A Miniaturized Extruder to Prototype Amorphous Solid Dispersions: Selection of Plasticizers for Hot Melt Extrusion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
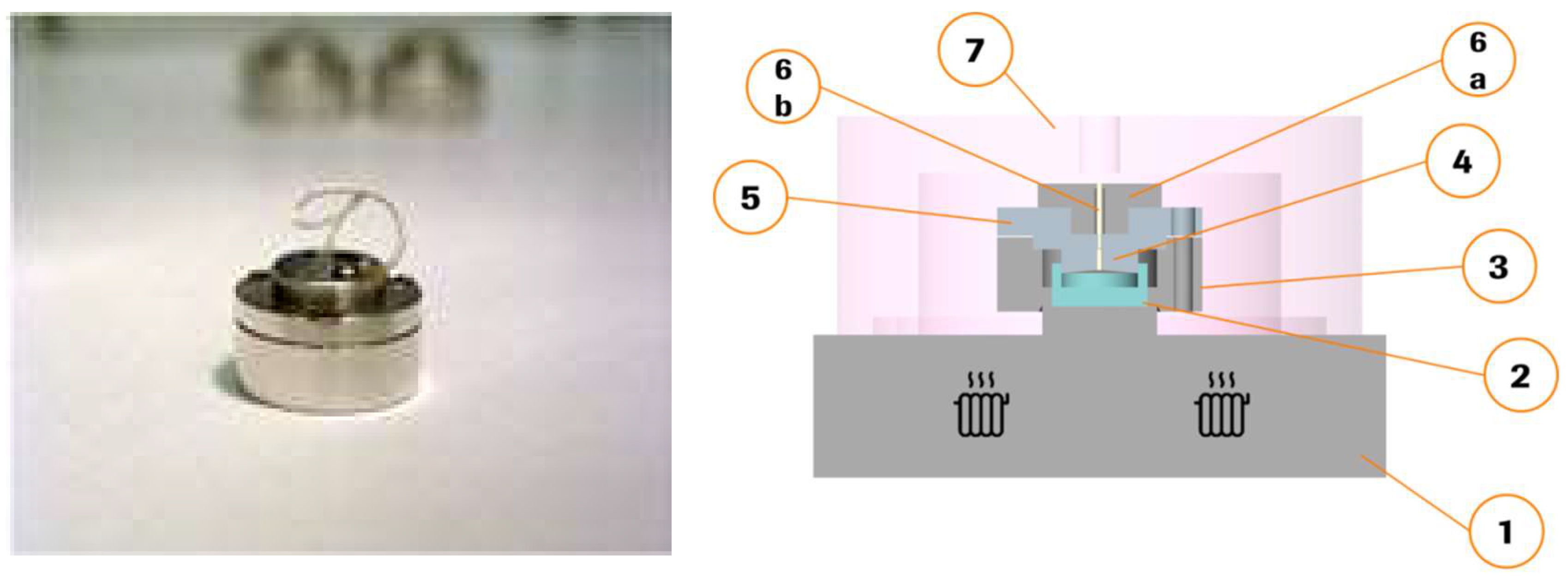
2.2. Miniaturized Extrusion
2.3. Investigating the Melt Behaviour
2.4. Manufacturing of Extrudates
2.5. Analytical Methods for Characterization
2.5.1. Atomic Force Microscopy (AFM)
2.5.2. Differential Scanning Calorimetry
2.5.3. Fourier Transformed Infrared Spectroscopy (FTIR)
2.5.4. X-ray Powder Diffraction (XRPD)
2.5.5. Two-Stage Dissolution Testing
2.6. Stability Testing
3. Results
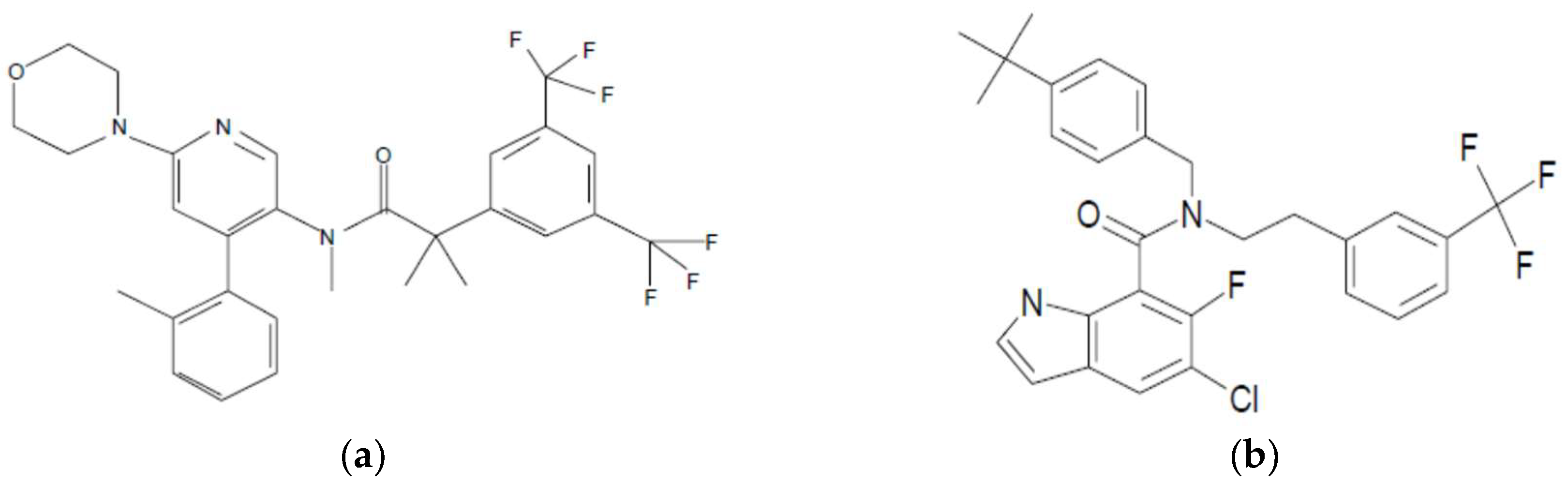
3.1. Selection and Properties of Compounds
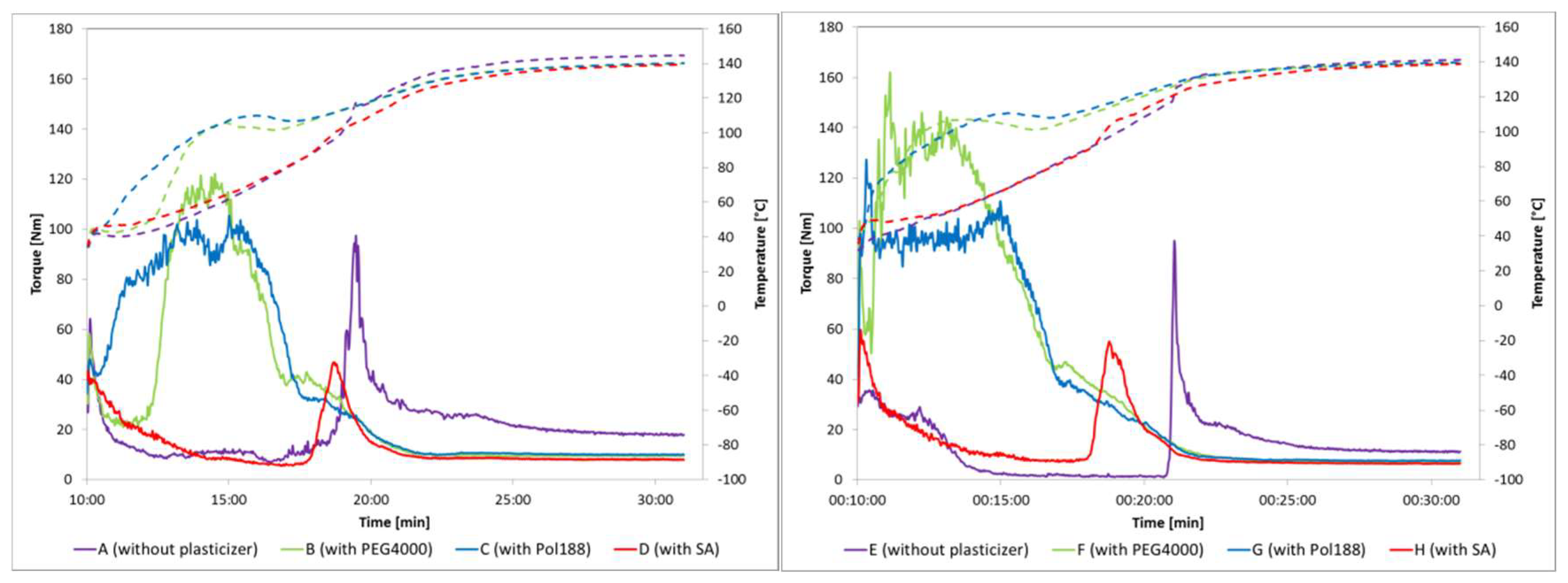
3.2. Torque Rheometery to Determine Impact of Plasticizers on Viscocity and Melt Behaviour
3.3. Preparation of Lab-Scale and MinEx Extrudates
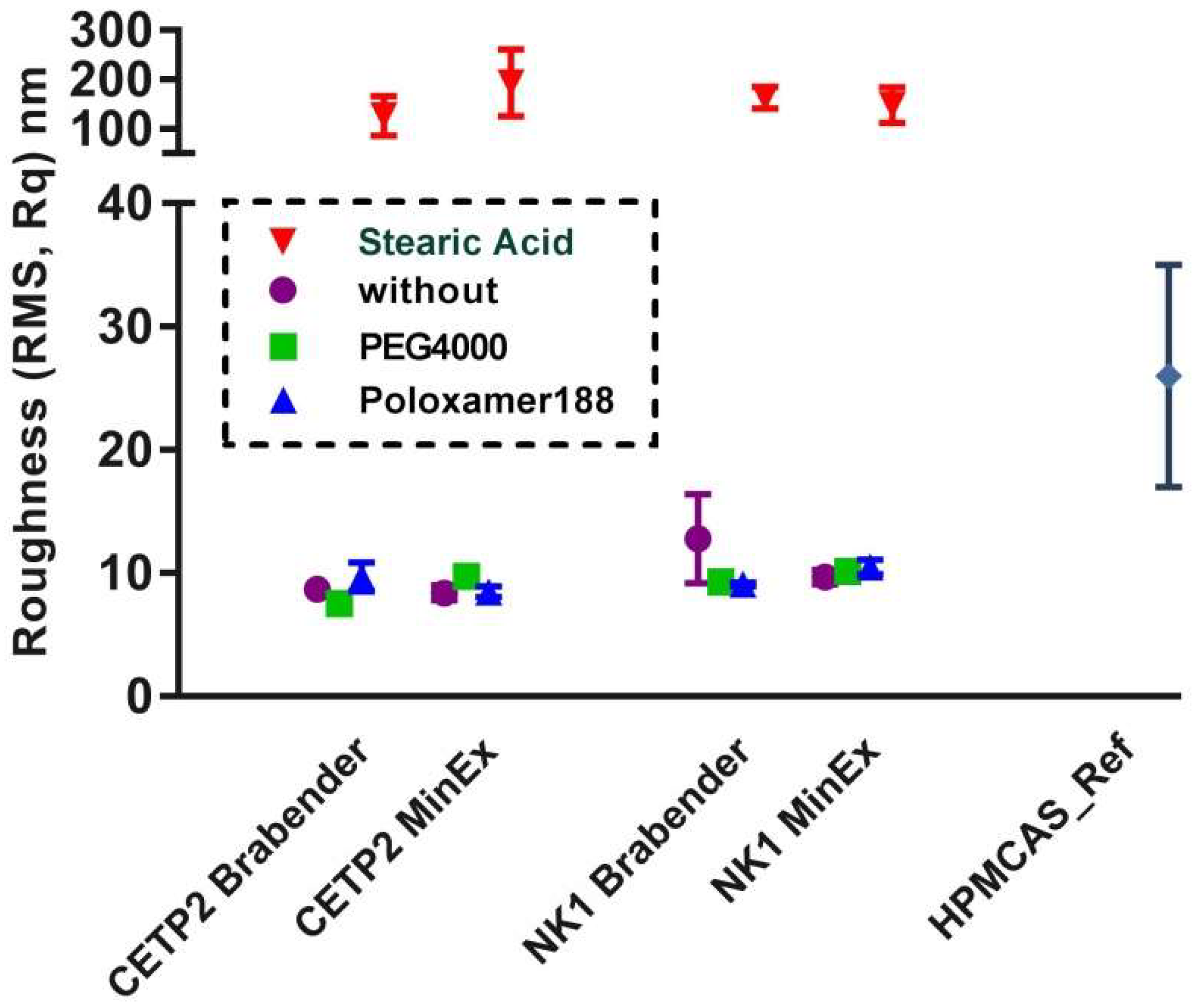
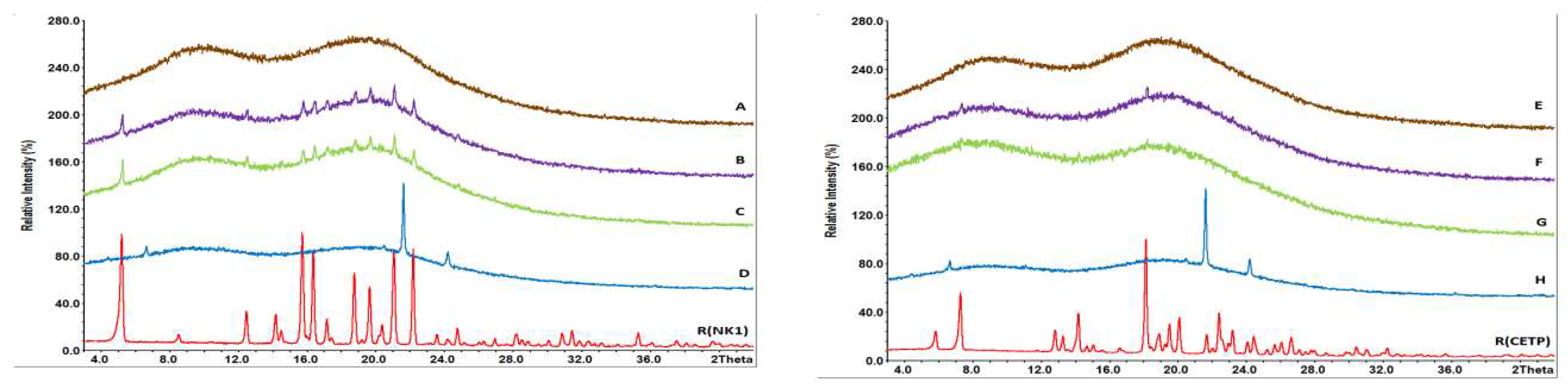
3.4. Nanostructure of MinEx and Lab-Scale Extrudates
3.5. Solid State Properties of MinEx Extrudates and Powders Derived from Lab-Scale Extrudates
3.6. Dissolution Properties of MinEx Extrudates and Powders Derived from Lab-Scale Extrusion
3.7. Phase Separation Processes on MinEx and Lab-Scale Extrudates (AFM)
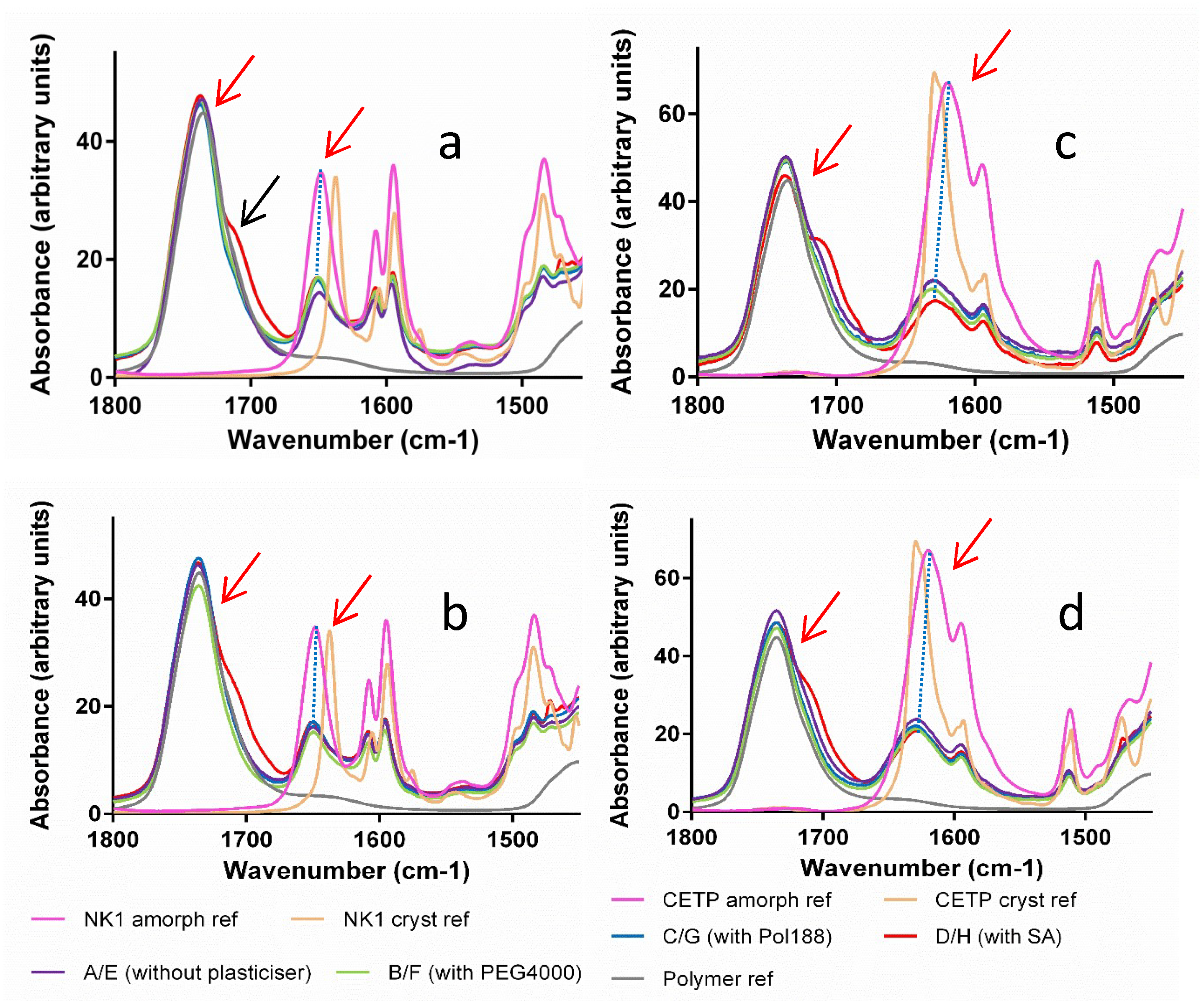
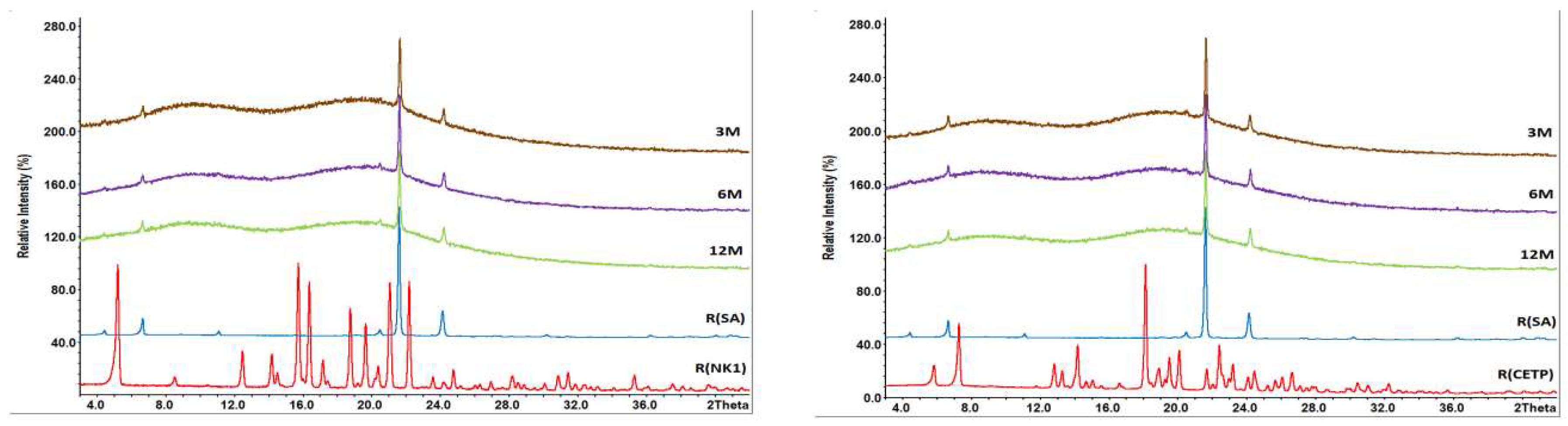
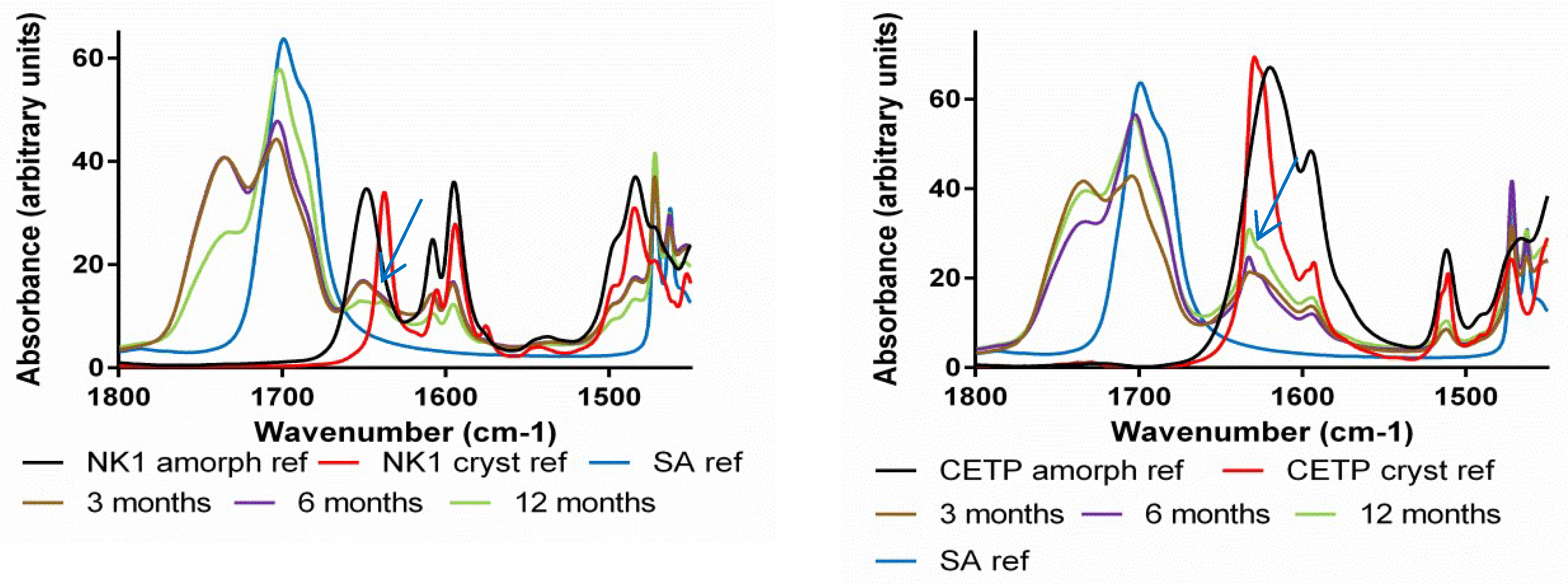
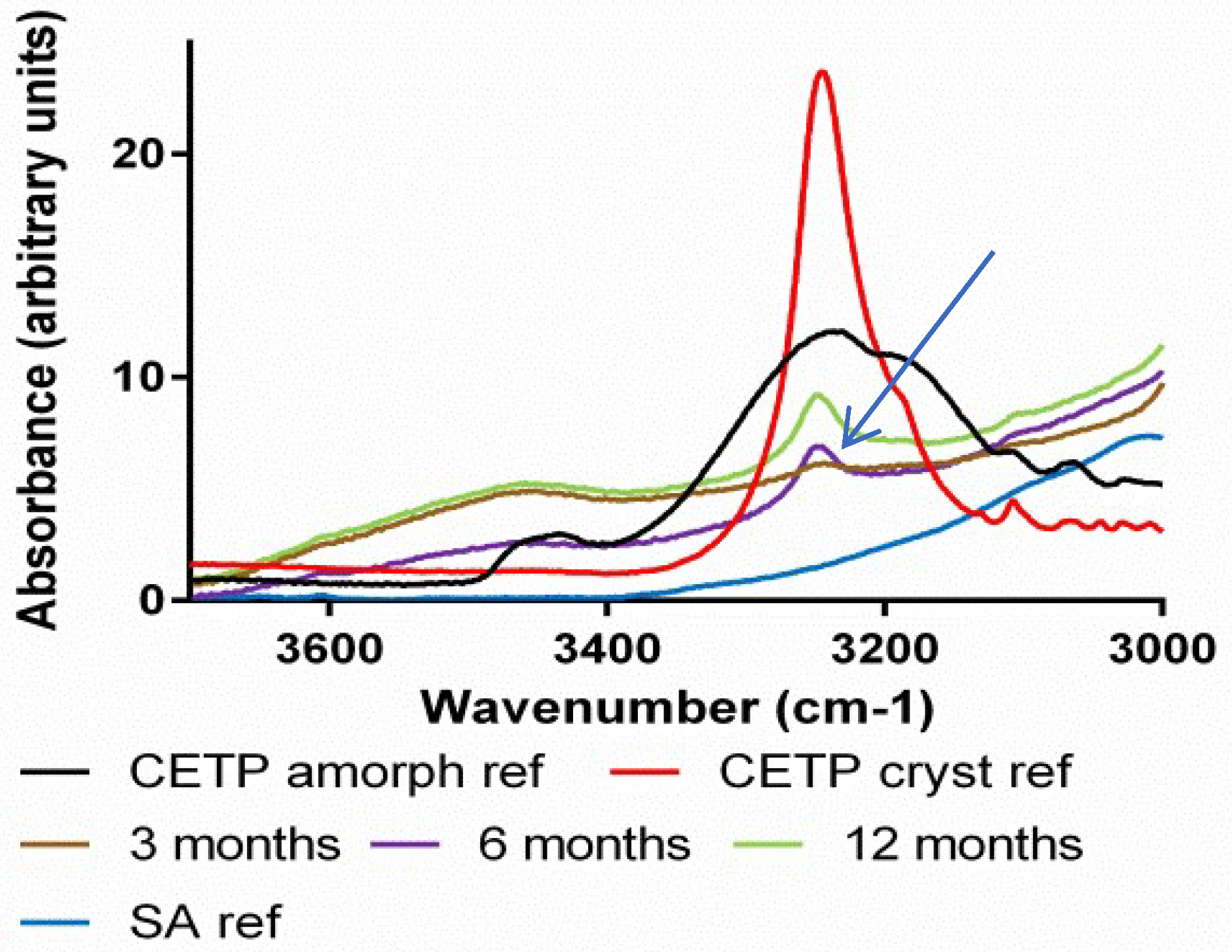
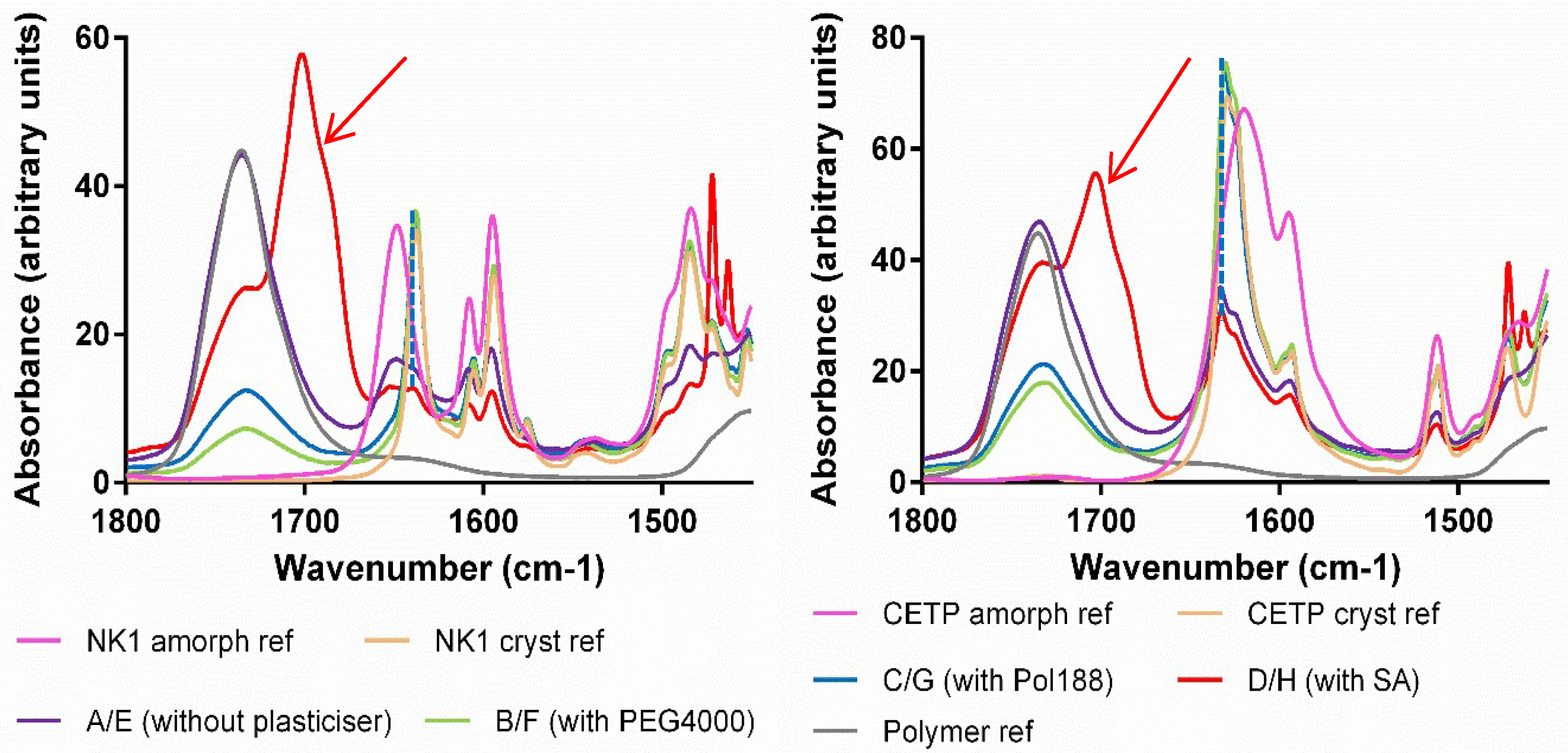
3.8. Macroscopic Physical Stability of Lab-Scale Extrudates with XRPD, DSC and FTIR.
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Appendix A



References
- Kawabata, Y.; Wada, K.; Nakatani, M.; Yamada, S.; Onoue, S. Formulation design for poorly water-soluble drugs based on biopharmaceutics classification system: Basic approaches and practical applications. Int. J. Pharm. 2011, 420, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Baghel, S.; Cathcart, H.; O’Reilly, N.J. Polymeric Amorphous Solid Dispersions: A Review of Amorphization, Crystallization, Stabilization, Solid-State Characterization, and Aqueous Solubilization of Biopharmaceutical Classification System Class II Drugs. J. Pharm. Sci. 2016, 105, 2527–2544. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Rohani, S. Polymorphism and Crystallization of Active Pharmaceutical Ingredients (APIs). Curr. Med. Chem. 2009, 16, 884–905. [Google Scholar] [CrossRef] [PubMed]
- Page, S.; Maurer, R.; Wyttenbach, N. Structured Development Approach for Amorphous Systems. In Formulating Poorly Water Soluble Drugs, 2nd ed.; Williams, R.O., III, Watts, A.B., Miller, D.A., Eds.; Springer: New York, NY, USA, 2016. [Google Scholar]
- Wyttenbach, N.; Janas, C.; Siam, M.; Lauer, M.E.; Jacob, L.; Scheubel, E.; Page, S. Miniaturized screening of polymers for amorphous drug stabilization (SPADS): Rapid assessment of solid dispersion systems. Eur. J. Pharm. Biopharm. 2013, 84, 583–598. [Google Scholar] [CrossRef] [PubMed]
- Paudel, A.; Nies, E.; Van den Mooter, G. Relating Hydrogen-Bonding Interactions with the Phase Behavior of Naproxen/PVP K 25 Solid Dispersions: Evaluation of Solution-Cast and Quench-Cooled Films. Mol. Pharm. 2012, 9, 3301–3317. [Google Scholar] [CrossRef] [PubMed]
- Lauer, M.; Grassmann, O.; Siam, M.; Tardio, J.; Jacob, L.; Page, S.; Kindt, J.; Engel, A.; Alsenz, J. Atomic Force Microscopy-Based Screening of Drug-Excipient Miscibility and Stability of Solid Dispersions. Pharm. Res. 2011, 28, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Lauer, M.E.; Siam, M.; Tardio, J.; Page, S.; Kindt, J.H.; Grassmann, O. Rapid Assessment of Homogeneity and Stability of Amorphous Solid Dispersions by Atomic Force Microscopy—From Bench to Batch. Pharm. Res. 2013, 30, 2010–2022. [Google Scholar] [CrossRef] [PubMed]
- Lamm, M.S.; DiNunzio, J.; Khawaja, N.N.; Crocker, L.S.; Pecora, A. Assessing Mixing Quality of a Copovidone-TPGS Hot Melt Extrusion Process with Atomic Force Microscopy and Differential Scanning Calorimetry. AAPS PharmSciTech 2016, 17, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Taylor, L.S. Nanoscale Infrared, Thermal, and Mechanical Characterization of Telaprevir-Polymer Miscibility in Amorphous Solid Dispersions Prepared by Solvent Evaporation. Mol. Pharm. 2016, 13, 1123–1136. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Van den Mooter, G. Spray drying formulation of amorphous solid dispersions. Adv. Drug Deliv. Rev. 2016, 100, 27–50. [Google Scholar] [CrossRef] [PubMed]
- Crowley, M.M.; Zhang, F.; Repka, M.A.; Thumma, S.; Upadhye, S.B.; Battu, S.K.; McGinity, J.W.; Martin, C. Pharmaceutical Applications of Hot-Melt Extrusion: Part I. Drug Dev. Ind. Pharm. 2007, 33, 909–926. [Google Scholar] [CrossRef] [PubMed]
- Patil, H.; Tiwari, R.V.; Repka, M.A. Hot-Melt Extrusion: From Theory to Application in Pharmaceutical Formulation. AAPS PharmSciTech 2016, 17, 20–42. [Google Scholar] [CrossRef] [PubMed]
- Vieira, M.G.A.; Silva, M.A.d.; Santos, L.O.d.; Beppu, M.M. Natural-based plasticizers and biopolymer films: A review. Eur. Polym. J. 2011, 47, 254–263. [Google Scholar] [CrossRef]
- LaFountaine, J.S.; McGinity, J.W.; Williams, R.O. Challenges and Strategies in Thermal Processing of Amorphous Solid Dispersions: A Review. AAPS PharmSciTech 2016, 17, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowska, P. The Effect of Concentration and Type of Plasticizer on the Mechanical Properties of Cellulose Acetate Butyrate Organic-Inorganic Hybrids. In Recent Advances in Plasticizers; Luqman, D.M., Ed.; InTech: Rijeka, Croatia, 2012. [Google Scholar] [CrossRef]
- Wypych, G.; Marcilla, A.; Beltrán, M. 5—Mechanisms Of Plasticizers Action A2. In Handbook of Plasticizers, 3rd ed.; ChemTec Publishing: Toronto, ON, Canada, 2017; pp. 119–134. [Google Scholar]
- Desai, D.; Sandhu, H.; Shah, N.; Malick, W.; Zia, H.; Phuapradit, W.; Vaka, S.R.K. Selection of Solid-State Plasticizers as Processing Aids for Hot-Melt Extrusion. J. Pharm. Sci. 2018, 107, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Van Renterghem, J.; Vervaet, C.; De Beer, T. Rheological Characterization of Molten Polymer-Drug Dispersions as a Predictive Tool for Pharmaceutical Hot-Melt Extrusion Processability. Pharm. Res. 2017, 34, 2312–2321. [Google Scholar] [CrossRef] [PubMed]
- A. Ashour, E.; Kulkarni, V.; Almutairy, B.; Park, J.-B.; Shah, S.P.; Majumdar, S.; Lian, Z.; Pinto, E.; Bi, V.; Durig, T.; et al. Influence of pressurized carbon dioxide on ketoprofen-incorporated hot-melt extruded low molecular weight hydroxypropylcellulose. Drug Dev. Ind. Pharm. 2016, 42, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Verreck, G.; Brewster, M.E.; Assche, I.V. The use of supercritical fluid technology to broaden the applicability of hot melt extrusion for drug delivery applications. Bull. Tech. Gattefossé 2012, 105, 28–42. [Google Scholar]
- Ghebremeskel, A.N.; Vemavarapu, C.; Lodaya, M. Use of surfactants as plasticizers in preparing solid dispersions of poorly soluble API: Selection of polymer-surfactant combinations using solubility parameters and testing the processability. Int. J. Pharm. 2007, 328, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Rath, B.; Dwivedi, A.D. Enhancement of dissolution rate and bioavailability of Paliperidone by Hot Melt Extrusion technique. J. Sci. Ind. Res. 2014, 73, 680–685. [Google Scholar]
- Kolhe, S.; Chaudhari, P.D.; More, D. Dissolution and Bioavailability Enhancement of Efavirenz by Hot Melt Extrusion Technique. IOSR J. Pharm. 2014, 4, 47–53. [Google Scholar]
- Schilling, S.U.; Lirola, H.L.; Shah, N.H.; Waseem Malick, A.; McGinity, J.W. Influence of plasticizer type and level on the properties of Eudragit® S100 matrix pellets prepared by hot-melt extrusion. J. Microencapsul. 2010, 27, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Repka, M.A.; Gerding, T.G.; Repka, S.L.; McGinity, J.W. Influence of Plasticizers and Drugs on the Physical-Mechanical Properties of Hydroxypropylcellulose Films Prepared by Hot Melt Extrusion. Drug Dev. Ind. Pharm. 1999, 25, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Repka, M.A.; McGinity, J.W. Influence of Vitamin E TPGS on the properties of hydrophilic films produced by hot-melt extrusion. Int. J. Pharm. 2000, 202, 63–70. [Google Scholar] [CrossRef]
- Repka, M.A.; McGinity, J.W. Physical-mechanical, moisture absorption and bioadhesive properties of hydroxypropylcellulose hot-melt extruded films. Biomaterials 2000, 21, 1509–1517. [Google Scholar] [CrossRef]
- Baird, J.A.; Van Eerdenbrugh, B.; Taylor, L.S. A classification system to assess the crystallization tendency of organic molecules from undercooled melts. J. Pharm. Sci. 2010, 99, 3787–3806. [Google Scholar] [CrossRef] [PubMed]
- Vertzoni, M.; Fotaki, N.; Nicolaides, E.; Reppas, C.; Kostewicz, E.; Stippler, E.; Leuner, C.; Dressman, J. Dissolution media simulating the intralumenal composition of the small intestine: Physiological issues and practical aspects. J. Pharm. Pharmacol. 2004, 56, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Ilasi, N.; Sekulic, S.S. Absolute molecular weight determination of hypromellose acetate succinate by size exclusion chromatography: Use of a multi angle laser light scattering detector and a mixed solvent. J. Pharm. Biomed. Anal. 2011, 56, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Sarode, A.L.; Obara, S.; Tanno, F.K.; Sandhu, H.; Iyer, R.; Shah, N. Stability assessment of hypromellose acetate succinate (HPMCAS) NF for application in hot melt extrusion (HME). Carbohydr. Polym. 2014, 101, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.; DiNunzio, J.; Eglesia, M.; Forster, S.; Lamm, M.; Lowinger, M.; Marsac, P.; McKelvey, C.; Meyer, R.; Schenck, L.; et al. Hot-Melt Extrusion for Solid Dispersions: Composition and Design Considerations. In Amorphous Solid Dispersions; Shah, N., Sandhu, H., Choi, D.S., Chokshi, H., Malick, A.W., Eds.; Springer: New York, NY, USA, 2014. [Google Scholar] [CrossRef]
- Wegiel, L.A.; Zhao, Y.H.; Mauer, L.J.; Edgar, K.J.; Taylor, L.S. Curcumin amorphous solid dispersions: The influence of intra and intermolecular bonding on physical stability. Pharm. Dev. Technol. 2014, 19, 976–986. [Google Scholar] [CrossRef] [PubMed]
- Moreno, E.; Cordobilla, R.; Calvet, T.; Cuevas-Diarte, M.A.; Gbabode, G.; Negrier, P.; Mondieig, D.; Oonk, H.A.J. Polymorphism of even saturated carboxylic acids from n-decanoic to n-eicosanoic acid. New J. Chem. 2007, 31, 947–957. [Google Scholar] [CrossRef]
- Malta, V.; Celotti, G.; Zannetti, R.; Martelli, A.F. Crystal structure of the C form of stearic acid. J. Chem. Soc. B 1971, 548–553. [Google Scholar] [CrossRef]
- Thumma, S.; ElSohly, M.A.; Zhang, S.Q.; Gul, W.; Repka, M.A. Influence of plasticizers on the stability and release of a prodrug of Delta(9)-tetrahydrocannabinol incorporated in poly (ethylene oxide) matrices. Eur. J. Pharm. Biopharm. 2008, 70, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Fung, M.H.; Suryanarayanan, R. Use of a Plasticizer for Physical Stability Prediction of Amorphous Solid Dispersions. Cryst. Growth Des. 2017, 17, 4315–4325. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Luo, R.; Chen, Y.; Ke, X.; Hu, D.R.; Han, M.M. Application of Carrier and Plasticizer to Improve the Dissolution and Bioavailability of Poorly Water-Soluble Baicalein by Hot Melt Extrusion. AAPS PharmSciTech 2014, 15, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Dunn, C.; Khadra, I.; Wilson, C.G.; Halbert, G.W. Statistical investigation of simulated fed intestinal media composition on the equilibrium solubility of oral drugs. Eur. J. Pharm. Sci. 2017, 99, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Hand, S.; Yang, J. Self-assembly of lamellar structures of fatty acids complexed with surfactant in aqueous solutions. Langmuir 1998, 14, 3597–3601. [Google Scholar] [CrossRef]
- Elvang, P.A.; Hinna, A.H.; Brouwers, J.; Hens, B.; Augustijns, P.; Brandl, M. Bile Salt Micelles and Phospholipid Vesicles Present in Simulated and Human Intestinal Fluids: Structural Analysis by Flow FieldeFlow Fractionation/Multiangle Laser Light Scattering. J. Pharm. Sci. 2016, 105, 2832–2839. [Google Scholar] [CrossRef] [PubMed]
- Khoshakhlagh, P.; Johnson, R.; Nawroth, T.; Langguth, P.; Schmueser, L.; Hellmann, N.; Decker, H.; Szekely, N.K. Nanoparticle structure development in the gastro-intestinal model fluid FaSSIF(mod6.5) from several phospholipids at various water content relevant for oral drug administration. Eur. J. Lipid Sci. Technol. 2014, 116, 1155–1166. [Google Scholar] [CrossRef]
- Windbergs, M.; Strachan, C.J.; Kleinebudde, P. Understanding the solid-state behaviour of triglyceride solid lipid extrudates and its influence on dissolution. Eur. J. Pharm. Biopharm. 2009, 71, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Witzleb, R.; Mullertz, A.; Kanikanti, V.R.; Hamann, H.J.; Kleinebudde, P. Dissolution of solid lipid extrudates in biorelevant media. Int. J. Pharm. 2012, 422, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.; Hathaway, R.; Leung, P.; Franz, R. Effect of Triacetin and Polyethylene Glycol-400 on Some Physical-Properties of Hydroxypropyl Methylcellulose Free Films. Int. J. Pharm. 1991, 73, 197–208. [Google Scholar] [CrossRef]





| Formulation | NK1 | CETP | HPMCAS * | PEG4000 | Pol188 ** | SA *** |
|---|---|---|---|---|---|---|
| A: NK1 + HPMCAS | 30.0 | 70.0 | ||||
| B: NK1 + HPMCAS + PEG4000 | 27.8 | 65.0 | 7.2 | |||
| C: NK1 + HPMCAS + Pol188 | 27.8 | 65.0 | 7.2 | |||
| D: NK1 + HPMCAS + SA | 27.8 | 65.0 | 7.2 | |||
| E: CETP + HPMCAS | 30.0 | 70.0 | ||||
| F: CETP + HPMCAS + PEG4000 | 27.8 | 65.0 | 7.2 | |||
| G: CETP + HPMCAS + Pol188 | 27.8 | 65.0 | 7.2 | |||
| H: CETP + HPMCAS + SA | 27.8 | 65.0 | 7.2 |
| HPLC Parameter | NK1 | CETP |
|---|---|---|
| Sample temperature | 25 °C | 25 °C |
| Mobile phase | 60% Acetonitrile/40% Water/0.1% TFA | 80% Acetonitrile/20% Water/0.1% TFA |
| Flow rate | 1.0 mL/min | 1.0 mL/min |
| Elution time | 5 min | 4 min |
| Injection volume | 10 mL | 10 mL |
| Formulation | Torque Rheometer | Lab-Scale Extrusion b | ||
|---|---|---|---|---|
| SESS (kJ/kg) | SME (kJ/kg) | Pdie c (bar) | Tdie d (°C) | |
| A: NK1 + HPMCAS | 683 | 6654 | 11.9 ± 2.8 | 157–161 |
| B: NK1 + HPMCAS + PEG4000 | 305 | 4616 | 6.2 ± 1.6 | 157 |
| C: NK1 + HPMCAS + Pol188 | 324 | 3714 | 5.5 ± 1.6 | 157 |
| D: NK1 + HPMCAS + SA | 263 | 3462 | 4.7 ± 1.3 | 157 |
| E: CETP + HPMCAS | 525 | 5042 | 10.4 ± 1.9 | 159–163 |
| F: CETP + HPMCAS + PEG4000 | 250 | 3082 | 6.1 ± 1.1 | 157–159 |
| G: CETP + HPMCAS + Pol188 | 260 | 3053 | 6.0 ± 1.1 | 157–160 |
| H: CETP + HPMCAS + SA | 222 | 2745 | 5.4 ± 0.9 | 157–160 |
| Formulation | MinEx Extrusion | Lab-Scale Extrusion a |
|---|---|---|
| A: NK1 + HPMCAS | Single Tg (67 °C) | Single Tg (59 °C) |
| B: NK1 + HPMCAS + PEG4000 | Single Tg (60 °C) | Single Tg (49 °C) |
| C: NK1 + HPMCAS + Pol188 | Single Tg (64 °C) | Single Tg (50 °C) |
| D: NK1 + HPMCAS + SA | Tg (54 °C), Tm (68 °C) | Single Tg (46 °C) |
| E: CETP + HPMCAS | Single Tg (54 °C) | Single Tg (55 °C) |
| F: CETP + HPMCAS + PEG4000 | Single Tg (49 °C) | Single Tg (53 °C) |
| G: CETP + HPMCAS + Pol188 | Single Tg (56 °C) | Single Tg (47 °C) |
| H: CETP + HPMCAS + SA | Tg (53 °C), Tm (65 °C) | Tg (52 °C), Tm (64 °C) |
| Storage Condition | 25 °C/60%RH | 40 °C/25%RH | 40 °C/75%RH | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Storage Time (Months) | 3 | 6 | 12 | 3 | 6 | 12 | 3 | 6 | 12 |
| A: NK1 + HPMCAS | AA | AA | AA | AA | AA | AA | AAa | AAa | AAc |
| B: NK1 + HPMCAS + PEG4000 | AA | AAc | CC | CAc | CCc | CC | CCc | CCc | CCc |
| C: NK1 + HPMCAS + Pol188 | AA | AAc | AA | CAc | CCc | CC | CCc | CCc | CCc |
| D: NK1 + HPMCAS + SA | AA | AA | AA | AA | AA | AA | AAa | AAc | AAc |
| E: CETP + HPMCAS | AA | AA | AA | AA | AA | AA | AAa | AAc | AAc |
| F: CETP + HPMCAS + PEG4000 | AA | AAa | CA | CAc | CCc | CC | CCc | CCc | CCc |
| G: CETP + HPMCAS + Pol188 | AA | AAa | CA | CAc | CCc | CC | CCc | CCc | CCc |
| H: CETP + HPMCAS + SA | AA | AA | AA | AA | AA | AA | AAc | AAc | AAc |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lauer, M.E.; Maurer, R.; De Paepe, A.T.; Stillhart, C.; Jacob, L.; James, R.; Kojima, Y.; Rietmann, R.; Kissling, T.; Van den Ende, J.A.; et al. A Miniaturized Extruder to Prototype Amorphous Solid Dispersions: Selection of Plasticizers for Hot Melt Extrusion. Pharmaceutics 2018, 10, 58. https://doi.org/10.3390/pharmaceutics10020058
Lauer ME, Maurer R, De Paepe AT, Stillhart C, Jacob L, James R, Kojima Y, Rietmann R, Kissling T, Van den Ende JA, et al. A Miniaturized Extruder to Prototype Amorphous Solid Dispersions: Selection of Plasticizers for Hot Melt Extrusion. Pharmaceutics. 2018; 10(2):58. https://doi.org/10.3390/pharmaceutics10020058
Chicago/Turabian StyleLauer, Matthias E., Reto Maurer, Anne T. De Paepe, Cordula Stillhart, Laurence Jacob, Rajesh James, Yuki Kojima, Rene Rietmann, Tom Kissling, Joost A. Van den Ende, and et al. 2018. "A Miniaturized Extruder to Prototype Amorphous Solid Dispersions: Selection of Plasticizers for Hot Melt Extrusion" Pharmaceutics 10, no. 2: 58. https://doi.org/10.3390/pharmaceutics10020058




