Molecular Mechanisms of Inhibitory Effects of Bovine Lactoferrin on Invasion of Oral Squamous Cell Carcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cells and Cell Culture
2.3. Wound Healing Assay
2.4. Chamber Migration Assay
2.5. In Vitro Invasion Assay
2.6. Reverse Transcription Polymerase Chain Reaction (RT-PCR)
2.7. Quantitative RT-PCR (qRT-PCR)
2.8. Western Blotting Analysis
2.9. Silencing by Small Interfering RNA (SiRNA)
2.10. Statistical Analysis
3. Results
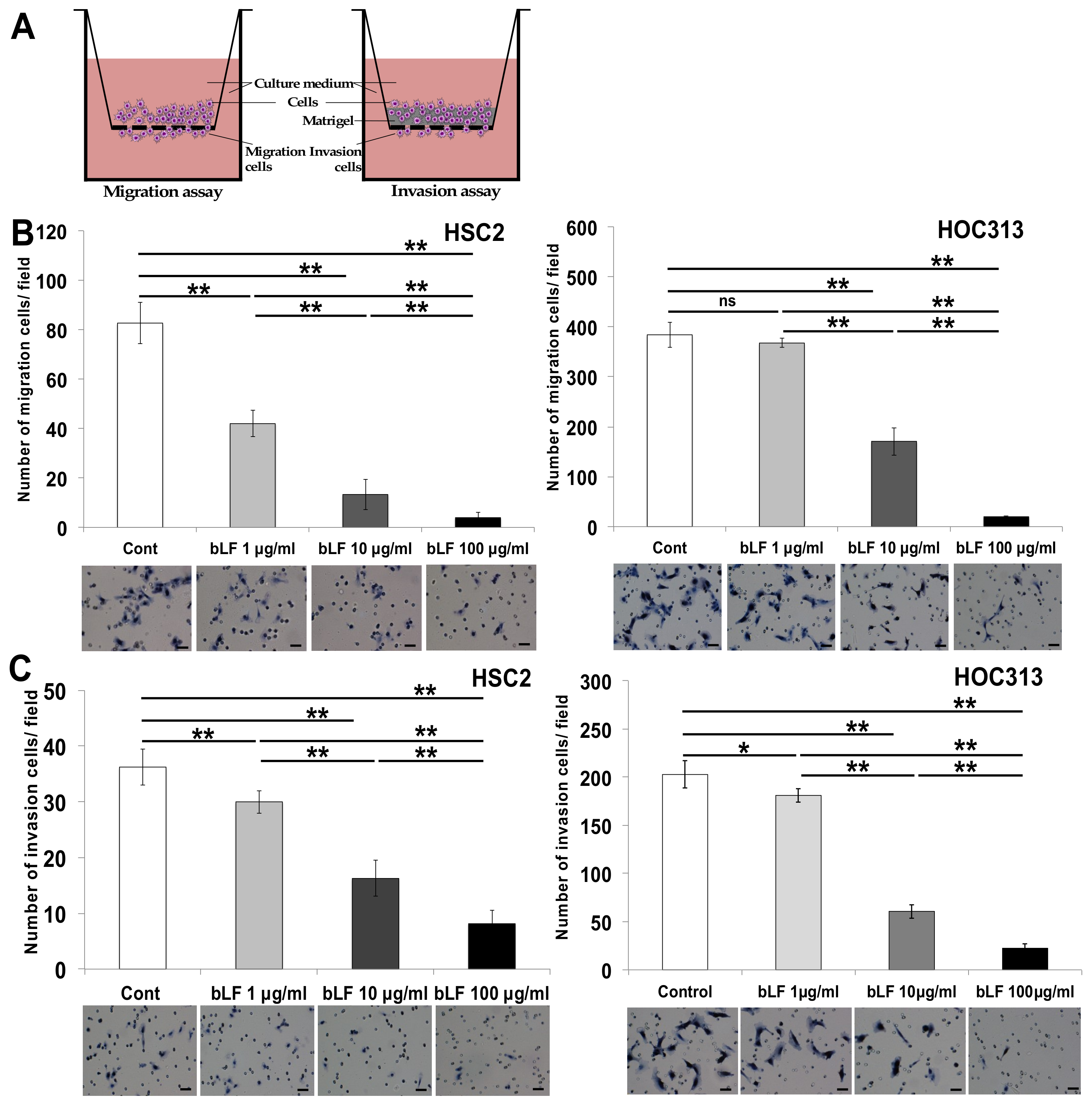
3.1. Bovine Lactoferrin Inhibits Invasion of OSCC Cells
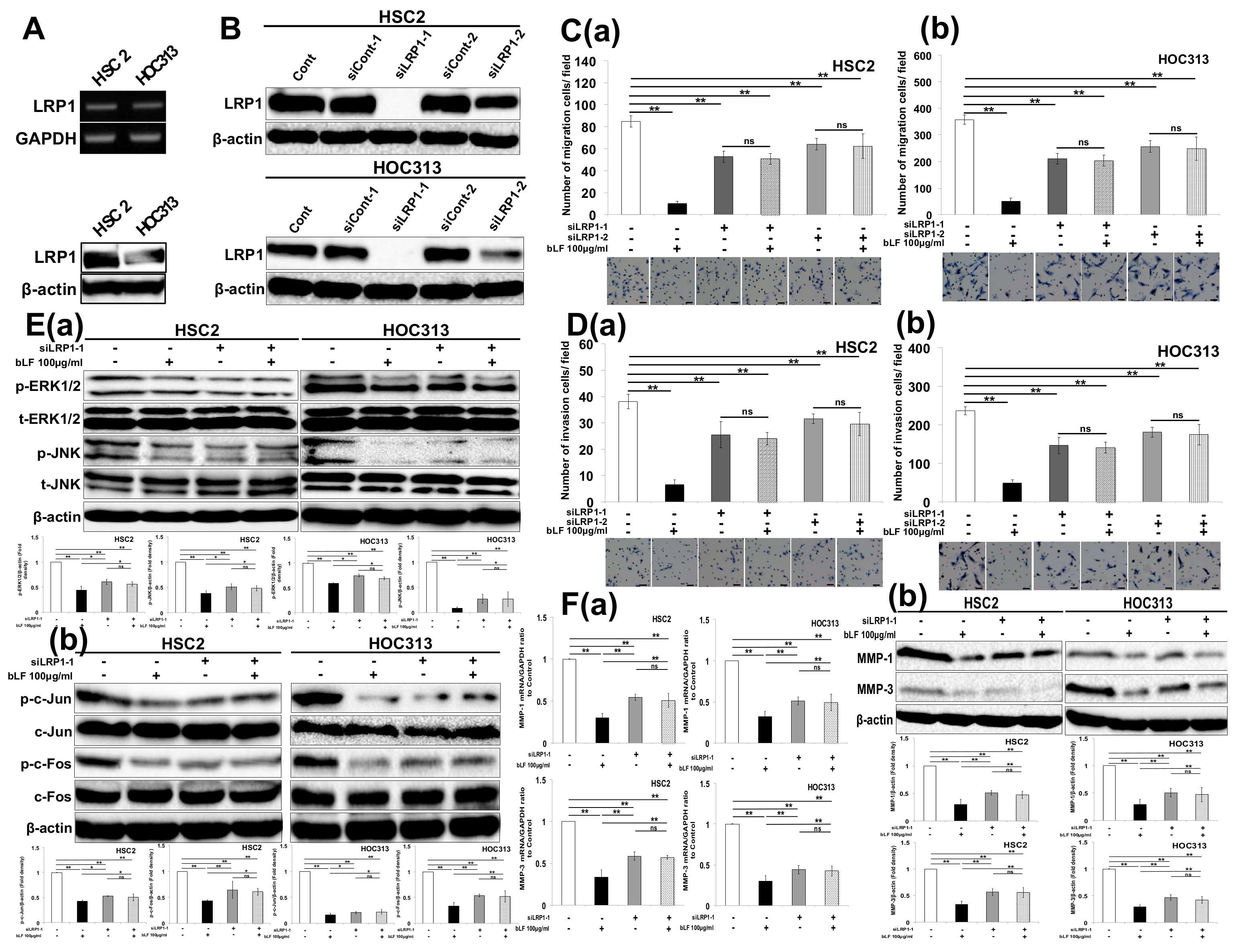
3.2. Bovine Lactoferrin Suppresses Invasion of OSCC Cells by Controlling AP-1 Activity to Suppress Expression of MMP-1 and MMP-3
3.3. Bovine Lactoferrin Requires Low-Density Lipoprotein Receptor-Related Protein 1 (LRP1) to Inhibit OSCC Cell Invasion
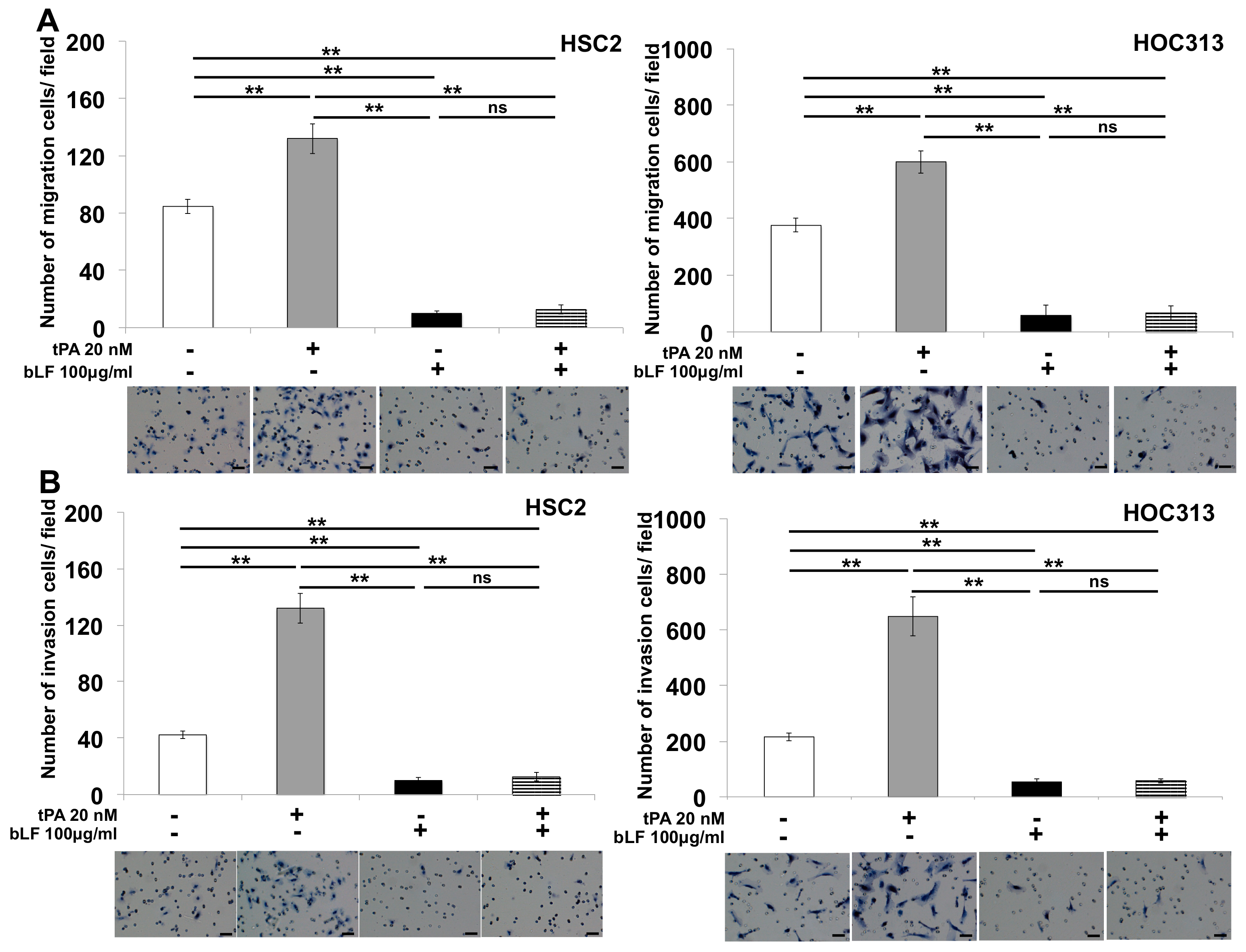
3.4. Bovine Lactoferrin Suppresses tPA-Induced Cell Invasion of OSCC by Reducing MMP-1 and MMP-3 Production
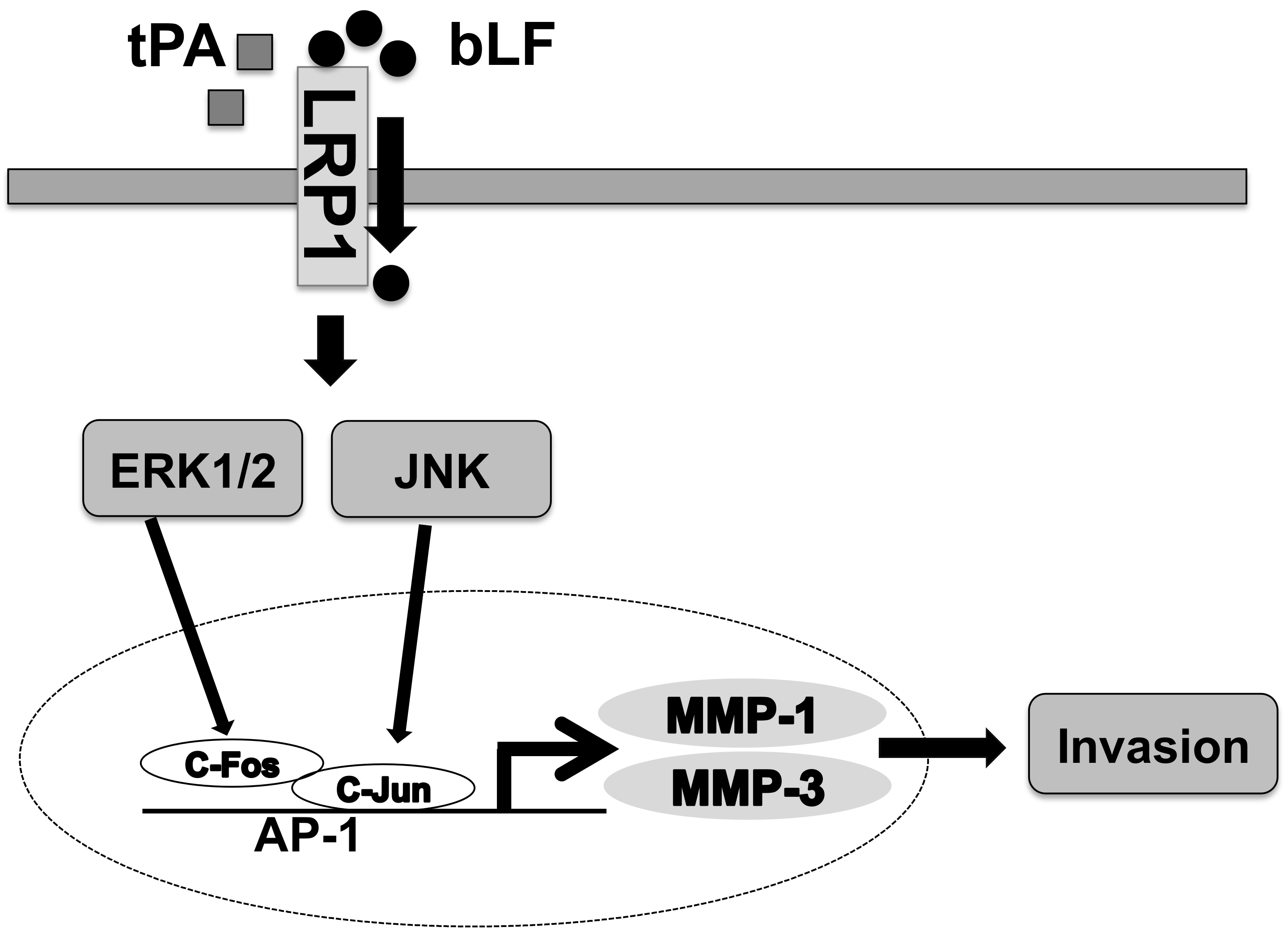
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gupta, S.; Kong, W.; Peng, Y.; Miao, Q.; Mackillop, W.J. Temporal trends in the incidence and survival of cancers of the upper aerodigestive tract in Ontario and the United States. Int. J. Cancer 2009, 125, 2159–2165. [Google Scholar] [CrossRef] [PubMed]
- Bozzuto, G.; Ruggieri, P.; Molinari, A. Molecular aspects of tumor cell migration and invasion. Ann. Dell’istituto Super. Sanita 2010, 46, 66–80. [Google Scholar] [CrossRef]
- Syrovets, T.; Simmet, T. Novel aspects and new roles for the serine protease plasmin. Cell Mol. Life Sci. 2004, 61, 873–885. [Google Scholar] [CrossRef] [PubMed]
- Baker, E.; Baker, H.M. Molecular structure, binding properties and dynamics of lactoferrin. Cell Mol. Life Sci. 2005, 62, 2531–2539. [Google Scholar] [CrossRef]
- Guillen, C.; McInnes, I.; Vaughan, D.; Speekenbrink, A.B.J.; Brock, J.H. The effects of local administration of lactoferrin on inflammation in murine autoimmune and infectious arthritis. Arthritis Rheum. 2000, 43, 2073–2080. [Google Scholar] [CrossRef]
- Rosa, L.; Tripepi, G.; Naldi, E.; Aimati, M.; Santangeli, S.; Venditto, F.; Caldarelli, M.; Valenti, P. Ambulatory COVID-19 patients treated with lactoferrin as a supplementary antiviral agent: A preliminary study. J. Clin. Med. 2021, 10, 4276. [Google Scholar] [CrossRef]
- Bellamy, W.; Takase, M.; Yamauchi, K.; Wakabayashi, H.; Kawase, K.; Tomita, M. Identification of the bactericidal domain of lactoferrin. Biochim. Biophys. Acta 1992, 1121, 130–136. [Google Scholar] [CrossRef]
- Shaheduzzaman, S.; Vishwanath, A.; Furusato, B.; Cullen, J.; Chen, Y.; Bañez, L.; Nau, M.; Ravindranath, L.; Kim, K.-H.; Mohammed, A.; et al. Silencing of lactotransferrin expression by methylation in prostate cancer progression. Cancer Biol. Ther. 2007, 6, 1088–1095. [Google Scholar] [CrossRef]
- Hirata, I.; Hoshimoto, M.; Saito, O.; Kayazawa, M.; Nishikawa, T.; Murano, M.; Toshina, K.; Wang, F.Y.; Matsuse, R. Usefulness of fecal lactoferrin and hemoglobin in diagnosis of colorectal diseases. World J. Gastroenterol. 2007, 13, 1569–1574. [Google Scholar] [CrossRef]
- Chea, C.; Miyauchi, M.; Inubushi, T.; Ayuningtyas, N.F.; Subarnbhesaj, A.; Nguyen, P.T.; Shrestha, M.; Haing, S.; Ohta, K.; Takata, T. Molecular mechanism of inhibitory effects of bovine lactoferrin on the growth of oral squamous cell carcinoma. PLoS ONE 2018, 13, e0191683. [Google Scholar] [CrossRef]
- Chea, C.; Miyauchi, M.; Inubushi, T.; Okamoto, K.; Haing, S.; Nguyen, P.T.; Imanaka, H.; Takata, T. Bovine lactoferrin reverses programming of epithelial-to-mesenchymal transition to mesenchymal-to-epithelial transition in oral squamous cell carcinoma. Biochem. Biophys. Res. Commun. 2018, 507, 142–147. [Google Scholar] [CrossRef]
- Gibbons, J.A.; Kanwar, R.K.; Kanwar, J.R. Lactoferrin and cancer in different cancer models. Front. Biosci. Sch. Ed. 2011, 3, 1080–1088. [Google Scholar] [CrossRef]
- Kanwar, J.R.; Mahidhara, G.; Kanwar, R.K. Novel alginate-enclosed chitosan–calcium phosphate-loaded iron-saturated bovine lactoferrin nanocarriers for oral delivery in colon cancer therapy. Nanomedicine 2012, 7, 1521–1550. [Google Scholar] [CrossRef]
- Samarasinghe, R.M.; Kanwar, R.K.; Kanwar, J.R. The effect of oral administration of iron saturated-bovine lactoferrin encapsulated chitosan-nanocarriers on osteoarthritis. Biomaterials 2014, 35, 7522–7534. [Google Scholar] [CrossRef]
- Tsuda, H.; Kozu, T.; Iinuma, G.; Ohashi, Y.; Saito, Y.; Saito, D.; Akasu, T.; Alexander, D.B.; Futakuchi, M.; Fukamachi, K.; et al. Cancer prevention by bovine lactoferrin: From animal studies to human trial. Biometals 2010, 23, 399–409. [Google Scholar] [CrossRef]
- Ye, Q.; Zheng, Y.; Fan, S.; Qin, Z.; Li, N.; Tang, A.; Ai, F.; Zhang, X.; Bian, Y.; Dang, W.; et al. Lactoferrin deficiency promotes colitis-associated colorectal dysplasia in mice. PLoS ONE 2014, 9, e103298. [Google Scholar] [CrossRef]
- Gibbons, J.A.; Kanwar, J.R.; Kanwar, R.K. Correction to: Iron-free and iron-saturated bovine lactoferrin inhibit survivin expression and differentially modulate apoptosis in breast cancer. BMC Cancer 2018, 18, 749. [Google Scholar] [CrossRef]
- Lee, W.; Mitchell, P.; Tjian, R. Purified transcription factor AP-1 interacts with TPA-inducible enhancer elements. Cell 1987, 49, 741–752. [Google Scholar] [CrossRef]
- Angel, P.; Karin, M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim. Biophys. Acta 1991, 1072, 129–157. [Google Scholar] [CrossRef]
- Ho, B.-Y.; Wu, Y.-M.; Chang, K.-J.; Pan, T.-M. Dimerumic acid inhibits SW620 cell invasion by attenuating H2O2-mediated MMP-7 expression via JNK/C-Jun and ERK/C-Fos activation in an AP-1-dependent manner. Int. J. Biol. Sci. 2011, 7, 869–880. [Google Scholar] [CrossRef]
- Shaulian, E.; Karin, M. AP-1 in cell proliferation and survival. Oncogene 2001, 20, 2390–2400. [Google Scholar] [CrossRef] [PubMed]
- Prusty, B.K.; Das, B.C. Constitutive activation of transcription factor AP-1 in cervical cancer and suppression of human papillomavirus (HPV) transcription and AP-1 activity in HeLa cells by curcumin. Int. J. Cancer 2004, 113, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Guo, J.-H.; Tu, X.-L.; Zhang, C.; Zhao, M.; Zhang, Q.-W.; Gao, F.-H. Tiron inhibits UVB-induced AP-1 binding sites transcriptional activation on MMP-1 and MMP-3 promoters by MAPK signaling pathway in human dermal fibroblasts. PLoS ONE 2016, 11, e0159998. [Google Scholar] [CrossRef] [PubMed]
- Yamano, E.; Miyauchi, M.; Furusyo, H.; Kawazoe, A.; Ishikado, A.; Makino, T.; Tanne, K.; Tanaka, E.; Takata, T. Inhibitory effects of orally administrated liposomal bovine lactoferrin on the LPS-induced osteoclastogenesis. Lab. Investig. J. Tech. Methods Pathol. 2010, 90, 1236–1246. [Google Scholar] [CrossRef] [PubMed]
- Dedieu, S.; Langlois, B.; Devy, J.; Sid, B.; Henriet, P.; Sartelet, H.; Bellon, G.; Emonard, H.; Martiny, L. LRP-1 silencing prevents malignant cell invasion despite increased pericellular proteolytic activities. Mol. Cell Biol. 2008, 28, 2980–2995. [Google Scholar] [CrossRef]
- Matrisian, L.M. The matrix-degrading metalloproteinases. Bioessays 1992, 14, 455–463. [Google Scholar] [CrossRef]
- Silence, J.; Lemmens, G.; Frederix, L.; Collen, D.; Lijnen, H.R. Regulation of gelatinase activity in mice with targeted inactivation of components of the plasminogen/plasmin system. Thromb. Haemost. 1998, 79, 1171–1176. [Google Scholar] [CrossRef]
- Fayard, B.; Bianchi, F.; Dey, J.; Moreno, E.; Djaffer, S.; Hynes, N.E.; Monard, D. The serine protease inhibitor protease Nexin-1 controls mammary cancer metastasis through LRP-1–Mediated MMP-9 expression. Cancer Res 2009, 69, 5690–5698. [Google Scholar] [CrossRef]
- Rosenthal, E.L.; Matrisian, L.M. Matrix metalloproteases in head and neck cancer. Head Neck 2006, 28, 639–648. [Google Scholar] [CrossRef]
- Hu, K.; Yang, J.; Tanaka, S.; Gonias, S.L.; Mars, W.M.; Liu, Y. Tissue-type plasminogen activator acts as a cytokine that triggers intracellular signal transduction and induces matrix metalloproteinase-9 gene expression. J. Biol. Chem. 2006, 281, 2120–2127. [Google Scholar] [CrossRef]
- Ishikado, A.; Uesaki, S.; Suido, H.; Nomura, Y.; Sumikawa, K.; Maeda, M.; Miyauchi, M.; Takata, T.; Makino, T. Human trial of liposomal lactoferrin supplementation for periodontal disease. Biol. Pharm. Bull. 2010, 33, 1758–1762. [Google Scholar] [CrossRef]
- Chea, C.; Haing, S.; Miyauchi, M.; Shrestha, M.; Imanaka, H.; Takata, T. Molecular mechanisms underlying the inhibitory effects of bovine lactoferrin on osteosarcoma. Biochem. Biophys. Res. Commun. 2018, 508, 946–952. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration—FDA, Agency Response Letter GRAS Notice No. GRN 000077. 2001. Available online: http://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/NoticeInventory/ucm154188.htm (accessed on 1 August 2016).
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific opinion on bovine lactoferrin. EFSA J. 2012, 10, 2701–2727. [Google Scholar] [CrossRef]
- Lauterbach, R.; Kamińska, E.; Michalski, P.; Lauterbach, J.P. Laktoferyna-glikoproteina o dużym potencjale tera-peutycznym [Lactoferrin—A glycoprotein of great therapeutic potentials]. Dev. Period Med. 2016, 20, 118–125. [Google Scholar]
- Troost, F.J.; Steijns, J.; Saris, W.H.M.; Brummer, R.-J.M. Gastric digestion of bovine lactoferrin in vivo in adults. J. Nutr. 2001, 131, 2101–2104. [Google Scholar] [CrossRef]
- Chourasia, M.K.; Jain, S.K. Pharmaceutical approaches to colon targeted drug delivery systems. J. Pharm. Pharm. Sci. 2003, 6, 33–66. [Google Scholar]
- Ishikado, A.; Imanaka, H.; Takeuchi, T.; Harada, E.; Makino, T. Liposomalization of lactoferrin enhanced it’s anti-inflammatory effects via oral administration. Biol. Pharm. Bull. 2005, 28, 1717–1721. [Google Scholar] [CrossRef]
- Saarialho-Kere, U.K.; Chang, E.S.; Welgus, H.G.; Parks, W.C. Distinct localization of collagenase and tissue inhibitor of metalloproteinases expression in wound healing associated with ulcerative pyogenic granuloma. J. Clin. Investig. 1992, 90, 1952–1957. [Google Scholar] [CrossRef]
- Rodriguez-Ochoa, N.; Cortes-Reynosa, P.; Rodriguez-Rojas, K.; de la Garza, M.; Salazar, E.P. Bovine holo-lactoferrin inhibits migration and invasion in MDA-MB-231 breast cancer cells. Mol. Biol. Rep. 2023, 50, 193–201. [Google Scholar] [CrossRef]
- El-Fakharany, E.M.; Abu-Serie, M.M.; Habashy, N.H.; Eltarahony, M. Augmenting apoptosis-mediated anticancer activity of lactoperoxidase and lactoferrin by nanocombination with copper and iron hybrid nanometals. Sci. Rep. 2022, 12, 13153. [Google Scholar] [CrossRef]
- Gutman, A.; Wasylyk, B. The collagenase gene promoter contains a TPA and oncogene-responsive unit encompassing the PEA3 and AP-1 binding sites. EMBO J. 1990, 9, 2241–2246. [Google Scholar] [CrossRef] [PubMed]
- Frisch, S.M.; Morisaki, J.H. Positive and negative transcriptional elements of the human type IV collagenase gene. Mol. Cell. Biol. 1990, 10, 6524–6532. [Google Scholar] [CrossRef] [PubMed]
- Nerlov, C.; Rørth, P.; Blasi, F.; Johnsen, M. Essential AP-1 and PEA3 binding elements in the human urokinase enhancer display cell type-specific activity. Oncogene 1991, 6, 1583–1592. [Google Scholar] [PubMed]
- Chen, J.-L.; Lai, C.-Y.; Ying, T.-H.; Lin, C.-W.; Wang, P.-H.; Yu, F.-J.; Liu, C.-J.; Hsieh, Y.-H. Modulating the ERK1/2–MMP1 axis through corosolic acid inhibits metastasis of human oral squamous cell carcinoma cells. Int. J. Mol. Sci. 2021, 22, 8641. [Google Scholar] [CrossRef]
- Wang, K.; Zheng, J.; Yu, J.; Wu, Y.; Guo, J.; Xu, Z.; Sun, X. Knockdown of MMP-1 inhibits the progression of colorectal cancer by suppressing the PI3K/Akt/c-myc signaling pathway and EMT. Oncol. Rep. 2020, 43, 1103–1112. [Google Scholar] [CrossRef]
- Zheng, G.; Lyons, J.G.; Tan, T.K.; Wang, Y.; Hsu, T.-T.; Min, D.; Succar, L.; Rangan, G.K.; Hu, M.; Henderson, B.R.; et al. Disruption of E-cadherin by matrix metalloproteinase directly mediates epithelial-mesenchymal transition downstream of transforming growth factor-β1 in renal tubular epithelial cells. Am. J. Pathol. 2009, 175, 580–591. [Google Scholar] [CrossRef]
- Khales, S.A.; Abbaszadegan, M.R.; Majd, A.; Forghanifard, M.M. TWIST1 upregulates matrix metalloproteinase (MMP) genes family in esophageal squamous carcinoma cells. Gene Expr. Patterns 2020, 37, 119127. [Google Scholar] [CrossRef]
- Murray, G.I.; Duncan, M.E.; O’Neil, P.; McKay, J.A.; Melvin, W.T.; Fothergill, J.E. Matrix metalloproteinase-1 is as-sociated with poor prognosis in oesophageal cancer. J. Pathol. 1998, 185, 256–261. [Google Scholar] [CrossRef]
- Shima, I.; Sasaguri, Y.; Kusukawa, J.; Yamana, H.; Fujita, H.; Kakegawa, T.; Morimatsu, M. Production of matrix met-alloproteinase-2 and metalloproteinase-3 related to malignant behavior of esophageal carcinoma. A clinicopathologic study. Cancer 1992, 70, 2747–2753. [Google Scholar] [CrossRef]
- Egeblad, M.; Werb, Z. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer 2002, 2, 161–174. [Google Scholar] [CrossRef]
- Cutler, S.J.; Doecke, J.D.; Ghazawi, I.; Yang, J.; Griffiths, L.R.; Spring, K.J.; Ralph, S.J.; Mellick, A.S. Novel STAT binding elements mediate IL-6 regulation of MMP-1 and MMP-3. Sci. Rep. 2017, 7, 8526. [Google Scholar] [CrossRef]
- Moestrup, S.K.; Gliemann, J.; Pallesen, G. Distribution of the 2-macroglobulin receptor/low density lipoprotein receptor-related protein in human tissues. Cell Tissue Res. 1992, 269, 375–382. [Google Scholar] [CrossRef]
- Herz, J.; Strickland, D.K. LRP: A multifunctional scavenger and signaling receptor. J. Clin. Investig. 2001, 108, 779–784. [Google Scholar] [CrossRef]
- Mantuano, E.; Jo, M.; Gonias, S.L.; Campana, W.M. Low density lipoprotein receptor-related protein (LRP1) regulates Rac1 and RhoA reciprocally to control schwann cell adhesion and migration. J. Biol. Chem. 2010, 285, 14259–14266. [Google Scholar] [CrossRef]
- Li, Y.; Marzolo, M.P.; van Kerkhof, P.; Strous, G.J.; Bu, G. The YXXL motif, but not the two NPXY motifs, serves as the dominant endocytosis signal for low density lipoprotein receptor-related protein. J. Biol. Chem. 2000, 275, 17187–17194. [Google Scholar] [CrossRef]
- Dedieu, S.; Langlois, B. LRP-1: A new modulator of cytoskeleton dynamics and adhesive complex turnover in cancer cells. Cell Adhes. Migr. 2008, 2, 77–80. [Google Scholar] [CrossRef]
- Song, H.; Li, Y.; Lee, J.; Schwartz, A.L.; Bu, G. Low-density lipoprotein receptor-related protein 1 promotes cancer cell migration and invasion by inducing the expression of matrix metalloproteinases 2 and 9. Cancer Res. 2009, 69, 879–886. [Google Scholar] [CrossRef]
- Li, Y.; Wood, N.; Grimsley, P.; Yellowlees, D.; Donnelly, P.K. In vitro invasiveness of human breast cancer cells is promoted by low density lipoprotein receptor-related protein. Invasion Metastasis 1998, 18, 240–251. [Google Scholar] [CrossRef]
- Pencheva, N.; Tran, H.; Buss, C.; Huh, D.; Drobnjak, M.; Busam, K.; Tavazoie, S.F. Convergent multi-miRNA targeting of ApoE drives LRP1/LRP8-dependent melanoma metastasis and angiogenesis. Cell 2012, 151, 1068–1082. [Google Scholar] [CrossRef]
- Ortiz-Zapater, E.; Peiró, S.; Roda, O.; Corominas, J.M.; Aguilar, S.; Ampurdanés, C.; Real, F.X.; Navarro, P. Tissue plasminogen activator induces pancreatic cancer cell proliferation by a non-catalytic mechanism that requires extracellular signal-regulated kinase 1/2 activation through epidermal growth factor receptor and annexin A2. Am. J. Pathol. 2007, 170, 1573–1584. [Google Scholar] [CrossRef]
- Willnow, T.; Goldstein, J.; Orth, K.; Brown, M.; Herz, J. Low density lipoprotein receptor-related protein and gp330 bind similar ligands, including plasminogen activator-inhibitor complexes and lactoferrin, an inhibitor of chylomicron remnant clearance. J. Biol. Chem. 1992, 267, 26172–26180. [Google Scholar] [CrossRef] [PubMed]
- Inubushi, T.; Kawazoe, A.; Miyauchi, M.; Kudo, Y.; Ao, M.; Ishikado, A.; Makino, T.; Takata, T. Molecular mechanisms of the inhibitory effects of bovine lactoferrin on lipopolysaccharide-mediated osteoclastogenesis. J. Biol. Chem. 2012, 287, 23527–23536. [Google Scholar] [CrossRef] [PubMed]
- Westermarck, J.; Kähäri, V.M. Regulation of matrix metalloproteinase expression in tumor invasion. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 1999, 13, 781–792. [Google Scholar] [CrossRef]
- Zheng, J.; Fan, H.; Chen, Y.; Ni, B.; Wang, S.; Sun, M.; Chen, D. Expression of MMP-1/PAR-1 and patterns of invasion in oral squamous cell carcinoma as potential prognostic markers. OncoTargets Ther. 2015, 8, 1619–1626. [Google Scholar] [CrossRef]
- Tadbir, A.A.; Purshahidi, S.; Ebrahimi, H.; Khademi, B.; Malekzadeh, M.; Mardani, M.; Taghva, M.; Sardari, Y. Serum level of MMP-3 in patients with oral squamous cell carcinoma—Lack of association with clinico-pathological features. Asian Pac. J. Cancer Prev. 2012, 13, 4545–4548. [Google Scholar] [CrossRef]
- Chen, P.; Shan, Z.; Zhao, J.; Li, F.; Zhang, W.; Yang, L.; Huang, Z. NFAT1 promotes cell motility through MMP-3 in esophageal squamous cell carcinoma. Biomed. Pharmacother. 2017, 86, 541–546. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chea, C.; Miyauchi, M.; Inubushi, T.; Okamoto, K.; Haing, S.; Takata, T. Molecular Mechanisms of Inhibitory Effects of Bovine Lactoferrin on Invasion of Oral Squamous Cell Carcinoma. Pharmaceutics 2023, 15, 562. https://doi.org/10.3390/pharmaceutics15020562
Chea C, Miyauchi M, Inubushi T, Okamoto K, Haing S, Takata T. Molecular Mechanisms of Inhibitory Effects of Bovine Lactoferrin on Invasion of Oral Squamous Cell Carcinoma. Pharmaceutics. 2023; 15(2):562. https://doi.org/10.3390/pharmaceutics15020562
Chicago/Turabian StyleChea, Chanbora, Mutsumi Miyauchi, Toshihiro Inubushi, Kana Okamoto, Sivmeng Haing, and Takashi Takata. 2023. "Molecular Mechanisms of Inhibitory Effects of Bovine Lactoferrin on Invasion of Oral Squamous Cell Carcinoma" Pharmaceutics 15, no. 2: 562. https://doi.org/10.3390/pharmaceutics15020562
APA StyleChea, C., Miyauchi, M., Inubushi, T., Okamoto, K., Haing, S., & Takata, T. (2023). Molecular Mechanisms of Inhibitory Effects of Bovine Lactoferrin on Invasion of Oral Squamous Cell Carcinoma. Pharmaceutics, 15(2), 562. https://doi.org/10.3390/pharmaceutics15020562





