Licochalcone A Suppresses Renal Cancer Cell Proliferation and Metastasis by Engagement of Sp1-Mediated LC3 Expression
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Reagent and Culture Medium
2.2. RCC Cell Culture
2.3. Cell Viability Assay
2.4. Cell Cytotoxicity Assay
2.5. Colony Formation Assay
2.6. Cell Cycle Phase
2.7. In Vitro Cell Migration and Invasion
2.8. siRNA and Plasmid Transfection
2.9. Western Blotting
2.10. Real-Time Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
2.11. Acidic Vesicular Organelle Staining
2.12. Observation of LC3 by Immunofluorescence Staining
2.13. Chromatin Immunoprecipitation (ChIP)
2.14. In Vivo Tumorigenesis Assay
2.15. Detection of Putative Transcription Factor Binding Sites of LC3 Promoter
2.16. Statistical Analysis
3. Results
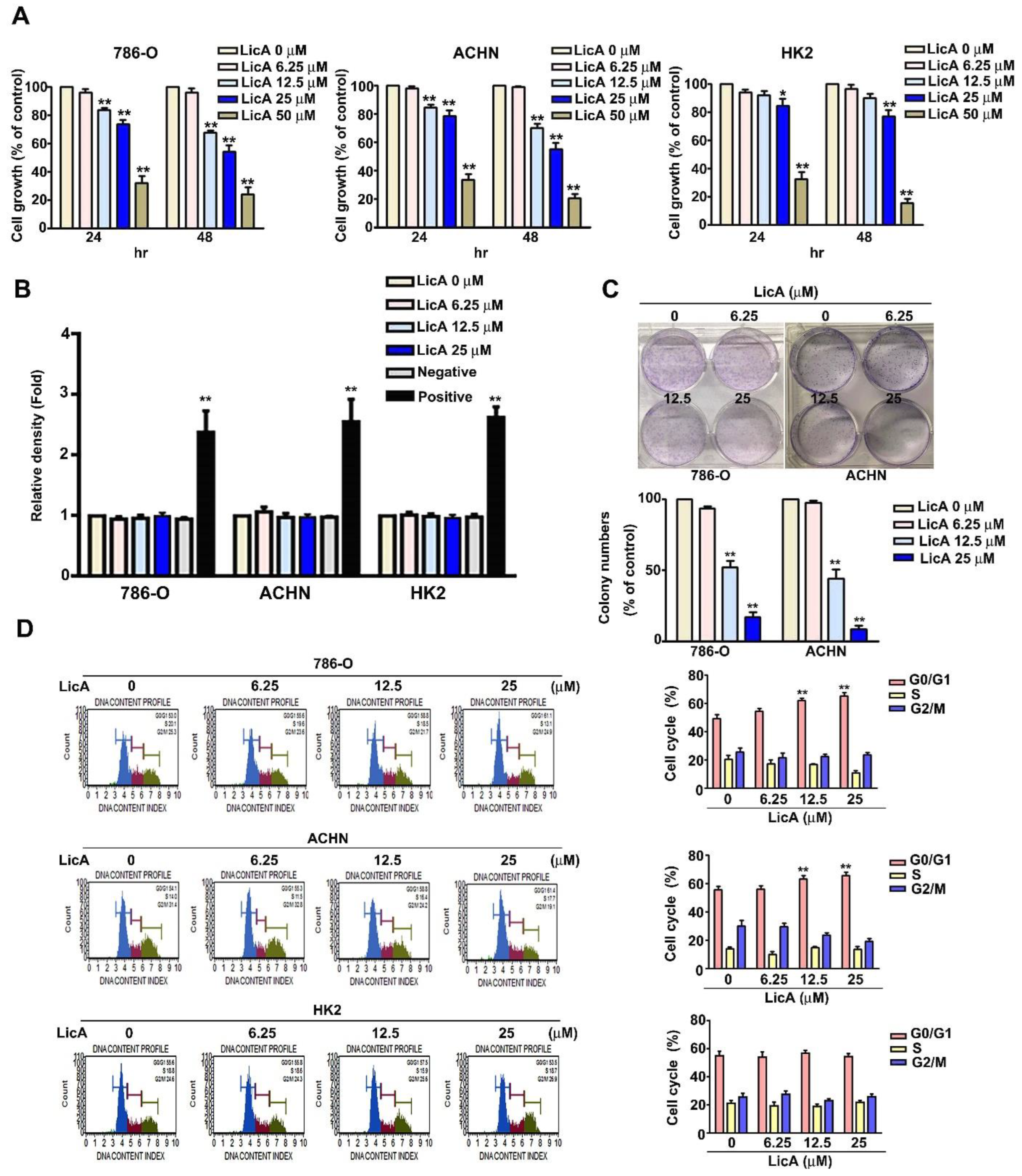
3.1. LicA Inhibited the Growth and Induced Cell Cycle Arrest of 786-O and ACHN Cells
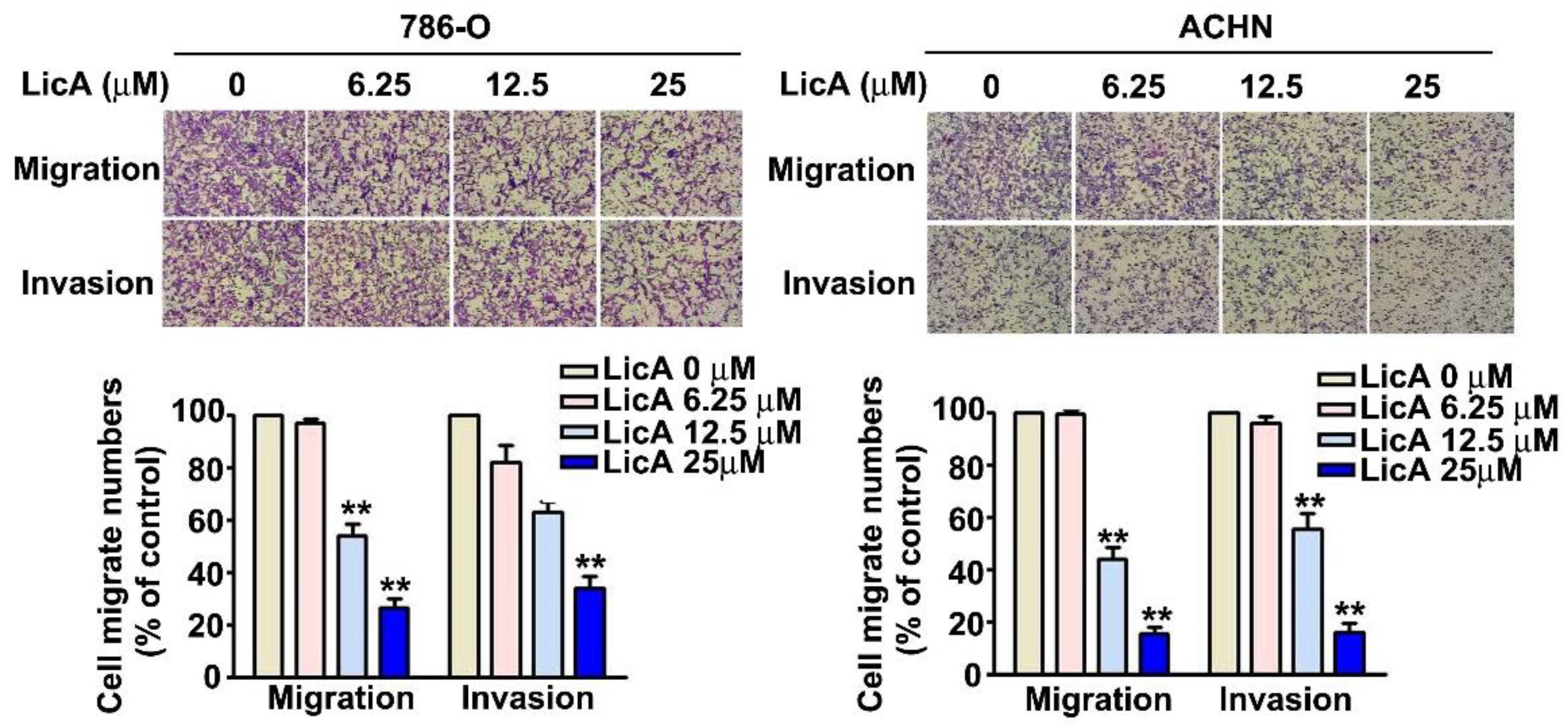
3.2. LicA Suppressed Invasion and Migration of 786-O and ACHN Cells
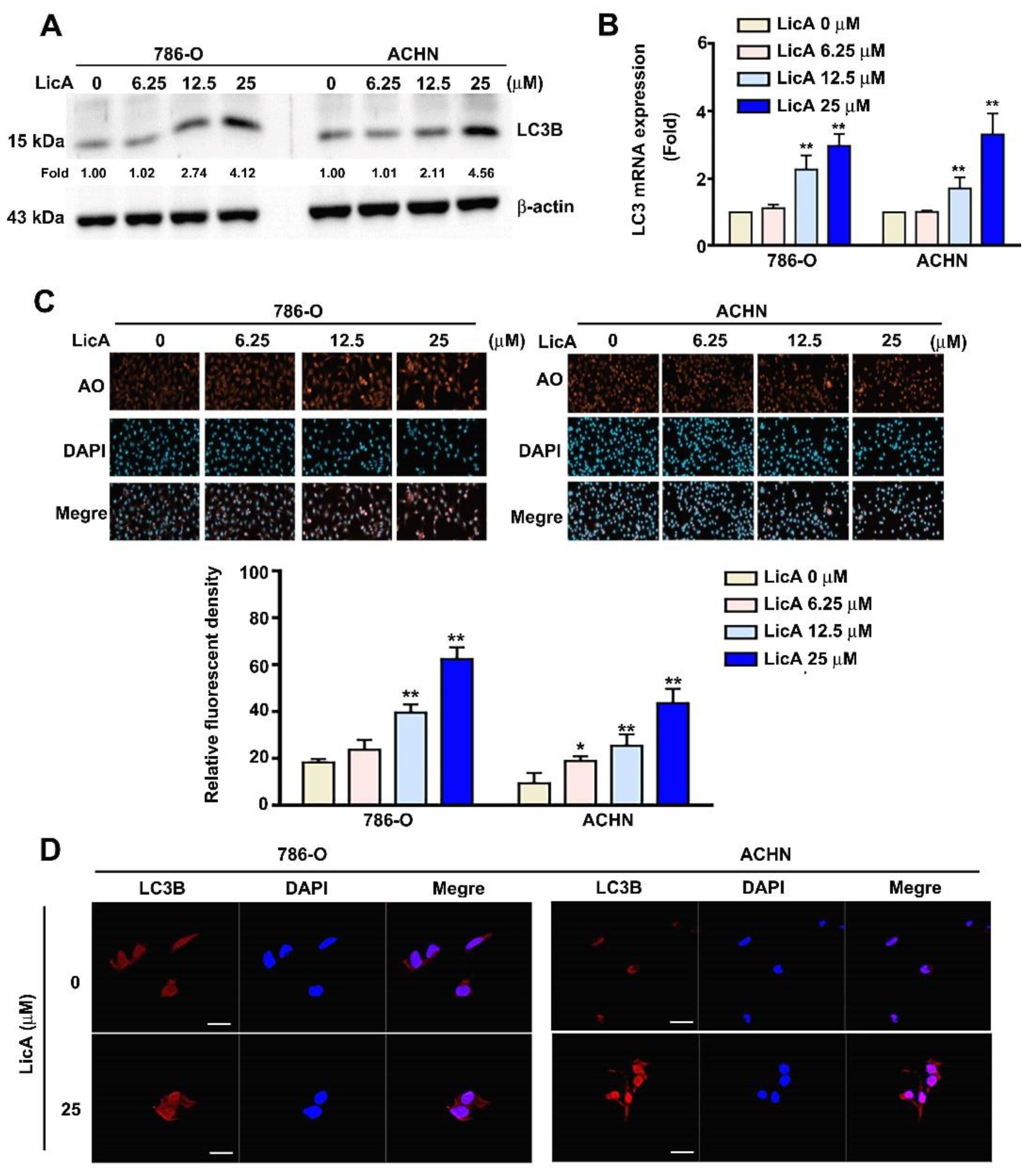
3.3. LicA Induced Autophagy of 786-O and ACHN Cells
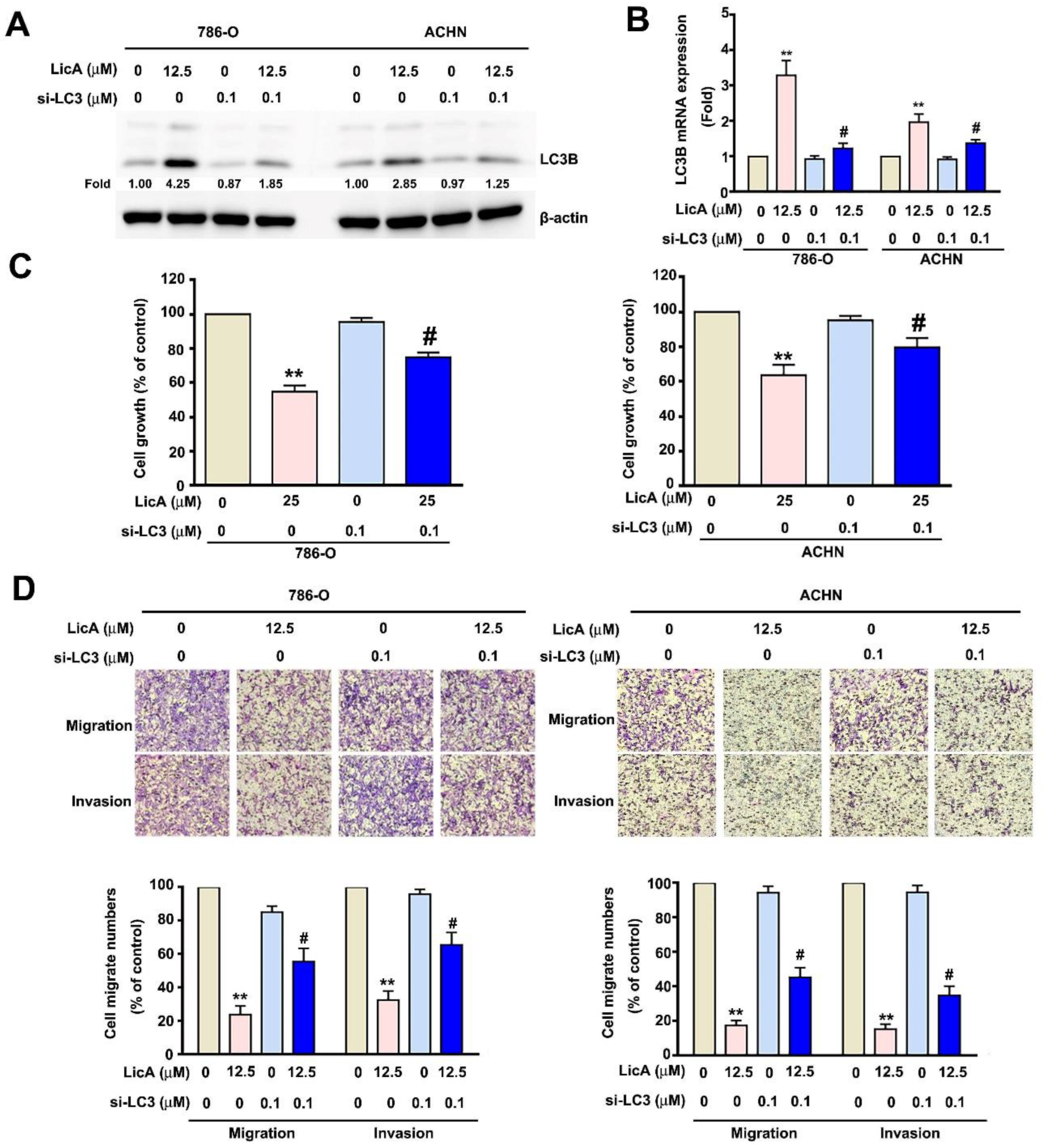
3.4. Downregulated Expression of LC3B Enhanced the Malignant Potential of 786-O and ACHN Cells
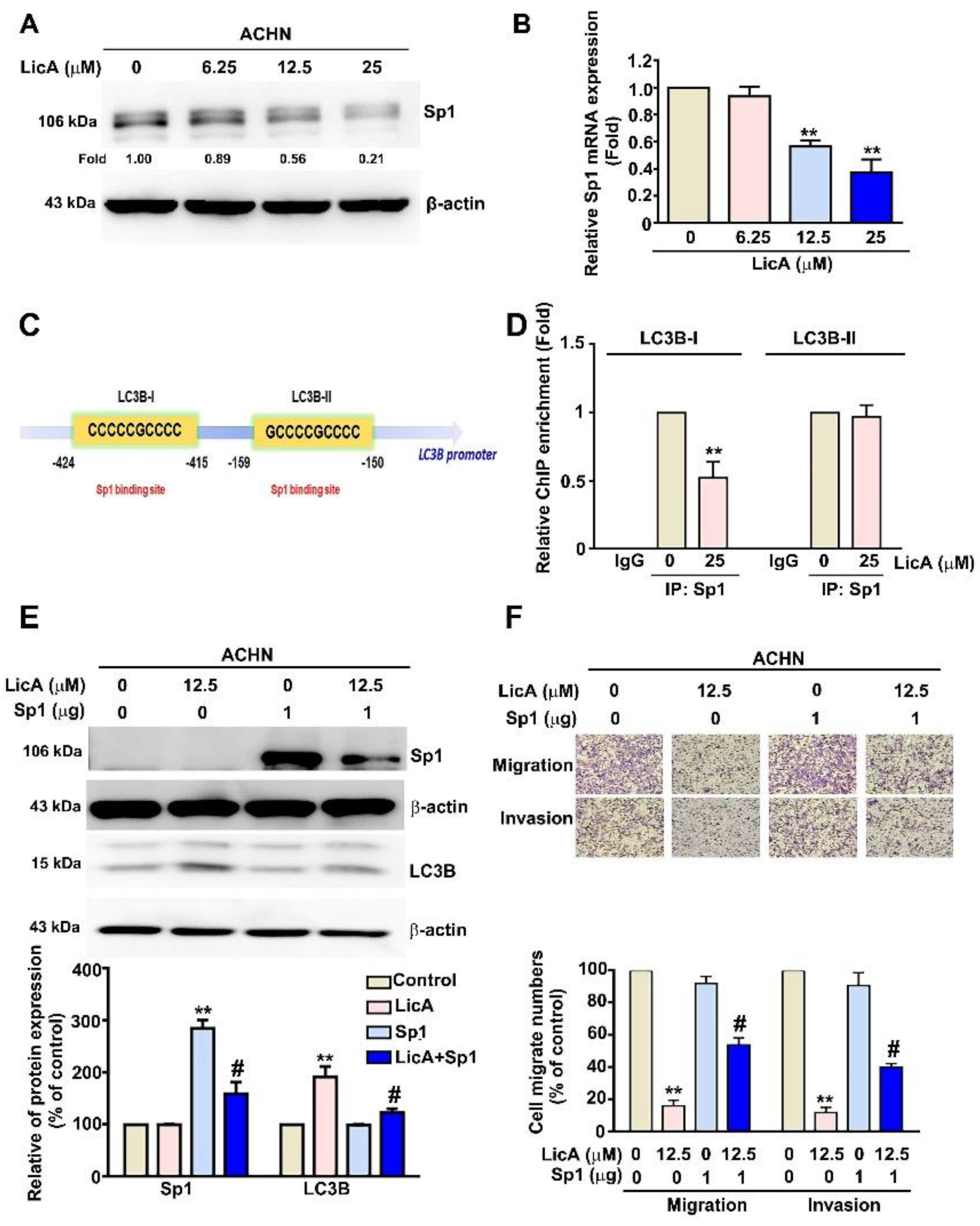
3.5. LicA Increased the Expression of LC3B by Inhibiting the Activation of Sp1 in ACHN Cells
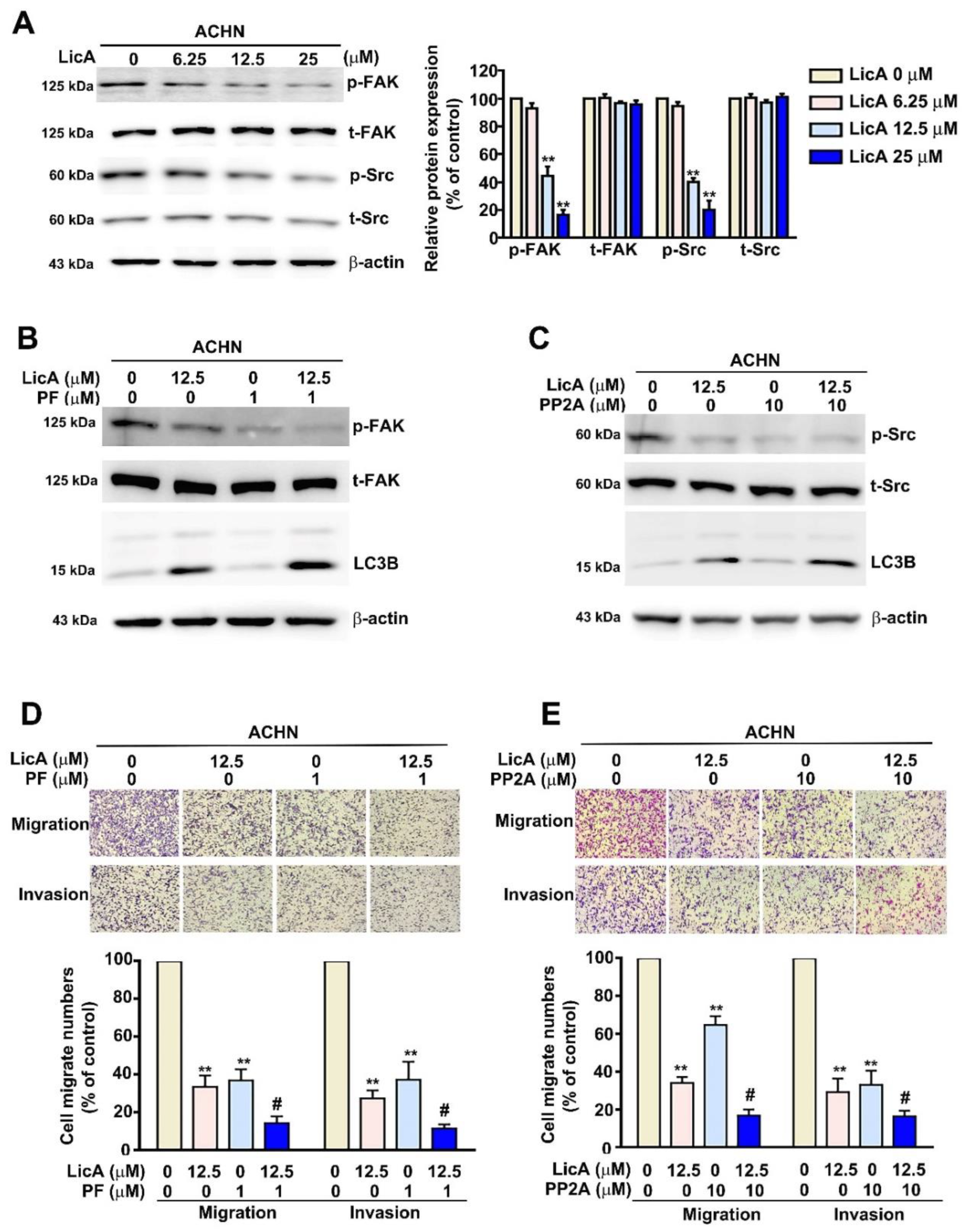
3.6. LicA Inhibited Tumorigenesis and Metastasis via the FAK/Src Signaling Pathway in ACHN Cells
3.7. Antitumorigenesis of LicA in 786-O Xenografts: In Vivo Animal Assay
4. Discussion
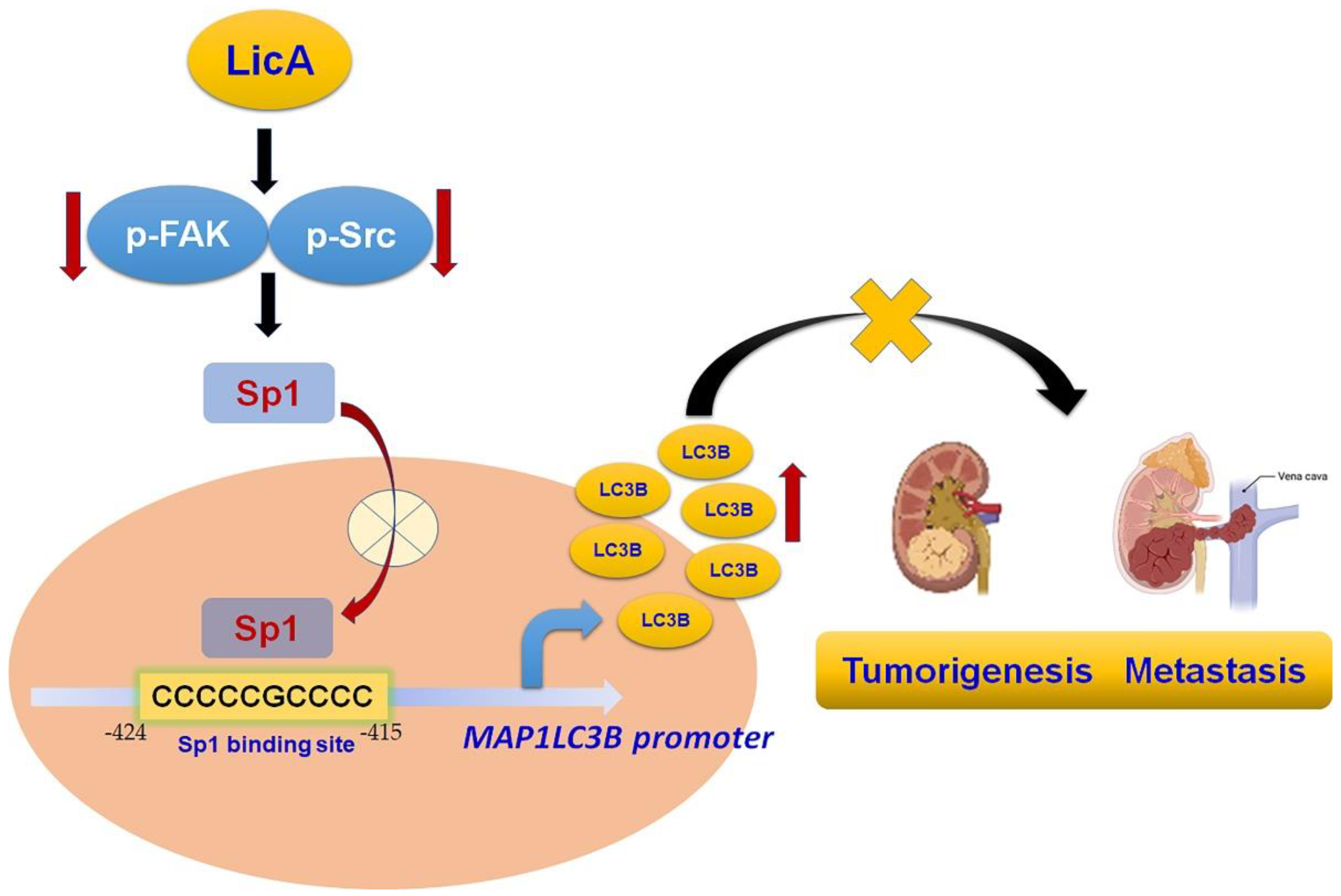
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Znaor, A.; Lortet-Tieulent, J.; Laversanne, M.; Jemal, A.; Bray, F. International variations and trends in renal cell carcinoma incidence and mortality. Eur. Urol. 2015, 67, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Bander, N.H.; Nanus, D.M. Renal-cell carcinoma. N. Engl. J. Med. 1996, 335, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Allard, C.B.; Gelpi-Hammerschmidt, F.; Harshman, L.C.; Choueiri, T.K.; Faiena, I.; Modi, P.; Chung, B.I.; Tinay, I.; Singer, E.A.; Chang, S.L. Contemporary trends in high-dose interleukin-2 use for metastatic renal cell carcinoma in the United States. Urol. Oncol. 2015, 33, 496.e11–496.e16. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.W.; Chen, P.N.; Lin, C.Y.; Hsieh, Y.S.; Chang, H.R. Everolimus suppresses invasion and migration of renal cell carcinoma by inhibiting FAK activity and reversing epithelial to mesenchymal transition in vitro and in vivo. Environ. Toxicol. 2017, 32, 1888–1898. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Escudier, B.; McDermott, D.F.; George, S.; Hammers, H.J.; Srinivas, S.; Tykodi, S.S.; Sosman, J.A.; Procopio, G.; Plimack, E.R.; et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2015, 373, 1803–1813. [Google Scholar] [CrossRef] [PubMed]
- Ogata, M.; Hino, S.; Saito, A.; Morikawa, K.; Kondo, S.; Kanemoto, S.; Murakami, T.; Taniguchi, M.; Tanii, I.; Yoshinaga, K.; et al. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol. Cell. Biol. 2006, 26, 9220–9231. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.L.; Deng, Q.; Chong, T.; Wang, Z.M. Autophagy suppresses the proliferation of renal carcinoma cell. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 343–350. [Google Scholar] [CrossRef]
- He, Y.H.; Tian, G. Autophagy as a Vital Therapy Target for Renal Cell Carcinoma. Front. Pharmacol. 2020, 11, 518225. [Google Scholar] [CrossRef]
- Xin, H.; Xu, W. Effect of licochalcone A on autophagy in renal cell carcinoma via PI3K/Akt/mTOR signaling pathway. Zhongguo Zhong Yao Za Zhi = Zhongguo Zhongyao Zazhi = China J. Chin. Mater. Med. 2018, 43, 3545–3552. [Google Scholar] [CrossRef]
- Martellucci, S.; Clementi, L.; Sabetta, S.; Mattei, V.; Botta, L.; Angelucci, A. Src Family Kinases as Therapeutic Targets in Advanced Solid Tumors: What We Have Learned so Far. Cancers 2020, 12, 1448. [Google Scholar] [CrossRef]
- Sato, F.; Sagara, A.; Tajima, K.; Miura, S.; Inaba, K.; Ando, Y.; Oku, T.; Murakami, T.; Kato, Y.; Yumoto, T. COL8A1 facilitates the growth of triple-negative breast cancer via FAK/Src activation. Breast Cancer Res. Treat. 2022, 194, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhan, W.; Guo, W.; Hu, F.; Qin, J.; Li, R.; Liao, X. MELK Accelerates the Progression of Colorectal Cancer via Activating the FAK/Src Pathway. Biochem. Genet. 2020, 58, 771–782. [Google Scholar] [CrossRef]
- Jung, O.; Lee, J.; Lee, Y.J.; Yun, J.M.; Son, Y.J.; Cho, J.Y.; Ryou, C.; Lee, S.Y. Timosaponin AIII inhibits migration and invasion of A549 human non-small-cell lung cancer cells via attenuations of MMP-2 and MMP-9 by inhibitions of ERK1/2, Src/FAK and beta-catenin signaling pathways. Bioorganic Med. Chem. Lett. 2016, 26, 3963–3967. [Google Scholar] [CrossRef]
- Sima, N.; Cheng, X.; Ye, F.; Ma, D.; Xie, X.; Lu, W. The overexpression of scaffolding protein NEDD9 promotes migration and invasion in cervical cancer via tyrosine phosphorylated FAK and SRC. PLoS ONE 2013, 8, e74594. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Guan, J.L. Signal transduction by focal adhesion kinase in cancer. Cancer Metastasis Rev. 2009, 28, 35–49. [Google Scholar] [CrossRef]
- Beraud, C.; Dormoy, V.; Danilin, S.; Lindner, V.; Bethry, A.; Hochane, M.; Coquard, C.; Barthelmebs, M.; Jacqmin, D.; Lang, H.; et al. Targeting FAK scaffold functions inhibits human renal cell carcinoma growth. Int. J. Cancer 2015, 137, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, L.; Xu, C.; Shi, J.; Chen, S.; Tan, M.; Chen, J.; Zou, L.; Chen, C.; Liu, Z.; et al. A Comprehensive Review for Phytochemical, Pharmacological, and Biosynthesis Studies on Glycyrrhiza spp. Am. J. Chin. Med. 2020, 48, 17–45. [Google Scholar] [CrossRef]
- Tsukiyama, R.; Katsura, H.; Tokuriki, N.; Kobayashi, M. Antibacterial activity of licochalcone A against spore-forming bacteria. Antimicrob. Agents Chemother. 2002, 46, 1226–1230. [Google Scholar] [CrossRef]
- Jiang, M.; Zhao, S.; Yang, S.; Lin, X.; He, X.; Wei, X.; Song, Q.; Li, R.; Fu, C.; Zhang, J.; et al. An “essential herbal medicine”-licorice: A review of phytochemicals and its effects in combination preparations. J. Ethnopharmacol. 2020, 249, 112439. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.S.; Thakur, K.; Hussain, S.S.; Zhang, J.G.; Xiao, G.R.; Wei, Z.J. Licochalcone A from licorice root, an inhibitor of human hepatoma cell growth via induction of cell apoptosis and cell cycle arrest. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2018, 120, 407–417. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, M.; Chen, L.; Zhou, L.; Bian, S.; Lv, Y. Licochalcone A restrains microphthalmia-associated transcription factor expression and growth by activating autophagy in melanoma cells via miR-142-3p/Rheb/mTOR pathway. Phytother. Res. PTR 2020, 34, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.J.; Wu, G.J.; Chen, R.J.; Chang, C.C.; Lien, L.M.; Chiu, C.C.; Tseng, M.F.; Huang, L.T.; Lin, K.H. Licochalcone A attenuates glioma cell growth in vitro and in vivo through cell cycle arrest. Food Funct. 2018, 9, 4500–4507. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.C.; Tsai, L.L.; Tsai, J.P.; Hsieh, S.C.; Yang, S.F.; Hsueh, J.T.; Hsieh, Y.H. Licochalcone A inhibits the migration and invasion of human lung cancer cells via inactivation of the Akt signaling pathway with downregulation of MMP-1/-3 expression. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2014, 35, 12139–12149. [Google Scholar] [CrossRef]
- Tsai, J.P.; Hsiao, P.C.; Yang, S.F.; Hsieh, S.C.; Bau, D.T.; Ling, C.L.; Pai, C.L.; Hsieh, Y.H. Licochalcone A suppresses migration and invasion of human hepatocellular carcinoma cells through downregulation of MKK4/JNK via NF-kappaB mediated urokinase plasminogen activator expression. PLoS ONE 2014, 9, e86537. [Google Scholar] [CrossRef]
- Tsai, J.P.; Lee, C.H.; Ying, T.H.; Lin, C.L.; Lin, C.L.; Hsueh, J.T.; Hsieh, Y.H. Licochalcone A induces autophagy through PI3K/Akt/mTOR inactivation and autophagy suppression enhances Licochalcone A-induced apoptosis of human cervical cancer cells. Oncotarget 2015, 6, 28851–28866. [Google Scholar] [CrossRef]
- Hung, T.W.; Chen, P.N.; Wu, H.C.; Wu, S.W.; Tsai, P.Y.; Hsieh, Y.S.; Chang, H.R. Kaempferol Inhibits the Invasion and Migration of Renal Cancer Cells through the Downregulation of AKT and FAK Pathways. Int. J. Med. Sci. 2017, 14, 984–993. [Google Scholar] [CrossRef]
- Liou, Y.F.; Hsieh, Y.S.; Hung, T.W.; Chen, P.N.; Chang, Y.Z.; Kao, S.H.; Lin, S.W.; Chang, H.R. Thymoquinone inhibits metastasis of renal cell carcinoma cell 786-O-SI3 associating with downregulation of MMP-2 and u-PA and suppression of PI3K/Src signaling. Int. J. Med. Sci. 2019, 16, 686–695. [Google Scholar] [CrossRef]
- Lee, C.C.; Tsai, J.P.; Lee, H.L.; Chen, Y.J.; Chen, Y.S.; Hsieh, Y.H.; Chen, J.C. Blockage of Autophagy Increases Timosaponin AIII-Induced Apoptosis of Glioma Cells In Vitro and In Vivo. Cells 2022, 12, 168. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.F.; Yang, S.F.; Chiou, H.L.; Hsu, W.H.; Hsu, J.C.; Liu, C.J.; Hsieh, Y.H. Licochalcone A inhibits the invasive potential of human glioma cells by targeting the MEK/ERK and ADAM9 signaling pathways. Food Funct. 2018, 9, 6196–6204. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.L.; Yang, S.F.; Hung, T.W.; Lin, C.L.; Hsieh, Y.H.; Chiou, H.L. Inhibition of eIF2alpha dephosphorylation accelerates pterostilbene-induced cell death in human hepatocellular carcinoma cells in an ER stress and autophagy-dependent manner. Cell Death Dis. 2019, 10, 418. [Google Scholar] [CrossRef]
- Ho, H.Y.; Lin, C.W.; Chien, M.H.; Reiter, R.J.; Su, S.C.; Hsieh, Y.H.; Yang, S.F. Melatonin suppresses TPA-induced metastasis by downregulating matrix metalloproteinase-9 expression through JNK/SP-1 signaling in nasopharyngeal carcinoma. J. Pineal. Res. 2016, 61, 479–492. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Y.; Park, K.I.; Kim, S.H.; Yu, S.N.; Park, S.G.; Kim, Y.W.; Seo, Y.K.; Ma, J.Y.; Ahn, S.C. Inhibition of Autophagy Promotes Salinomycin-Induced Apoptosis via Reactive Oxygen Species-Mediated PI3K/AKT/mTOR and ERK/p38 MAPK-Dependent Signaling in Human Prostate Cancer Cells. Int. J. Mol. Sci. 2017, 18, 1088. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.C.; Yang, S.F.; Chiou, H.L.; Hsieh, S.C.; Wen, S.H.; Lu, K.H.; Hsieh, Y.H. Licochalcone A-Induced Apoptosis Through the Activation of p38MAPK Pathway Mediated Mitochondrial Pathways of Apoptosis in Human Osteosarcoma Cells In Vitro and In Vivo. Cells 2019, 8, 1441. [Google Scholar] [CrossRef]
- Messeguer, X.; Escudero, R.; Farre, D.; Nunez, O.; Martinez, J.; Alba, M.M. PROMO: Detection of known transcription regulatory elements using species-tailored searches. Bioinformatics 2002, 18, 333–334. [Google Scholar] [CrossRef]
- Catalano, M.; D’Alessandro, G.; Lepore, F.; Corazzari, M.; Caldarola, S.; Valacca, C.; Faienza, F.; Esposito, V.; Limatola, C.; Cecconi, F.; et al. Autophagy induction impairs migration and invasion by reversing EMT in glioblastoma cells. Mol. Oncol. 2015, 9, 1612–1625. [Google Scholar] [CrossRef]
- Lv, Q.; Wang, W.; Xue, J.; Hua, F.; Mu, R.; Lin, H.; Yan, J.; Lv, X.; Chen, X.; Hu, Z.W. DEDD interacts with PI3KC3 to activate autophagy and attenuate epithelial-mesenchymal transition in human breast cancer. Cancer Res. 2012, 72, 3238–3250. [Google Scholar] [CrossRef]
- Black, A.R.; Black, J.D.; Azizkhan-Clifford, J. Sp1 and kruppel-like factor family of transcription factors in cell growth regulation and cancer. J. Cell Physiol. 2001, 188, 143–160. [Google Scholar] [CrossRef]
- Slack, J.K.; Adams, R.B.; Rovin, J.D.; Bissonette, E.A.; Stoker, C.E.; Parsons, J.T. Alterations in the focal adhesion kinase/Src signal transduction pathway correlate with increased migratory capacity of prostate carcinoma cells. Oncogene 2001, 20, 1152–1163. [Google Scholar] [CrossRef]
- Lin, J.A.; Fang, S.U.; Su, C.L.; Hsiao, C.J.; Chang, C.C.; Lin, Y.F.; Cheng, C.W. Silencing glucose-regulated protein 78 induced renal cell carcinoma cell line G1 cell-cycle arrest and resistance to conventional chemotherapy. Urol. Oncol. 2014, 32, 29.e1–29.e11. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Zhang, G.; Zhu, Z.; Zheng, Y.; Yan, F.; Pan, C.; Wang, Z.; Li, X.; Wang, F.; Meng, P.; et al. Nobiletin Inhibits Cell Viability via the SRC/AKT/STAT3/YY1AP1 Pathway in Human Renal Carcinoma Cells. Front. Pharmacol. 2019, 10, 690. [Google Scholar] [CrossRef]
- Xie, J.; Qian, Y.Y.; Yang, Y.; Peng, L.J.; Mao, J.Y.; Yang, M.R.; Tian, Y.; Sheng, J. Isothiocyanate From Moringa oleifera Seeds Inhibits the Growth and Migration of Renal Cancer Cells by Regulating the PTP1B-dependent Src/Ras/Raf/ERK Signaling Pathway. Front. Cell Dev. Biol. 2021, 9, 790618. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.J.; Chae, J.I.; Yoon, G.; Kim, K.H.; Cho, J.H.; Cho, S.S.; Cho, Y.S.; Shim, J.H. Licochalcone A, a natural chalconoid isolated from Glycyrrhiza inflata root, induces apoptosis via Sp1 and Sp1 regulatory proteins in oral squamous cell carcinoma. Int. J. Oncol. 2014, 45, 667–674. [Google Scholar] [CrossRef]
- Hao, W.; Yuan, X.; Yu, L.; Gao, C.; Sun, X.; Wang, D.; Zheng, Q. Licochalcone A-induced human gastric cancer BGC-823 cells apoptosis by regulating ROS-mediated MAPKs and PI3K/AKT signaling pathways. Sci. Rep. 2015, 5, 10336. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.Y.; Hao, M.; Yang, X.Y.; Ba, Q.; Li, M.; Ni, S.J.; Wang, L.S.; Du, X. Licochalcone A inhibits growth of gastric cancer cells by arresting cell cycle progression and inducing apoptosis. Cancer Lett. 2011, 302, 69–75. [Google Scholar] [CrossRef]
- Qiu, C.; Zhang, T.; Zhang, W.; Zhou, L.; Yu, B.; Wang, W.; Yang, Z.; Liu, Z.; Zou, P.; Liang, G. Licochalcone A Inhibits the Proliferation of Human Lung Cancer Cell Lines A549 and H460 by Inducing G2/M Cell Cycle Arrest and ER Stress. Int. J. Mol. Sci. 2017, 18, 1761. [Google Scholar] [CrossRef]
- de Heer, P.; Koudijs, M.M.; van de Velde, C.J.; Aalbers, R.I.; Tollenaar, R.A.; Putter, H.; Morreau, J.; van de Water, B.; Kuppen, P.J. Combined expression of the non-receptor protein tyrosine kinases FAK and Src in primary colorectal cancer is associated with tumor recurrence and metastasis formation. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2008, 34, 1253–1261. [Google Scholar] [CrossRef]
- Hiscox, S.; Jordan, N.J.; Morgan, L.; Green, T.P.; Nicholson, R.I. Src kinase promotes adhesion-independent activation of FAK and enhances cellular migration in tamoxifen-resistant breast cancer cells. Clin. Exp. Metastasis 2007, 24, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Chen, M.C.; Chiu, T.H.; Li, Y.H.; Yu, W.C.; Liao, W.L.; Oner, M.; Yu, C.R.; Wu, C.C.; Yang, T.Y.; et al. Arecoline Promotes Migration of A549 Lung Cancer Cells through Activating the EGFR/Src/FAK Pathway. Toxins 2019, 11, 185. [Google Scholar] [CrossRef] [PubMed]
- Chiu, K.Y.; Chen, T.H.; Wen, C.L.; Lai, J.M.; Cheng, C.C.; Liu, H.C.; Hsu, S.L.; Tzeng, Y.M. Antcin-H Isolated from Antrodia cinnamomea Inhibits Renal Cancer Cell Invasion Partly through Inactivation of FAK-ERK-C/EBP-beta/c-Fos-MMP-7 Pathways. Evid.-Based Complement. Altern. Med. Ecam 2017, 2017, 5052870. [Google Scholar] [CrossRef]
- Suske, G. The Sp-family of transcription factors. Gene 1999, 238, 291–300. [Google Scholar] [CrossRef]
- Bai, H.; Zhou, M.; Zhou, H.; Han, Q.; Xu, H.; Xu, P.; Chen, B. Licochalcone A suppresses Sp1 expression with potential anti-myeloma activity. Cancer Commun. 2021, 41, 1239–1242. [Google Scholar] [CrossRef]
- Cho, S.G.; Yi, Z.; Pang, X.; Yi, T.; Wang, Y.; Luo, J.; Wu, Z.; Li, D.; Liu, M. Kisspeptin-10, a KISS1-derived decapeptide, inhibits tumor angiogenesis by suppressing Sp1-mediated VEGF expression and FAK/Rho GTPase activation. Cancer Res. 2009, 69, 7062–7070. [Google Scholar] [CrossRef] [PubMed]
- Fulciniti, M.; Amin, S.; Nanjappa, P.; Rodig, S.; Prabhala, R.; Li, C.; Minvielle, S.; Tai, Y.T.; Tassone, P.; Avet-Loiseau, H.; et al. Significant biological role of sp1 transactivation in multiple myeloma. Clin. Cancer Res. 2011, 17, 6500–6509. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, D.; Knebelmann, B.; Cohen, H.T.; Ananth, S.; Sukhatme, V.P. The von Hippel-Lindau tumor suppressor gene product interacts with Sp1 to repress vascular endothelial growth factor promoter activity. Mol. Cell. Biol. 1997, 17, 5629–5639. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Niu, X.; Pan, H.; Zhou, Y.; Zhang, Z.; Qu, P.; Zhou, J. Tumor-suppressing effects of microRNA-429 in human renal cell carcinoma via the downregulation of Sp1. Oncol. Lett. 2016, 12, 2906–2911. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tseng, T.-Y.; Lee, C.-H.; Lee, H.-L.; Su, C.-Y.; Kao, C.-Y.; Tsai, J.-P.; Hsieh, Y.-H. Licochalcone A Suppresses Renal Cancer Cell Proliferation and Metastasis by Engagement of Sp1-Mediated LC3 Expression. Pharmaceutics 2023, 15, 684. https://doi.org/10.3390/pharmaceutics15020684
Tseng T-Y, Lee C-H, Lee H-L, Su C-Y, Kao C-Y, Tsai J-P, Hsieh Y-H. Licochalcone A Suppresses Renal Cancer Cell Proliferation and Metastasis by Engagement of Sp1-Mediated LC3 Expression. Pharmaceutics. 2023; 15(2):684. https://doi.org/10.3390/pharmaceutics15020684
Chicago/Turabian StyleTseng, Tsai-Yi, Chien-Hsing Lee, Hsiang-Lin Lee, Chien-Yu Su, Cheng-Yen Kao, Jen-Pi Tsai, and Yi-Hsien Hsieh. 2023. "Licochalcone A Suppresses Renal Cancer Cell Proliferation and Metastasis by Engagement of Sp1-Mediated LC3 Expression" Pharmaceutics 15, no. 2: 684. https://doi.org/10.3390/pharmaceutics15020684
APA StyleTseng, T.-Y., Lee, C.-H., Lee, H.-L., Su, C.-Y., Kao, C.-Y., Tsai, J.-P., & Hsieh, Y.-H. (2023). Licochalcone A Suppresses Renal Cancer Cell Proliferation and Metastasis by Engagement of Sp1-Mediated LC3 Expression. Pharmaceutics, 15(2), 684. https://doi.org/10.3390/pharmaceutics15020684








