- Authier, F.; Danielsen, G.M.; Kouach, M.; Briand, G.; Chauvet, G. Identification of insulin domains important for binding to and degradation by endosomal acidic insulinase. Endocrinology 2001, 142, 276–289. [Google Scholar]
- Smedsrod, B.; Einarsson, M. Clearance of tissue plasminogen activator by mannose and galactose receptors in the liver. Thromb. Haemost. 1990, 63, 60–66. [Google Scholar]
- FDA. Guidance for Industry General Considerations for Pediatric Pharmacokinetic Studies for Drugs and Biological Products. Available online: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm072114.pdf (accessed on 1 December 2010).
- Lobo, E.D.; Hansen, R.J.; Balthasar, J.P. Antibody pharmacokinetics and pharmacodynamics. J. Pharm. Sci. 2004, 93, 2645–2668. [Google Scholar] [CrossRef]
- Tang, L.; Persky, A.M.; Hochhaus, G.; Meibohm, B. Pharmacokinetic aspects of biotechnology products. J. Pharm. Sci. 2004, 93, 2184–2204. [Google Scholar]
- Reilly, R.M.; Sandhu, J.; Alvarez-Diez, T.M.; Gallinger, S.; Kirsh, J.; Stern, H. Problems of delivery of monoclonal antibodies. Pharmaceutical and pharmacokinetic solutions. Clin. Pharmacokinet. 1995, 28, 126–142. [Google Scholar] [CrossRef]
- Zito, S.W. Pharmaceutical Biotechnology: A Programmed Text; Technomic Pub.Co.: Lancaster, PA, USA, 1997; pp. 184–186. [Google Scholar]
- Tan, A.C.; Russel, F.G.; Thien, T.; Benraad, T.J. Atrial natriuretic peptide. An overview of clinical pharmacology and pharmacokinetics. Clin. Pharmacokinet. 1993, 24, 28–45. [Google Scholar]
- Colburn, W. Peptide, Peptoid, and Protein Pharmacokinetics/Pharmacodynamics; Harvey Whitney Books: Cincinnati, OH, USA, 1991; Volume 3, pp. 94–115. [Google Scholar]
- Eppler, S.M.; Combs, D.L.; Henry, T.D.; Lopez, J.J.; Ellis, S.G.; Yi, J.H.; Annex, B.H.; McCluskey, E.R.; Zioncheck, T.F. A target-mediated model to describe the pharmacokinetics and hemodynamic effects of recombinant human vascular endothelial growth factor in humans. Clin. Pharmacol. Ther. 2002, 72, 20–32. [Google Scholar] [CrossRef]
- Piscitelli, S.C.; Reiss, W.G.; Figg, W.D.; Petros, W.P. Pharmacokinetic studies with recombinant cytokines. Scientific issues and practical considerations. Clin. Pharmacokinet. 1997, 32, 368–381. [Google Scholar] [CrossRef]
- Soy, D.; Aweeka, F.T.; Church, J.A.; Cunningham, C.K.; Palumbo, P.; Kosel, B.W.; Sheiner, L.B. Population pharmacokinetics of enfuvirtide in pediatric patients with human immunodeficiency virus: searching for exposure-response relationships. Clin. Pharmacol. Ther. 2003, 74, 569–580. [Google Scholar] [CrossRef]
- Toon, S. The relevance of pharmacokinetics in the development of biotechnology products. Eur. J. Drug. Metab. Pharmacokinet. 1996, 21, 93–103. [Google Scholar] [CrossRef]
- Johnson, V.; Maack, T. Renal extraction, filtration, absorption, and catabolism of growth hormone. Am. J. Physiol. 1977, 233, F185–F196. [Google Scholar]
- Rabkin, R.; Ryan, M.P.; Duckworth, W.C. The renal metabolism of insulin. Diabetologia 1984, 27, 351–357. [Google Scholar] [CrossRef]
- Takagi, A.; Masuda, H.; Takakura, Y.; Hashida, M. Disposition characteristics of recombinant human interleukin-11 after a bolus intravenous administration in mice. J. Pharmacol. Exp. Ther. 1995, 275, 537–543. [Google Scholar]
- Carone, F.A.; Peterson, D.R. Hydrolysis and transport of small peptides by the proximal tubule. Am. J. Physiol. 1980, 238, F151–F158. [Google Scholar]
- Carone, F.A.; Peterson, D.R.; Flouret, G. Renal tubular processing of small peptide hormones. J. Lab. Clin. Med. 1982, 100, 1–14. [Google Scholar]
- Maack, T.; Johnson, V.; Kau, S. T.; Figueiredo, J.; Sigulem, D. Renal filtration, transport, and metabolism of low-molecular-weight proteins: A review. Kidney. Int. 1979, 16, 251–270. [Google Scholar] [CrossRef]
- Nielsen, S.; Nielsen, J.T.; Christensen, E.I. Luminal and basolateral uptake of insulin in isolated, perfused, proximal tubules. Am. J. Physiol. 1987, 253, F857–F867. [Google Scholar]
- Authier, F.; Posner, B.I.; Bergeron, J.J. Endosomal proteolysis of internalized proteins. FEBS Lett. 1996, 389, 55–60. [Google Scholar] [CrossRef]
- Meibohm, B.; Derendorf, H. Pharmacokinetics and Pharmacodynamics of Biotech Drugs; Wiley-VCH: Weinheim, Germany, 2003; pp. 35–36. [Google Scholar]
- Racine-Poon, A.; Botta, L.; Chang, T.W.; Davis, F.M.; Gygax, D.; Liou, R.S.; Rohane, P.; Staehelin, T.; van Steijn, A.M.; Frank, W. Efficacy, pharmacodynamics, and pharmacokinetics of CGP 51901, an anti-immunoglobulin E chimeric monoclonal antibody, in patients with seasonal allergic rhinitis. Clin. Pharmacol. Ther. 1997, 62, 675–690. [Google Scholar] [CrossRef]
- Schulman, E.S. Development of a monoclonal anti-immunoglobulin E antibody (omalizumab) for the treatment of allergic respiratory disorders. Am. J. Respir. Crit. Care Med. 2001, 164, S6–11. [Google Scholar] [CrossRef]
- Kovarik, J.M.; Offner, G.; Broyer, M.; Niaudet, P.; Loirat, C.; Mentser, M.; Lemire, J.; Crocker, J.F.; Cochat, P.; Clark, G.; Gerbeau, C.; Chodoff, L.; Korn, A.; Hall, M. A rational dosing algorithm for basiliximab (Simulect) in pediatric renal transplantation based on pharmacokinetic-dynamic evaluations. Transplantation 2002, 74, 966–971. [Google Scholar] [CrossRef]
- Kovarik, J.M.; Kahan, B.D.; Rajagopalan, P.R.; Bennett, W.; Mulloy, L.L.; Gerbeau, C.; Hall, M.L. Population pharmacokinetics and exposure-response relationships for basiliximab in kidney transplantation. The U.S. Simulect Renal Transplant Study Group. Transplantation 1999, 68, 1288–1294. [Google Scholar] [CrossRef]
- Kovarik, J.M.; Gridelli, B.G.; Martin, S.; Rodeck, B.; Melter, M.; Dunn, S.P.; Merion, R.M.; Tzakis, A.G.; Alonso, E.; Bucuvalas, J.; Sharp, H.; Gerbeau, C.; Chodoff, L.; Korn, A.; Hall, M. Basiliximab in pediatric liver transplantation: A pharmacokinetic-derived dosing algorithm. Pediatr. Transplant 2002, 6, 224–230. [Google Scholar] [CrossRef]
- Hocker, B.; Kovarik, J.M.; Daniel, V.; Opelz, G.; Fehrenbach, H.; Holder, M.; Hoppe, B.; Hoyer, P.; Jungraithmayr, T.C.; Kopf-Shakib, S.; Laube, G.F.; Muller-Wiefel, D.E.; Offner, G.; Plank, C.; Schroder, M.; Weber, L.T.; Zimmerhackl, L.B.; Tonshoff, B. Pharmacokinetics and immunodynamics of basiliximab in pediatric renal transplant recipients on mycophenolate mofetil comedication. Transplantation 2008, 86, 1234–1240. [Google Scholar] [CrossRef]
- Kovarik, J.M.; Pescovitz, M.D.; Sollinger, H.W.; Kaplan, B.; Legendre, C.; Salmela, K.; Book, B.K.; Gerbeau, C.; Girault, D.; Somberg, K. Differential influence of azathioprine and mycophenolate mofetil on the disposition of basiliximab in renal transplant patients. Clin. Transplant 2001, 15, 123–130. [Google Scholar] [CrossRef]
- Pescovitz, M.D.; Knechtle, S.; Alexander, S.R.; Colombani, P.; Nevins, T.; Nieforth, K.; Bouw, M.R. Safety and pharmacokinetics of daclizumab in pediatric renal transplant recipients. Pediatr. Transplant 2008, 12, 447–455. [Google Scholar] [CrossRef]
- Saez-Llorens, X.; Castano, E.; Null, D.; Steichen, J.; Sanchez, P.J.; Ramilo, O.; Top, F.H., Jr.; Connor, E. Safety and pharmacokinetics of an intramuscular humanized monoclonal antibody to respiratory syncytial virus in premature infants and infants with bronchopulmonary dysplasia. The MEDI-493 Study Group. Pediatr. Infect. Dis. J. 1998, 17, 787–791. [Google Scholar]
- Saez-Llorens, X.; Moreno, M.T.; Ramilo, O.; Sanchez, P.J.; Top, F.H., Jr.; Connor, E.M. Safety and pharmacokinetics of palivizumab therapy in children hospitalized with respiratory syncytial virus infection. Pediatr. Infect. Dis. J. 2004, 23, 707–712. [Google Scholar] [CrossRef]
- Burns, J.C.; Best, B.M.; Mejias, A.; Mahony, L.; Fixler, D.E.; Jafri, H.S.; Melish, M.E.; Jackson, M.A.; Asmar, B.I.; Lang, D.J.; Connor, J.D.; Capparelli, E.V.; Keen, M.L.; Mamun, K.; Keenan, G.F.; Ramilo, O. Infliximab treatment of intravenous immunoglobulin-resistant Kawasaki disease. J. Pediatr. 2008, 153, 833–838. [Google Scholar]
- Ruperto, N.; Lovell, D.J.; Cuttica, R.; Wilkinson, N.; Woo, P.; Espada, G.; Wouters, C.; Silverman, E.D.; Balogh, Z.; Henrickson, M.; Apaz, M.T.; Baildam, E.; Fasth, A.; Gerloni, V.; Lahdenne, P.; Prieur, A.M.; Ravelli, A.; Saurenmann, R.K.; Gamir, M.L.; Wulffraat, N.; Marodi, L.; Petty, R.E.; Joos, R.; Zulian, F.; McCurdy, D.; Myones, B.L.; Nagy, K.; Reuman, P.; Szer, I.; Travers, S.; Beutler, A.; Keenan, G.; Clark, J.; Visvanathan, S.; Fasanmade, A.; Raychaudhuri, A.; Mendelsohn, A.; Martini, A.; Giannini, E.H. A randomized, placebo-controlled trial of infliximab plus methotrexate for the treatment of polyarticular-course juvenile rheumatoid arthritis. Arthritis Rheum. 2007, 56, 3096–3106. [Google Scholar] [CrossRef]
- Ternant, D.; Mulleman, D.; Degenne, D.; Willot, S.; Guillaumin, J.M.; Watier, H.; Goupille, P.; Paintaud, G. An enzyme-linked immunosorbent assay for therapeutic drug monitoring of infliximab. Ther. Drug Monit. 2006, 28, 169–174. [Google Scholar] [CrossRef]
- Cornillie, F.; Shealy, D.; D'Haens, G.; Geboes, K.; Van Assche, G.; Ceuppens, J.; Wagner, C.; Schaible, T.; Plevy, S.E.; Targan, S.R.; Rutgeerts, P. Infliximab induces potent anti-inflammatory and local immunomodulatory activity but no systemic immune suppression in patients with Crohn's disease. Aliment. Pharmacol. Ther. 2001, 15, 463–473. [Google Scholar] [CrossRef]
- St Clair, E.W.; Wagner, C.L.; Fasanmade, A.A.; Wang, B.; Schaible, T.; Kavanaugh, A.; Keystone, E.C. The relationship of serum infliximab concentrations to clinical improvement in rheumatoid arthritis: results from ATTRACT, a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2002, 46, 1451–1459. [Google Scholar]
- Sleight, B.S.; Chan, K.W.; Braun, T.M.; Serrano, A.; Gilman, A.L. Infliximab for GVHD therapy in children. Bone Marrow Transplant. 2007, 40, 473–480. [Google Scholar] [CrossRef]
- Buckwalter, M.; Dowell, J.A.; Korth-Bradley, J.; Gorovits, B.; Mayer, P.R. Pharmacokinetics of gemtuzumab ozogamicin as a single-agent treatment of pediatric patients with refractory or relapsed acute myeloid leukemia. J. Clin. Pharmacol. 2004, 44, 873–880. [Google Scholar] [CrossRef]
- Dowell, J.A.; Korth-Bradley, J.; Liu, H.; King, S.P.; Berger, M.S. Pharmacokinetics of gemtuzumab ozogamicin, an antibody-targeted chemotherapy agent for the treatment of patients with acute myeloid leukemia in first relapse. J. Clin. Pharmacol. 2001, 41, 1206–1214. [Google Scholar] [CrossRef]
- Angiolillo, A.L.; Yu, A.L.; Reaman, G.; Ingle, A.M.; Secola, R.; Adamson, P.C. A phase II study of Campath-1H in children with relapsed or refractory acute lymphoblastic leukemia: a Children's Oncology Group report. Pediatr. Blood Cancer 2009, 53, 978–983. [Google Scholar] [CrossRef]
- Mould, D.R.; Baumann, A.; Kuhlmann, J.; Keating, M.J.; Weitman, S.; Hillmen, P.; Brettman, L.R.; Reif, S.; Bonate, P.L. Population pharmacokinetics-pharmacodynamics of alemtuzumab (Campath) in patients with chronic lymphocytic leukaemia and its link to treatment response. Br. J. Clin. Pharmacol. 2007, 64, 278–291. [Google Scholar] [CrossRef]
- Montillo, M.; Tedeschi, A.; Miqueleiz, S.; Veronese, S.; Cairoli, R.; Intropido, L.; Ricci, F.; Colosimo, A.; Scarpati, B.; Montagna, M.; Nichelatti, M.; Regazzi, M.; Morra, E. Alemtuzumab as consolidation after a response to fludarabine is effective in purging residual disease in patients with chronic lymphocytic leukemia. J. Clin. Oncol. 2006, 24, 2337–2342. [Google Scholar]
- Hale, G.; Rebello, P.; Brettman, L.R.; Fegan, C.; Kennedy, B.; Kimby, E.; Leach, M.; Lundin, J.; Mellstedt, H.; Moreton, P.; Rawstron, A.C.; Waldmann, H.; Osterborg, A.; Hillmen, P. Blood concentrations of alemtuzumab and antiglobulin responses in patients with chronic lymphocytic leukemia following intravenous or subcutaneous routes of administration. Blood 2004, 104, 948–955. [Google Scholar] [CrossRef]
- Trippett, T.M.; Herzog, C.; Whitlock, J.A.; Wolff, J.; Kuttesch, J.; Bagatell, R.; Hunger, S.P.; Boklan, J.; Smith, A.A.; Arceci, R.J.; Katzenstein, H.M.; Harbison, C.; Zhou, X.; Lu, H.; Langer, C.; Weber, M.; Gore, L. Phase I and pharmacokinetic study of cetuximab and irinotecan in children with refractory solid tumors: A study of the pediatric oncology experimental therapeutic investigators' consortium. J. Clin. Oncol. 2009, 27, 5102–5108. [Google Scholar] [CrossRef]
- Tan, A.R.; Moore, D.F.; Hidalgo, M.; Doroshow, J.H.; Poplin, E.A.; Goodin, S.; Mauro, D.; Rubin, E.H. Pharmacokinetics of cetuximab after administration of escalating single dosing and weekly fixed dosing in patients with solid tumors. Clin. Cancer Res. 2006, 12, 6517–6522. [Google Scholar] [CrossRef]
- Glade Bender, J.L.; Adamson, P.C.; Reid, J.M.; Xu, L.; Baruchel, S.; Shaked, Y.; Kerbel, R.S.; Cooney-Qualter, E.M.; Stempak, D.; Chen, H.X.; Nelson, M.D.; Krailo, M.D.; Ingle, A.M.; Blaney, S.M.; Kandel, J.J.; Yamashiro, D.J. Phase I trial and pharmacokinetic study of bevacizumab in pediatric patients with refractory solid tumors: A Children's Oncology Group Study. J. Clin. Oncol. 2008, 26, 399–405. [Google Scholar] [CrossRef]
- Gordon, M.S.; Margolin, K.; Talpaz, M.; Sledge, G.W., Jr.; Holmgren, E.; Benjamin, R.; Stalter, S.; Shak, S.; Adelman, D. Phase I safety and pharmacokinetic study of recombinant human anti-vascular endothelial growth factor in patients with advanced cancer. J. Clin. Oncol. 2001, 19, 843–850. [Google Scholar]
- Lu, J.F.; Bruno, R.; Eppler, S.; Novotny, W.; Lum, B.; Gaudreault, J. Clinical pharmacokinetics of bevacizumab in patients with solid tumors. Cancer Chemother. Pharmacol. 2008, 62, 779–786. [Google Scholar] [CrossRef]
- Hyams, J.S.; Wilson, D.C.; Thomas, A.; Heuschkel, R.; Mitton, S.; Mitchell, B.; Daniels, R.; Libonati, M.A.; Zanker, S.; Kugathasan, S. Natalizumab therapy for moderate to severe Crohn disease in adolescents. J. Pediatr. Gastroenterol. Nutr. 2007, 44, 185–191. [Google Scholar] [CrossRef]
- Freeman, B.B., 3rd; Hinds, P.; Iacono, L.C.; Razzouk, B.I.; Burghen, E.; Stewart, C.F. Pharmacokinetics and pharmacodynamics of intravenous epoetin alfa in children with cancer. Pediatr. Blood Cancer 2006, 47, 572–579. [Google Scholar] [CrossRef]
- Lim, V.S.; DeGowin, R.L.; Zavala, D.; Kirchner, P.T.; Abels, R.; Perry, P.; Fangman, J. Recombinant human erythropoietin treatment in pre-dialysis patients. A double-blind placebo-controlled trial. Ann. Intern. Med. 1989, 110, 108–114. [Google Scholar] [CrossRef]
- Evans, J.H.; Brocklebank, J.T.; Bowmer, C.J.; Ng, P.C. Pharmacokinetics of recombinant human erythropoietin in children with renal failure. Nephrol. Dial. Transplant 1991, 6, 709–714. [Google Scholar] [CrossRef]
- Widness, J.A.; Veng-Pedersen, P.; Peters, C.; Pereira, L.M.; Schmidt, R.L.; Lowe, L.S. Erythropoietin pharmacokinetics in premature infants: developmental, nonlinearity, and treatment effects. J. Appl. Physiol. 1996, 80, 140–148. [Google Scholar]
- Ohls, R.K.; Veerman, M.W.; Christensen, R.D. Pharmacokinetics and effectiveness of recombinant erythropoietin administered to preterm infants by continuous infusion in total parenteral nutrition solution. J. Pediatr. 1996, 128, 518–523. [Google Scholar] [CrossRef]
- Knebel, W.; Palmen, M.; Dowell, J.A.; Gastonguay, M. Population pharmacokinetic modeling of epoetin delta in pediatric patients with chronic kidney disease. J. Clin. Pharmacol. 2008, 48, 837–848. [Google Scholar] [CrossRef]
- Smith, W.B.; Dowell, J.A.; Pratt, R.D. Pharmacokinetics and pharmacodynamics of epoetin delta in two studies in healthy volunteers and two studies in patients with chronic kidney disease. Clin. Ther. 2007, 29, 1368–1380. [Google Scholar] [CrossRef]
- Lerner, G.; Kale, A.S.; Warady, B.A.; Jabs, K.; Bunchman, T.E.; Heatherington, A.; Olson, K.; Messer-Mann, L.; Maroni, B.J. Pharmacokinetics of darbepoetin alfa in pediatric patients with chronic kidney disease. Pediatr. Nephrol. 2002, 17, 933–937. [Google Scholar] [CrossRef]
- Macdougall, I.C.; Gray, S.J.; Elston, O.; Breen, C.; Jenkins, B.; Browne, J.; Egrie, J. Pharmacokinetics of novel erythropoiesis stimulating protein compared with epoetin alfa in dialysis patients. J. Am. Soc. Nephrol. 1999, 10, 2392–2395. [Google Scholar]
- Ateshkadi, A.; Johnson, C.A.; Oxton, L.L.; Hammond, T.G.; Bohenek, W.S.; Zimmerman, S.W. Pharmacokinetics of intraperitoneal, intravenous, and subcutaneous recombinant human erythropoietin in patients on continuous ambulatory peritoneal dialysis. Am. J. Kidney Dis. 1993, 21, 635–642. [Google Scholar]
- Macdougall, I.C.; Roberts, D.E.; Coles, G.A.; Williams, J.D. Clinical pharmacokinetics of epoetin (recombinant human erythropoietin). Clin. Pharmacokinet. 1991, 20, 99–113. [Google Scholar] [CrossRef]
- Blumer, J.; Berg, S.; Adamson, P.C.; Loew, T.; Rossi, G.; Hastings, C. Pharmacokinetic evaluation of darbepoetin alfa for the treatment of pediatric patients with chemotherapy-induced anemia. Pediatr. Blood Cancer 2007, 49, 687–693. [Google Scholar] [CrossRef]
- De Palo, T.; Giordano, M.; Palumbo, F.; Bellantuono, R.; Messina, G.; Colella, V.; Caringella, A.D. Clinical experience with darbepoietin alfa (NESP) in children undergoing hemodialysis. Pediatr. Nephrol. 2004, 19, 337–340. [Google Scholar]
- Faulkner, L.B.; Tucci, F.; Tamburini, A.; Tintori, V.; Lippi, A.A.; Bambi, F.; Malentacca, F.; Azzari, C.; Gelli, A.M.; Genovese, F.; Bernini, G. G-CSF serum pharmacokinetics during peripheral blood progenitor cell mobilization: Neutrophil count-adjusted dosage might potentially improve mobilization and be more cost-effective. Bone Marrow Transplant. 1998, 21, 1091–1095. [Google Scholar]
- Wells, R.J.; Weck, P.K.; Baehner, R.L.; Krivit, W.; Raney, R.B.; Ortega, J.A.; Bernstein, I.O.; Lampkin, B.; Whisnant, J.K.; Sather, H.N. Interferon-alpha n1 in children with recurrent acute lymphocytic leukemia: a phase I study of pharmacokinetics and tolerance. J. Interferon Res. 1988, 8, 309–318. [Google Scholar]
- Knost, J.A.; Sherwin, S.A.; Abrams, P.G.; Ochs, J.J.; Foon, K.A.; Williams, R.; Tuttle, R.; Oldham, R.K. The treatment of cancer patients with human lymphoblastoid interferon. A comparison of two routes of administration. Cancer Immunol. Immunother. 1983, 15, 144–148. [Google Scholar]
- Schwarz, K.B.; Mohan, P.; Narkewicz, M.R.; Molleston, J.P.; Nash, S.R.; Hu, S.; Wang, K.; Gries, J.M. Safety, efficacy and pharmacokinetics of peginterferon alpha2a (40 kd) in children with chronic hepatitis C. J. Pediatr. Gastroenterol. Nutr. 2006, 43, 499–505. [Google Scholar] [CrossRef]
- Zeuzem, S.; Feinman, S.V.; Rasenack, J.; Heathcote, E.J.; Lai, M.Y.; Gane, E.; O'Grady, J.; Reichen, J.; Diago, M.; Lin, A.; Hoffman, J.; Brunda, M.J. Peginterferon alfa-2a in patients with chronic hepatitis C. N. Engl. J. Med. 2000, 343, 1666–1672. [Google Scholar] [CrossRef]
- Gonzalez-Peralta, R.P.; Kelly, D.A.; Haber, B.; Molleston, J.; Murray, K.F.; Jonas, M.M.; Shelton, M.; Mieli-Vergani, G.; Lurie, Y.; Martin, S.; Lang, T.; Baczkowski, A.; Geffner, M.; Gupta, S.; Laughlin, M. Interferon alfa-2b in combination with ribavirin for the treatment of chronic hepatitis C in children: efficacy, safety, and pharmacokinetics. Hepatology 2005, 42, 1010–1018. [Google Scholar] [CrossRef]
- Villar, A.; Aronis, S.; Morfini, M.; Santagostino, E.; Auerswald, G.; Thomsen, H.F.; Erhardtsen, E.; Giangrande, P.L. Pharmacokinetics of activated recombinant coagulation factor VII (NovoSeven) in children vs. adults with haemophilia A. Haemophilia 2004, 10, 352–359. [Google Scholar]
- Murry, D.J.; Crom, W.R.; Reddick, W.E.; Bhargava, R.; Evans, W.E. Liver volume as a determinant of drug clearance in children and adolescents. Drug Metab. Disposition 1995, 23, 1110–1116. [Google Scholar]
- Klitgaard, T.; Nielsen, T.G. Overview of the human pharmacokinetics of recombinant activated factor VII. Br. J. Clin. Pharmacol. 2008, 65, 3–11. [Google Scholar] [CrossRef]
- Blanchette, V.S.; Shapiro, A.D.; Liesner, R.J.; Hernandez Navarro, F.; Warrier, I.; Schroth, P.C.; Spotts, G.; Ewenstein, B.M. Plasma and albumin-free recombinant factor VIII: pharmacokinetics, efficacy and safety in previously treated pediatric patients. J. Thromb. Haemost. 2008, 6, 1319–1326. [Google Scholar] [CrossRef]
- Tarantino, M.D.; Collins, P.W.; Hay, C.R.; Shapiro, A.D.; Gruppo, R.A.; Berntorp, E.; Bray, G.L.; Tonetta, S.A.; Schroth, P.C.; Retzios, A.D.; Rogy, S.S.; Sensel, M.G.; Ewenstein, B.M. Clinical evaluation of an advanced category antihaemophilic factor prepared using a plasma/albumin-free method: Pharmacokinetics, efficacy, and safety in previously treated patients with haemophilia A. Haemophilia 2004, 10, 428–437. [Google Scholar] [CrossRef]
- Gale, R.F.; Hird, M.F.; Colvin, B.T. Management of a premature infant with moderate haemophilia A using recombinant factor VIII. Haemophilia 1998, 4, 850–853. [Google Scholar]
- Carlsson, M.; Berntorp, E.; Björkman, S.; Lethagen, S.; Ljung, R. Improved cost-effectiveness by pharmacokinetic dosing of factor VIII in prophylactic treatment of haemophilia A. Haemophilia 2007, 3, 96–101. [Google Scholar]
- Bolon-Larger, M.; Chamouard, V.; Bressolle, F.; Boulieu, R. A limited sampling strategy for estimating individual pharmacokinetic parameters of coagulation factor VIII in patients with hemophilia A. Ther. Drug Monit. 2007, 29, 20–26. [Google Scholar] [CrossRef]
- Bjorkman, S.; Folkesson, A.; Jonsson, S. Pharmacokinetics and dose requirements of factor VIII over the age range 3-74 years: a population analysis based on 50 patients with long-term prophylactic treatment for haemophilia A. Eur. J. Clin. Pharmacol. 2009, 65, 989–998. [Google Scholar] [CrossRef]
- Matucci, M.; Messori, A.; Donati-Cori, G.; Longo, G.; Vannini, S.; Morfini, M.; Tendi, E.; Rossi-Ferrini, P.L. Kinetic evaluation of four Factor VIII concentrates by model-independent methods. Scand. J. Haematol. 1985, 34, 22–28. [Google Scholar]
- van Dijk, K.; van der Bom, J.G.; Lenting, P.J.; de Groot, P.G.; Mauser-Bunschoten, E.P.; Roosendaal, G.; Grobbee, D.E.; van den Berg, H.M. Factor VIII half-life and clinical phenotype of severe hemophilia A. Haematologica 2005, 90, 494–498. [Google Scholar]
- Bjorkman, S.; Folkesson, A.; Berntorp, E. In vivo recovery of factor VIII and factor IX: intra- and interindividual variance in a clinical setting. Haemophilia 2007, 13, 2–8. [Google Scholar] [CrossRef]
- Ahnstrom, J.; Berntorp, E.; Lindvall, K.; Bjorkman, S. A 6-year follow-up of dosing, coagulation factor levels and bleedings in relation to joint status in the prophylactic treatment of haemophilia. Haemophilia 2004, 10, 689–697. [Google Scholar] [CrossRef]
- Bjorkman, S.; Shapiro, A.D.; Berntorp, E. Pharmacokinetics of recombinant factor IX in relation to age of the patient: implications for dosing in prophylaxis. Haemophilia 2001, 7, 133–139. [Google Scholar] [CrossRef]
- Danne, T.; Datz, N.; Endahl, L.; Haahr, H.; Nestoris, C.; Westergaard, L.; Fjording, M.S.; Kordonouri, O. Insulin detemir is characterized by a more reproducible pharmacokinetic profile than insulin glargine in children and adolescents with type 1 diabetes: results from a randomized, double-blind, controlled trial. Pediatr. Diabetes 2008, 9, 554–560. [Google Scholar] [CrossRef]
- Danne, T. Flexibility of rapid-acting insulin analogues in children and adolescents with diabetes mellitus. Clin. Ther. 2007, 29 (Suppl. D), S145–S152. [Google Scholar] [CrossRef]
- Mortensen, H.B.; Lindholm, A.; Olsen, B.S.; Hylleberg, B. Rapid appearance and onset of action of insulin aspart in paediatric subjects with type 1 diabetes. Eur. J. Pediatr. 2000, 159, 483–488. [Google Scholar] [CrossRef]
- Danne, T.; Becker, R.H.; Heise, T.; Bittner, C.; Frick, A.D.; Rave, K. Pharmacokinetics, prandial glucose control, and safety of insulin glulisine in children and adolescents with type 1 diabetes. Diabetes Care 2005, 28, 2100–2105. [Google Scholar]
- Malloy, J.; Capparelli, E.; Gottschalk, M.; Guan, X.; Kothare, P.; Fineman, M. Pharmacology and tolerability of a single dose of exenatide in adolescent patients with type 2 diabetes mellitus being treated with metformin: a randomized, placebo-controlled, single-blind, dose-escalation, crossover study. Clin. Ther. 2009, 31, 806–815. [Google Scholar] [CrossRef]
- Buse, J.B.; Henry, R.R.; Han, J.; Kim, D.D.; Fineman, M.S.; Baron, A.D. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care 2004, 27, 2628–2635. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Ratner, R.E.; Han, J.; Kim, D.D.; Fineman, M.S.; Baron, A.D. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 2005, 28, 1092–1100. [Google Scholar] [CrossRef]
- Kendall, D.M.; Riddle, M.C.; Rosenstock, J.; Zhuang, D.; Kim, D.D.; Fineman, M.S.; Baron, A.D. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care 2005, 28, 1083–1091. [Google Scholar] [CrossRef]
- Walvoord, E.C.; de la Pena, A.; Park, S.; Silverman, B.; Cuttler, L.; Rose, S.R.; Cutler, G.; Drop, S.; Chipman, J.J. Inhaled growth hormone (GH) compared with subcutaneous GH in children with GH deficiency: pharmacokinetics, pharmacodynamics, and safety. J. Clin. Endocrinol. Metab. 2009, 94, 2052–2059. [Google Scholar]
- Peter, F.; Savoy, C.; Ji, H.J.; Juhasz, M.; Bidlingmaier, M.; Saenger, P. Pharmacokinetic and pharmacodynamic profile of a new sustained-release GH formulation, LB03002, in children with GH deficiency. Eur. J. Endocrinol. 2009, 160, 349–355. [Google Scholar]
- Bidlingmaier, M.; Kim, J.; Savoy, C.; Kim, M.J.; Ebrecht, N.; de la Motte, S.; Strasburger, C.J. Comparative pharmacokinetics and pharmacodynamics of a new sustained-release growth hormone (GH), LB03002, versus daily GH in adults with GH deficiency. J. Clin. Endocrinol. Metab. 2006, 91, 2926–2930. [Google Scholar] [CrossRef]
- Kemp, S.F.; Fielder, P.J.; Attie, K.M.; Blethen, S.L.; Reiter, E.O.; Ford, K.M.; Marian, M.; Dao, L.N.; Lee, H.J.; Saenger, P. Pharmacokinetic and pharmacodynamic characteristics of a long-acting growth hormone (GH) preparation (nutropin depot) in GH-deficient children. J. Clin. Endocrinol. Metab. 2004, 89, 3234–3240. [Google Scholar]
- Houdijk, E.C.; Herdes, E.; Delemarre-Van de Waal, H.A. Pharmacokinetics and pharmacodynamics of recombinant human growth hormone by subcutaneous jet- or needle-injection in patients with growth hormone deficiency. Acta Paediatr. 1997, 86, 1301–1307. [Google Scholar] [CrossRef]
- Cairo, M.S.; Davenport, V.; Bessmertny, O.; Goldman, S.C.; Berg, S.L.; Kreissman, S.G.; Laver, J.; Shen, V.; Secola, R.; van de Ven, C.; Reaman, G.H. Phase I/II dose escalation study of recombinant human interleukin-11 following ifosfamide, carboplatin and etoposide in children, adolescents and young adults with solid tumours or lymphoma: a clinical, haematological and biological study. Br. J. Haematol. 2005, 128, 49–58. [Google Scholar] [CrossRef]
- Aoyama, K.; Uchida, T.; Takanuki, F.; Usui, T.; Watanabe, T.; Higuchi, S.; Toyoki, T.; Mizoguchi, H. Pharmacokinetics of recombinant human interleukin-11 (rhIL-11) in healthy male subjects. Br. J. Clin. Pharmacol. 1997, 43, 571–578. [Google Scholar]
- Yim, D.S.; Zhou, H.; Buckwalter, M.; Nestorov, I.; Peck, C.C.; Lee, H. Population pharmacokinetic analysis and simulation of the time-concentration profile of etanercept in pediatric patients with juvenile rheumatoid arthritis. J. Clin. Pharmacol. 2005, 45, 246–256. [Google Scholar] [CrossRef]
- Zhou, H.; Buckwalter, M.; Boni, J.; Mayer, P.; Raible, D.; Wajdula, J.; Fatenejad, S.; Sanda, M. Population-based pharmacokinetics of the soluble TNFr etanercept: A clinical study in 43 patients with ankylosing spondylitis compared with post hoc data from patients with rheumatoid arthritis. Int. J. Clin. Pharmacol. Ther. 2004, 42, 267–276. [Google Scholar]
- Lee, H.; Kimko, H.C.; Rogge, M.; Wang, D.; Nestorov, I.; Peck, C.C. Population pharmacokinetic and pharmacodynamic modeling of etanercept using logistic regression analysis. Clin. Pharmacol. Ther. 2003, 73, 348–365. [Google Scholar] [CrossRef]
- Wiznia, A.; Church, J.; Emmanuel, P.; Eppes, S.; Rowell, L.; Evans, C.; Bertasso, A. Safety and efficacy of enfuvirtide for 48 weeks as part of an optimized antiretroviral regimen in pediatric human immunodeficiency virus 1-infected patients. Pediatr. Infect. Dis. J. 2007, 26, 799–805. [Google Scholar] [CrossRef]
- Church, J.A.; Cunningham, C.; Hughes, M.; Palumbo, P.; Mofenson, L.M.; Delora, P.; Smith, E.; Wiznia, A.; Purdue, L.; Hawkins, E.; Sista, P. Safety and antiretroviral activity of chronic subcutaneous administration of T-20 in human immunodeficiency virus 1-infected children. Pediatr. Infect. Dis. J. 2002, 21, 653–659. [Google Scholar] [CrossRef]
- Church, J.A.; Hughes, M.; Chen, J.; Palumbo, P.; Mofenson, L.M.; Delora, P.; Smith, E.; Wiznia, A.; Hawkins, E.; Sista, P.; Cunningham, C.K. Long term tolerability and safety of enfuvirtide for human immunodeficiency virus 1-infected children. Pediatr. Infect. Dis. J. 2004, 23, 713–718. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, T.; Bertasso, A.; Evans, C.; Dorr, A.; Kolis, S.J.; Salgo, M.; Patel, I. Population pharmacokinetics of enfuvirtide in HIV-1-infected pediatric patients over 48 weeks of treatment. J. Clin. Pharmacol. 2007, 47, 510–517. [Google Scholar] [CrossRef]
- Patel, I.H.; Zhang, X.; Nieforth, K.; Salgo, M.; Buss, N. Pharmacokinetics, pharmacodynamics and drug interaction potential of enfuvirtide. Clin. Pharmacokinet. 2005, 44, 175–186. [Google Scholar] [CrossRef]
- Mould, D.R.; Zhang, X.; Nieforth, K.; Salgo, M.; Buss, N.; Patel, I.H. Population pharmacokinetics and exposure-response relationship of enfuvirtide in treatment-experienced human immunodeficiency virus type 1-infected patients. Clin. Pharmacol. Ther. 2005, 77, 515–528. [Google Scholar] [CrossRef]
- Vieira Pinheiro, J.P.; Lanversa, C.; Wurthwein, G.; Beier, R.; Casimiro da Palma, J.; von Stackelberg, A.; Boos, J. Drug monitoring of PEG-asparaginase treatment in childhood acute lymphoblastic leukemia and non-Hodgkin's lymphoma. Leuk. Lymphoma. 2002, 43, 1911–1920. [Google Scholar] [CrossRef]
- Baillargeon, J.; Langevin, A.M.; Lewis, M.; Thomas, P.J.; Mullins, J.; Dugan, J.; Pollock, B.H. L-asparaginase as a marker of chemotherapy dose modification in children with acute lymphoblastic leukemia. Cancer 2005, 104, 2858–2861. [Google Scholar] [CrossRef]
- Anderson, B.J.; Allegaert, K.; Holford, N.H. Population clinical pharmacology of children: modelling covariate effects. Eur. J. Pediatr. 2006, 165, 819–829. [Google Scholar] [CrossRef]
- Anderson, B.J.; Holford, N.H. Mechanism-based concepts of size and maturity in pharmacokinetics. Annu. Rev. Pharmacol. Toxicol. 2008, 48, 303–332. [Google Scholar] [CrossRef]
- Anderson, B.J.; Holford, N.H. Mechanistic basis of using body size and maturation to predict clearance in humans. Drug Metab. Pharmacokinet. 2009, 24, 25–36. [Google Scholar] [CrossRef]
- Sehgal, N. Hospitalization rate surges 119% in pre-teens due to eating disorders--study. Available online: www.themoneytimes.com/featured/20101129/hospitalization-rate-surges-119-preteens-dueeating-disordersstudy-id-10143305.htm (accessed on 3 December 2010).
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Shi, R.; Derendorf, H. Pediatric Dosing and Body Size in Biotherapeutics. Pharmaceutics 2010, 2, 389-418. https://doi.org/10.3390/pharmaceutics2040389
Shi R, Derendorf H. Pediatric Dosing and Body Size in Biotherapeutics. Pharmaceutics. 2010; 2(4):389-418. https://doi.org/10.3390/pharmaceutics2040389
Chicago/Turabian StyleShi, Rong, and Hartmut Derendorf. 2010. "Pediatric Dosing and Body Size in Biotherapeutics" Pharmaceutics 2, no. 4: 389-418. https://doi.org/10.3390/pharmaceutics2040389
APA StyleShi, R., & Derendorf, H. (2010). Pediatric Dosing and Body Size in Biotherapeutics. Pharmaceutics, 2(4), 389-418. https://doi.org/10.3390/pharmaceutics2040389




