An Inactivated Novel Genotype Fowl Adenovirus 4 Protects Chickens against the Hydropericardium Syndrome That Recently Emerged in China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fowl Adenovirus Serotype 4
2.2. Detection of the Titration of FAdV-4
2.3. Production of Inactivated FAdV-4 Vaccine
2.4. Experiment Animals
2.5. Immunization and Challenge
2.6. Sample Collection
2.7. Antibody and Cytokine Assay
2.8. Real-Time PCR for Virus Shedding
2.9. Histopathology
2.10. Statistical Analysis
3. Results
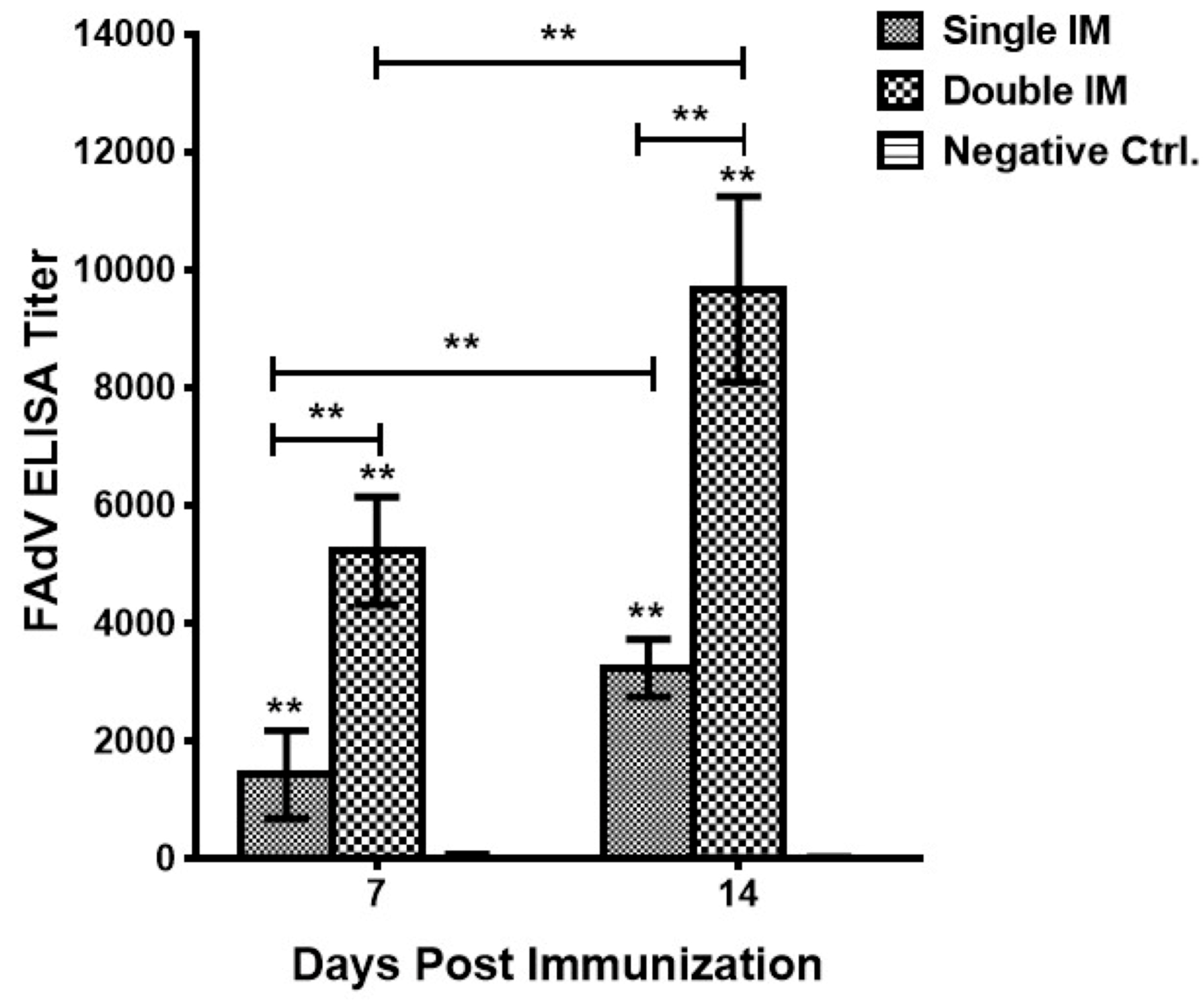
3.1. Antibody Responses of Vaccinated Chickens
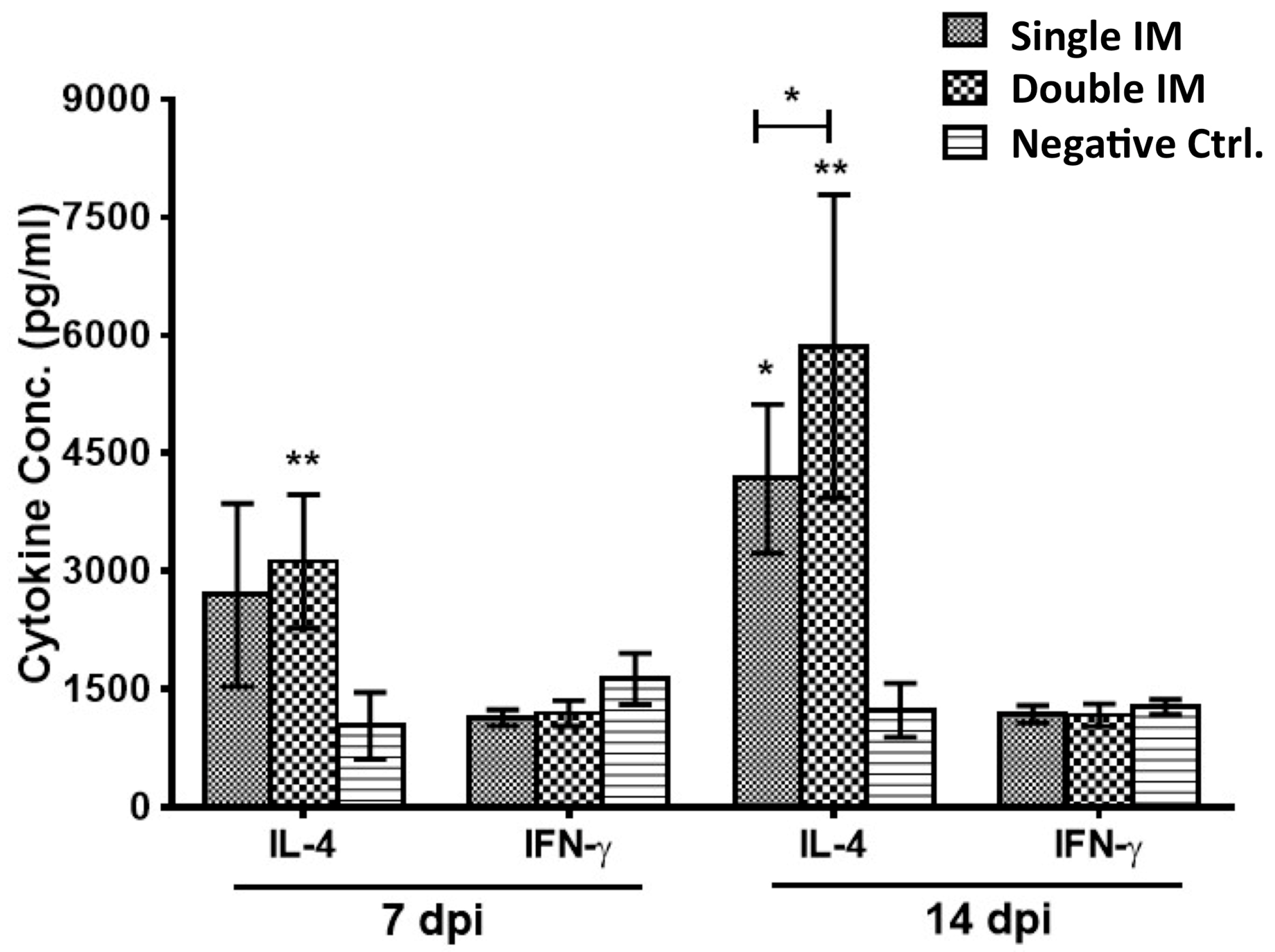
3.2. Cytokine Production of SPF Chickens
3.3. Protective Efficacy of the Vaccine
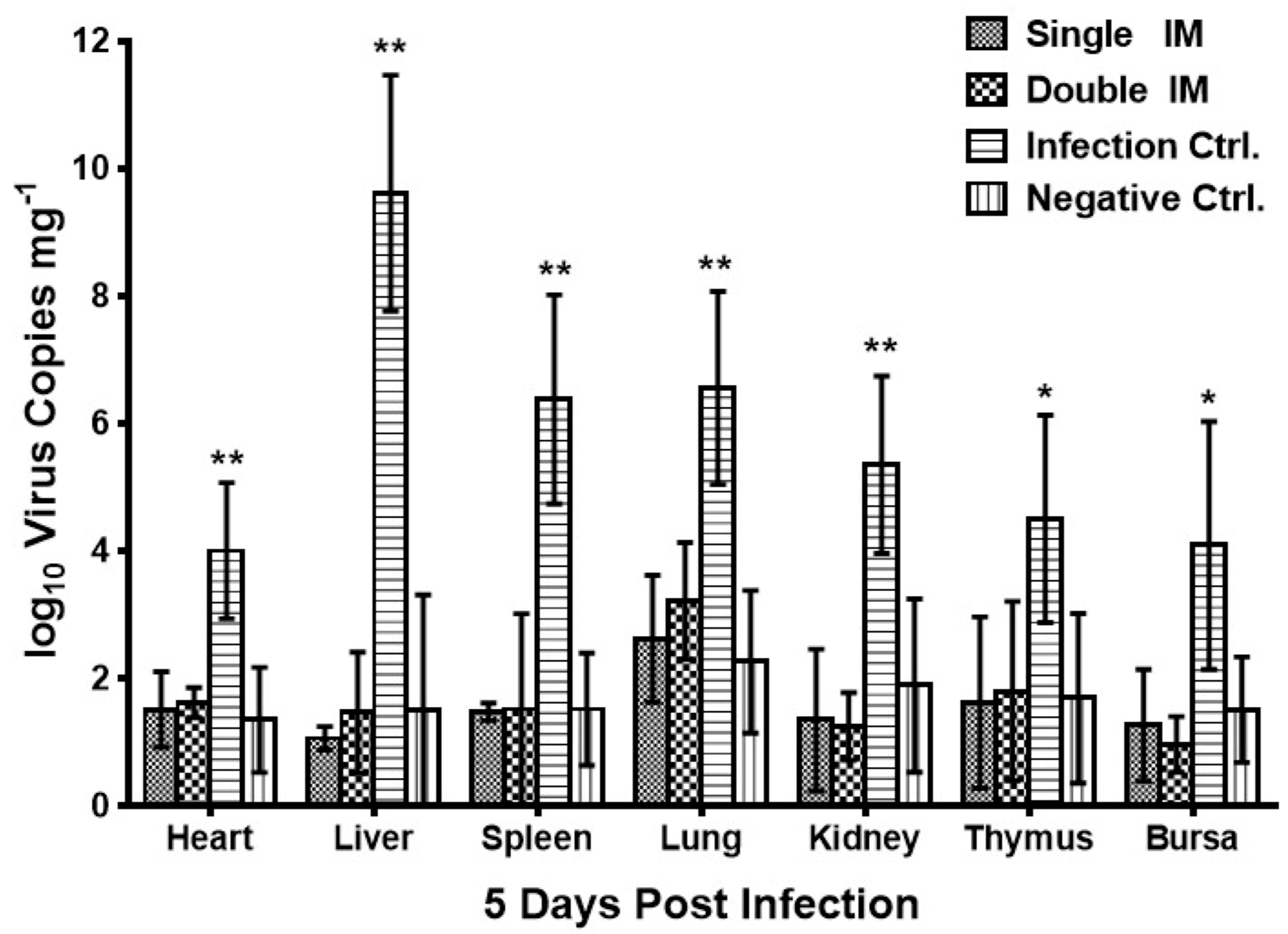
3.4. Virus Shedding of HLJFAd15 in Different Tissues
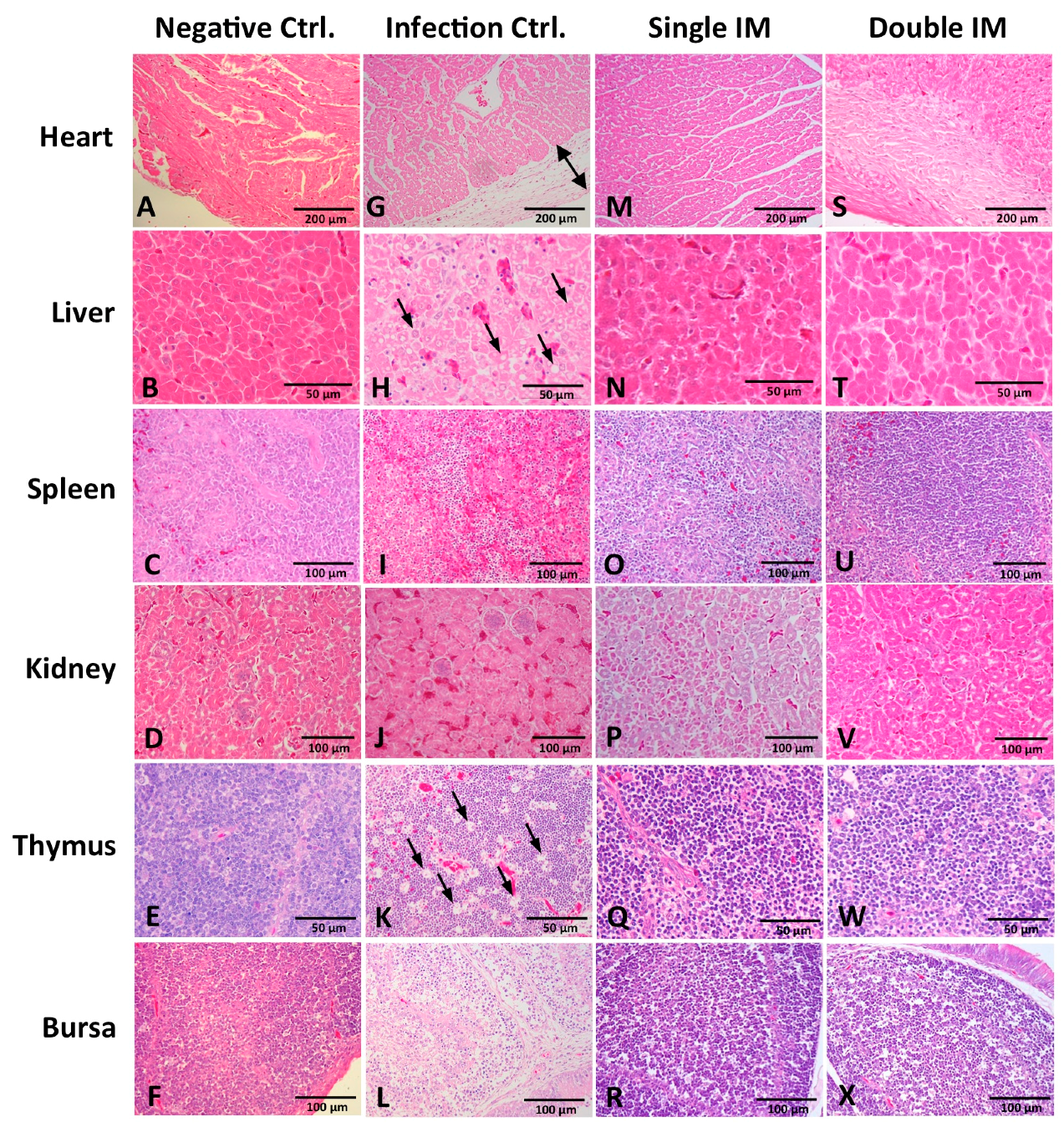
3.5. Histopathology in Different Tissues
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hess, M. Detection and differentiation of avian adenoviruses: A review. Avian Pathol. 2000, 29, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Domanska-Blicharz, K.; Tomczyk, G.; Smietanka, K.; Kozaczynski, W.; Minta, Z. Molecular characterization of fowl adenoviruses isolated from chickens with gizzard erosions. Poult. Sci. 2011, 90, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Marek, A.; Nolte, V.; Schachner, A.; Berger, E.; Schlötterer, C.; Hess, M. Two fiber genes of nearly equal lengths are a common and distinctive feature of fowl adenovirus C members. Vet. Microbiol. 2012, 156, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Khawaja, D.A.; Ahmad, S.; Rauf, A.M.; Zulfiqar, M.; Mahmood, S.M.; Mahmood, H.M. Isolation of an adenovirus from hydropericardium syndrome in broiler chicks. Pak. J. Vet. Res. 1988, 1, 2–17. [Google Scholar]
- Hess, M.; Raue, R.; Prusas, C. Epidemiological studies on fowl adenoviruses isolated from cases of infectious hydropericardium. Avian Pathol. 1999, 28, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Schade, B.; Schmitt, F.; Böhm, B.; Alex, M.; Fux, R.; Cattoli, G.; Terregino, C.; Monne, I.; Currie, R.J.; Olias, P. Adenoviral gizzard erosion in broiler chickens in Germany. Avian Dis. 2013, 57, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Dar, A.; Gomis, S.; Shirley, I.; Mutwiri, G.; Brownlie, R.; Potter, A.; Gerdts, V.; Tikoo, S.K. Pathotypic and molecular characterization of a fowl adenovirus associated with inclusion body hepatitis in Saskatchewan chickens. Avian Dis. 2012, 56, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Niczyporuk, J.S. Phylogenetic and geographic analysis of fowl adenovirus field strains isolated from poultry in Poland. Arch. Virol. 2016, 161, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Kaján, G.L.; Kecskeméti, S.; Harrach, B.; Benkő, M. Molecular typing of fowl adenoviruses, isolated in Hungary recently, reveals high diversity. Vet. Microbiol. 2013, 167, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Toro, H.; Prusas, C.; Raue, R.; Cerda, L.; Geisse, C.; González, C.; Hess, M. Characterization of fowl adenoviruses from outbreaks of inclusion body hepatitis/hydropericardium syndrome in Chile. Avian Dis. 1999, 43, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Joubert, H.W.; Aitchison, H.; Maartens, L.H.; Venter, E.H. Molecular differentiation and pathogenicity of Aviadenoviruses isolated during an outbreak of inclusion body hepatitis in South Africa. J. S. Afr. Vet. Assoc. 2014, 85, 1058. [Google Scholar] [CrossRef] [PubMed]
- Vera-Hernández, P.F.; Morales-Garzón, A.; Cortés-Espinosa, D.V.; Galiote-Flores, A.; García-Barrera, L.J.; Rodríguez-Galindo, E.T.; Toscano-Contreras, A.; Lucio-Decanini, E.; Absalón, A.E. Clinicopathological characterization and genomic sequence differences observed in a highly virulent fowl Aviadenovirus serotype 4. Avian Pathol. 2016, 45, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Gowthaman, V.; Singh, S.D.; Dhama, K.; Barathidasan, R.; Kumar, M.A.; Desingu, P.A.; Mahajan, N.K.; Ramakrishnan, M.A. Fowl adenovirus (FAdV) in India: Evidence for emerging role as primary respiratory pathogen in chickens. Pak. J. Biol. Sci. 2012, 15, 900–903. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.S.; Kye, S.J.; Kim, J.Y.; Jeon, W.J.; Lee, E.K.; Park, K.Y.; Sung, H.W. Epidemiological investigation of outbreaks of fowl adenovirus infection in commercial chickens in Korea. Poult. Sci. 2012, 91, 2502–2506. [Google Scholar] [CrossRef] [PubMed]
- Mase, M.; Nakamura, K. Phylogenetic analysis of fowl adenoviruses isolated from chickens with gizzard erosion in Japan. J. Vet. Med. Sci. 2014, 76, 1535–1538. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Liu, L.; Gao, Y.; Liu, C.; Qi, X.; Zhang, Y.; Wang, Y.; Li, K.; Gao, L.; Wang, X.; et al. Characterization of a hypervirulent fowl adenovirus 4 with the novel genotype newly prevalent in China and establishment of reproduction infection model of hydropericardium syndrome in chickens. Poult. Sci. 2017, 96, 1581–1588. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, M.K.; Hussain, I.; Arshad, M.; Muhammad, G. Preparation and evaluation of chicken embryo-adapted fowl adenovirus serotype 4 vaccine in broiler chickens. Trop. Anim. Health Prod. 2011, 43, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Schonewille, E.; Jaspers, R.; Paul, G.; Hess, M. Specific-pathogen-free chickens vaccinated with a live FAdV-4 vaccine are fully protected against a severe challenge even in the absence of neutralizing antibodies. Avian Dis. 2010, 54, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Lim, T.H.; Lee, D.H.; Youn, H.N.; Yuk, S.S.; Kim, B.Y.; Choi, S.W.; Jung, C.H.; Han, J.H.; Song, C.S. An inactivated oil-emulsion fowl Adenovirus serotype 4 vaccine provides broad cross-protection against various serotypes of fowl Adenovirus. Vaccine 2014, 32, 3564–3568. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.S.; Ashraf, A.; Rahman, M.; Khan, M.I.; Qureshi, J.A. A subunit vaccine against hydropericardium syndrome using adenovirus penton capsid protein. Vaccine 2012, 30, 7153–7156. [Google Scholar] [CrossRef] [PubMed]
- Schachner, A.; Marek, A.; Jaskulska, B.; Bilic, I.; Hess, M. Recombinant FAdV-4 fiber-2 protein protects chickens against hepatitis-hydropericardium syndrome (HHS). Vaccine 2014, 32, 1086–1092. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhong, Q.; Zhao, Y.; Hu, Y.X.; Zhang, G.Z. Pathogenicity and complete genome characterization of fowl adenoviruses isolated from chickens associated with inclusion body hepatitis and hydropericardium syndrome in China. PLoS ONE 2015, 10, e0133073. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, J.; Qiu, L.; Han, Z.; Liu, S. Fowl adenovirus species C serotype 4 is attributed to the emergence of hepatitis-hydropericardium syndrome in chickens in China. Infect. Genet. Evol. 2016, 45, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Liang, G.; Zhang, J.; Wang, W.; Song, N.; Wang, P.; Zheng, W.; Xie, Q.; Shao, H.; Wan, Z.; et al. Outbreaks of serotype 4 fowl adenovirus with novel genotype, China. Emerg. Microbes Infect. 2016, 5, e50. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Luo, L.; Luo, Q.; Zhang, T.; Zhao, K.; Wang, H.; Zhang, R.; Lu, Q.; Pan, Z.; Shao, H.; et al. Genome sequence of a fowl adenovirus serotype 4 strain lethal to chickens, isolated from China. Genome Announc. 2016, 4. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wan, W.; Gao, D.; Li, Y.; Yang, X.; Liu, H.; Yao, H.; Chen, L.; Wang, C.; Zhao, J. Genetic characterization of novel fowl aviadenovirus 4 isolates from outbreaks of hepatitis-hydropericardium syndrome in broiler chickens in China. Emerg. Microbes Infect. 2016, 5, e117. [Google Scholar] [CrossRef] [PubMed]
- Niczyporuk, J.S. Molecular characterisation of fowl adenovirus type 7 isolated from poultry associated with inclusion body hepatitis in Poland. Arch. Virol. 2017, 162, 1325–1333. [Google Scholar] [CrossRef] [PubMed]
- Reed, L.J.; Muench, H. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 1938, 27, 493–497. [Google Scholar]
- Pan, Q.; Yang, Y.; Shi, Z.; Liu, L.; Gao, Y.; Qi, X.; Liu, C.; Zhang, Y.; Cui, H.; Wang, X. Different dynamic distribution in chickens and ducks of the hypervirulent, novel genotype fowl adenovirus serotype 4 recently emerged in China. Front. Microbiol. 2017, 8, 1005. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Gao, H.; Gao, L.; Qi, X.; Gao, Y.; Qin, L.; Wang, Y.; Wang, X. Enhancement of humoral and cellular immunity in chickens against reticuloendotheliosis virus by DNA prime-protein boost vaccination. Vaccine 2013, 31, 1944–1949. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Gao, Y.; Ni, W.; Sun, M.; Wang, Y.; Yin, C.; Qi, X.; Gao, H.; Wang, X. Development and application of real-time PCR for detection of subgroup J avian leukosis virus. J. Clin. Microbiol. 2013, 51, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Ahmed, K.A.; Ayalew, L.E.; Popowich, S.; Kurukulasuriya, S.; Goonewardene, K.; Gunawardana, T.; Karunarathna, R.; Ojkic, D.; Tikoo, S.K.; et al. Immunogenicity and protective efficacy of virus-like particles and recombinant fiber proteins in broiler-breeder vaccination against fowl adenovirus (FAdV)-8b. Vaccine 2017, 35, 2716–2722. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Sharif, S.; Nagy, E. Oral inoculation of chickens with a candidate fowl adenovirus 9 vector. Clin. Vaccine Immunol. 2013, 20, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Valdivia-Olarte, H.; Requena, D.; Ramirez, M.; Saravia, L.E.; Izquierdo, R.; Falconi-Agapito, F.; Zavaleta, M.; Best, I.; Fernández-Díaz, M.; Zimic, M. Design of a predicted MHC restricted short peptide immunodiagnostic and vaccine candidate for fowl adenovirus C in chicken infection. Bioinformation 2015, 11, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Fingerut, E.; Gutter, B.; Gallili, G.; Michael, A.; Pitcovski, J. A subunit vaccine against the adenovirus egg-drop syndrome using part of its fiber protein. Vaccine 2003, 21, 2761–2766. [Google Scholar] [CrossRef]
- Shah, M.S.; Ashraf, A.; Khan, M.I.; Rahman, M.; Habib, M.; Qureshi, J.A. Molecular cloning, expression and characterization of 100K gene of fowl adenovirus-4 for prevention and control of hydropericardium syndrome. Biologicals 2016, 44, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Sarfraz, M.; Suleman, M.; Tikoo, S.K.; Wheler, C.; Potter, A.A.; Gerdts, V.; Dar, A. Immune responses to in ovo vaccine formulations containing inactivated fowl adenovirus 8b with poly[di(sodium carboxylatoethylphenoxy)]phosphazene (PCEP) and avian β defensin as adjuvants in chickens. Vaccine 2017, 35, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Changjing, L.; Haiying, L.; Dongdong, W.; Jingjing, W.; Youming, W.; Shouchun, W.; Jida, L.; Ping, L.; Jianlin, W.; Shouzhen, X.; et al. Characterization of fowl adenoviruses isolated between 2007 and 2014 in China. Vet. Microbiol. 2016, 197, 62–67. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, Q.; Yang, Y.; Gao, Y.; Qi, X.; Liu, C.; Zhang, Y.; Cui, H.; Wang, X. An Inactivated Novel Genotype Fowl Adenovirus 4 Protects Chickens against the Hydropericardium Syndrome That Recently Emerged in China. Viruses 2017, 9, 216. https://doi.org/10.3390/v9080216
Pan Q, Yang Y, Gao Y, Qi X, Liu C, Zhang Y, Cui H, Wang X. An Inactivated Novel Genotype Fowl Adenovirus 4 Protects Chickens against the Hydropericardium Syndrome That Recently Emerged in China. Viruses. 2017; 9(8):216. https://doi.org/10.3390/v9080216
Chicago/Turabian StylePan, Qing, Yanchao Yang, Yulong Gao, Xiaole Qi, Changjun Liu, Yanping Zhang, Hongyu Cui, and Xiaomei Wang. 2017. "An Inactivated Novel Genotype Fowl Adenovirus 4 Protects Chickens against the Hydropericardium Syndrome That Recently Emerged in China" Viruses 9, no. 8: 216. https://doi.org/10.3390/v9080216
APA StylePan, Q., Yang, Y., Gao, Y., Qi, X., Liu, C., Zhang, Y., Cui, H., & Wang, X. (2017). An Inactivated Novel Genotype Fowl Adenovirus 4 Protects Chickens against the Hydropericardium Syndrome That Recently Emerged in China. Viruses, 9(8), 216. https://doi.org/10.3390/v9080216




