Identification of Vimentin as a Potential Therapeutic Target against HIV Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Cultures and Reagents
2.2. Comparative Proteomics
2.3. Western Blotting to Detect Vimentin
2.4. Construction of the Expression Transfer Plasmid Encoding Short Hairpin RNA Targeting Vimentin and the Mock Transfer Plasmid
2.5. Construction of the Transfer Plasmid Encoding the eGFP Reporter Gene
2.6. Production of Lentiviral Vector Particles
2.7. Generation of the Vimentin Stable Knockdown Cell Line (MT4sh/Vim) and the MT4mock Cell Line
2.8. Early Steps of the HIV-1 Replication Assay
2.9. HIV-1 Replication Assay
2.10. Cytotoxicity Assay
2.11. Transmission Electron Microscopy
2.12. Immunofluorescence Analysis
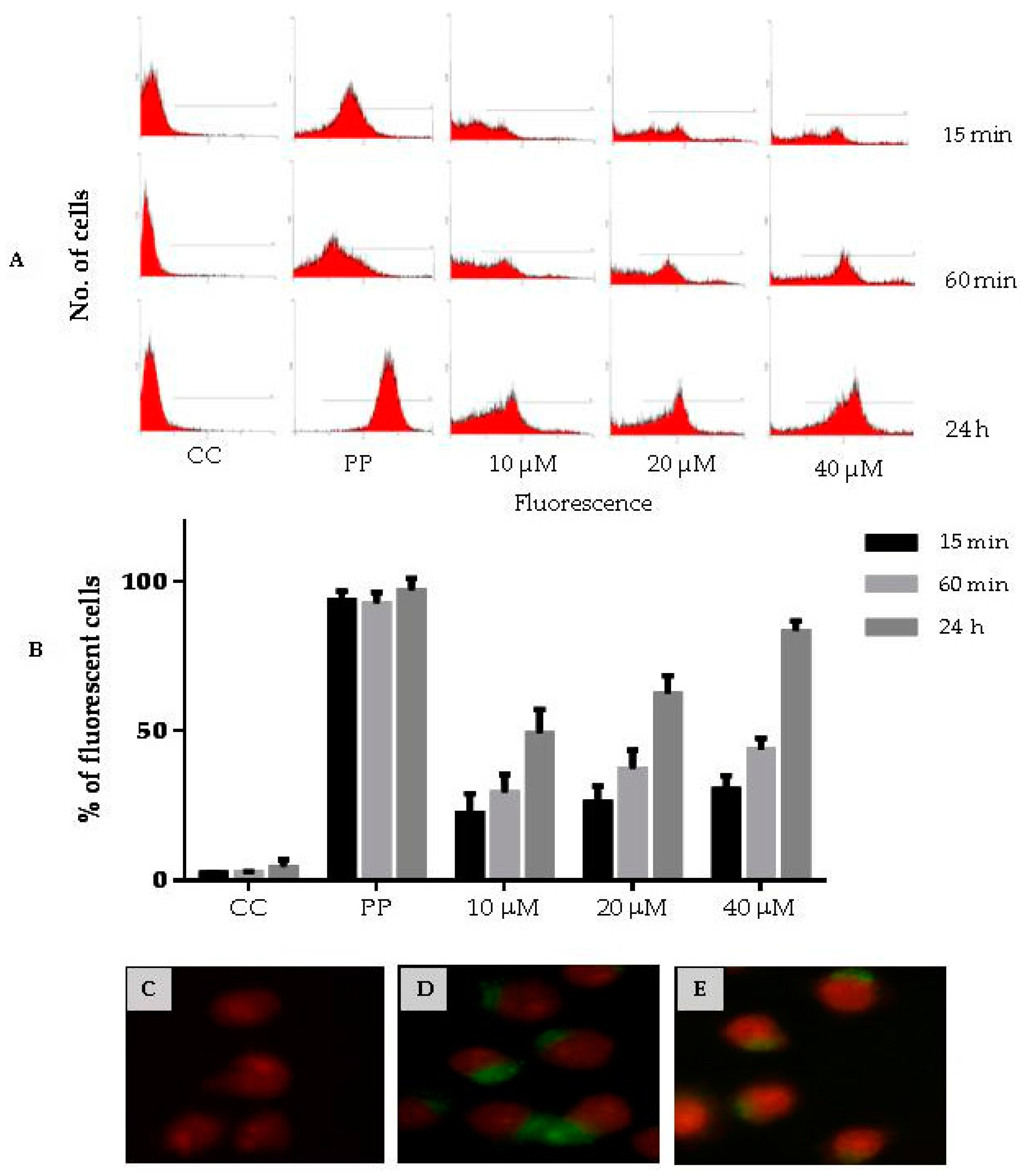
2.13. Cell Penetration Assays
2.14. Statistical Analysis
3. Results
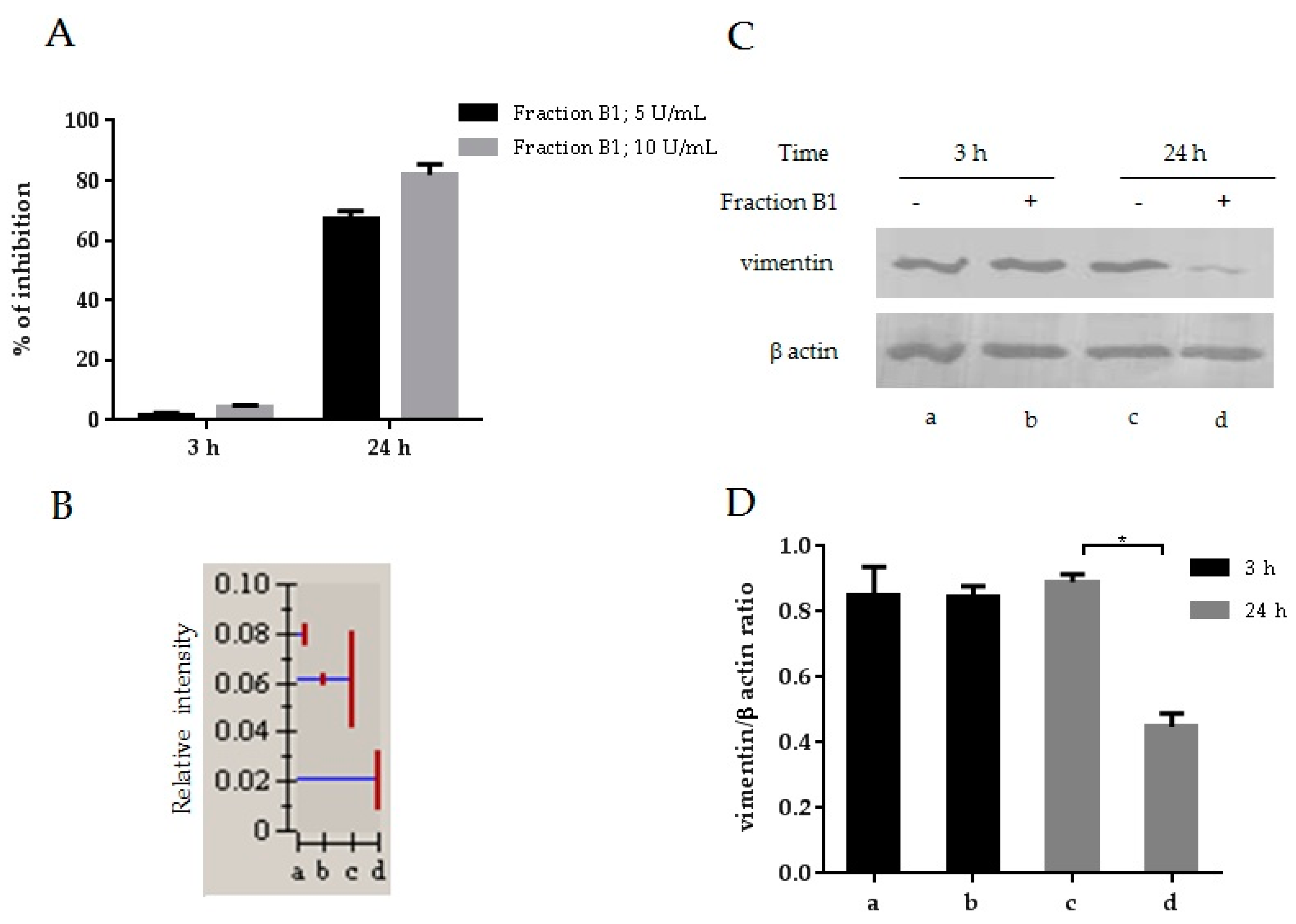
3.1. HIV-1 Inhibition and Downmodulation of Vimentin in MT4 Cells Treated with an Anti-HIV Fraction
3.2. Generation of Vimentin Stable Knockdown and Mock Cell Lines
3.3. Effect of Vimentin Knockdown on Early Steps of HIV-1 Replication
3.4. Inhibition of Replication Competent HIV-1 in Vimentin Knockdown Cell Line
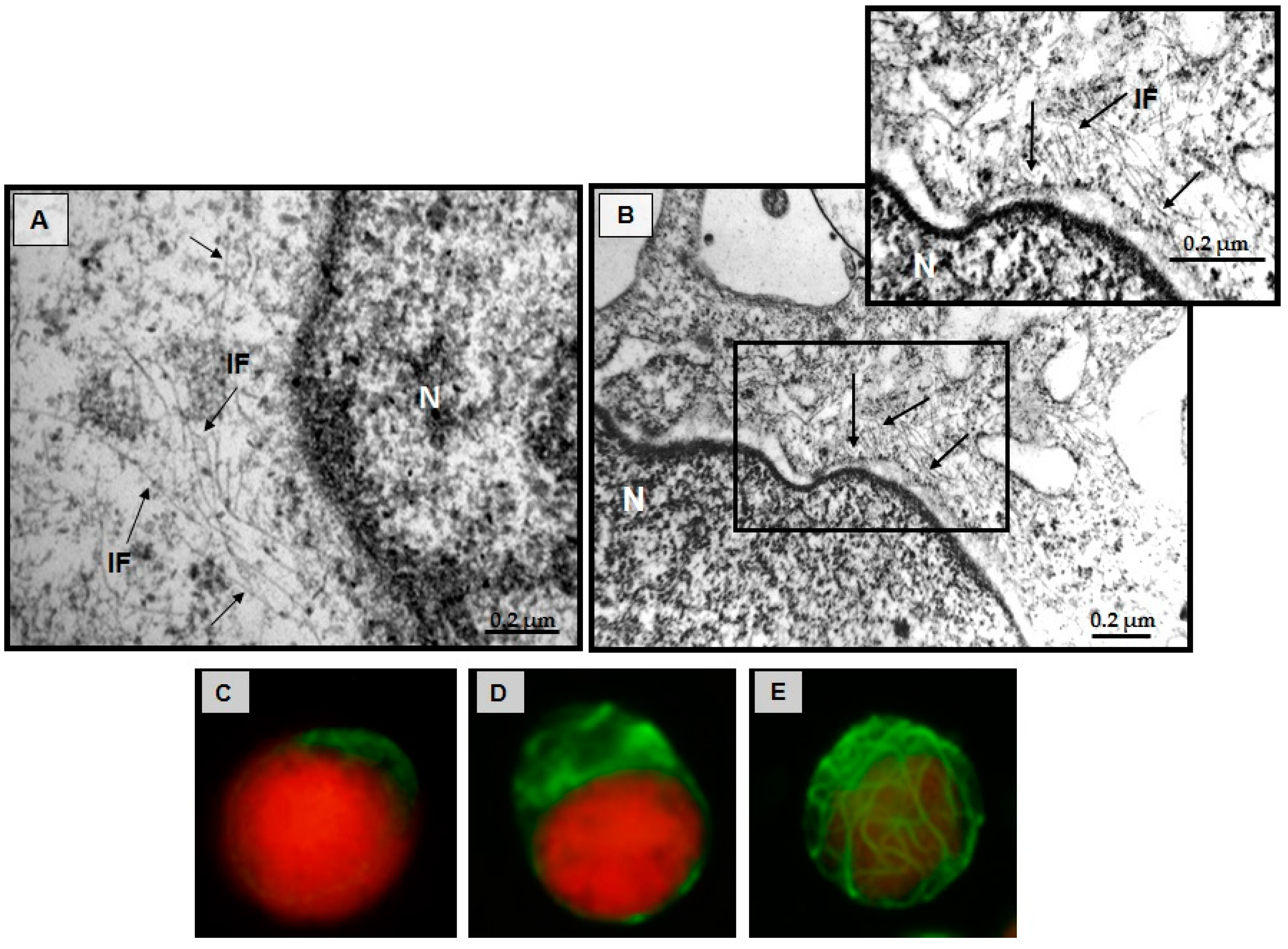
3.5. Structural Changes of Intermediate Filaments in MT4 Cells
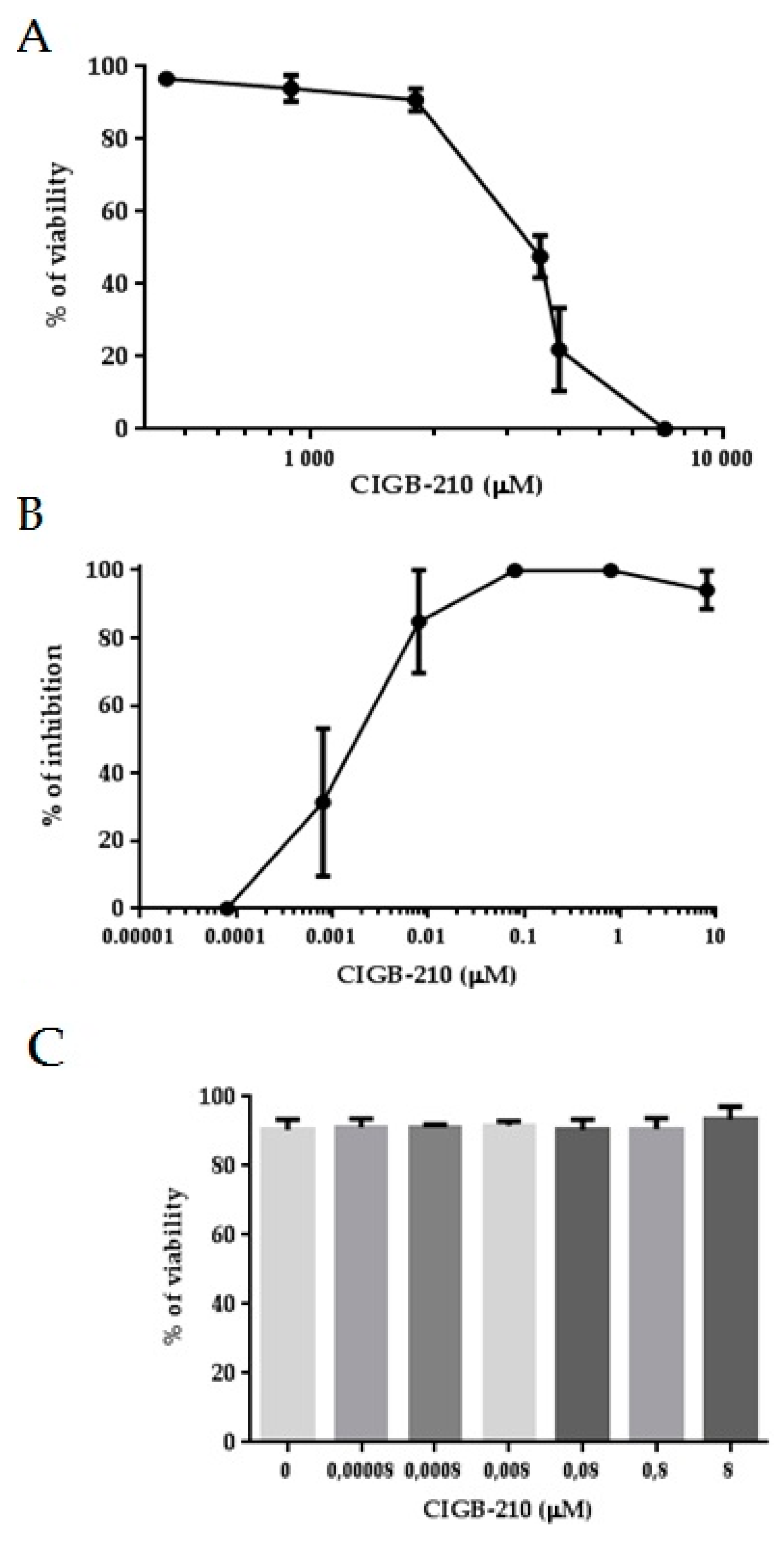
3.6. HIV-1 Inhibition by a Synthetic Peptide
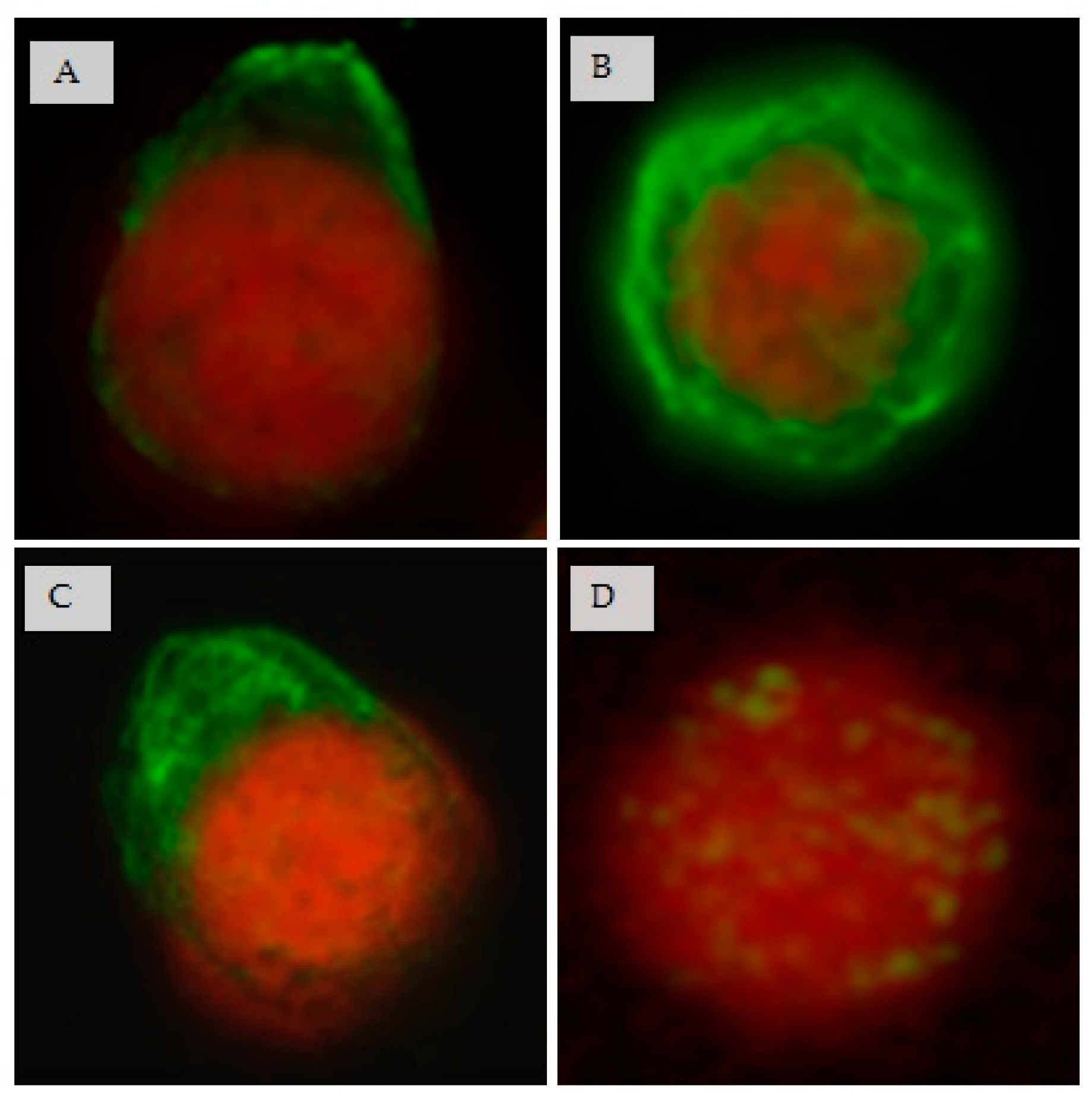
3.7. Effect of CIGB-210 on Intermediate Filaments in MT4 Cells
3.8. Cell Penetration of CIGB-210
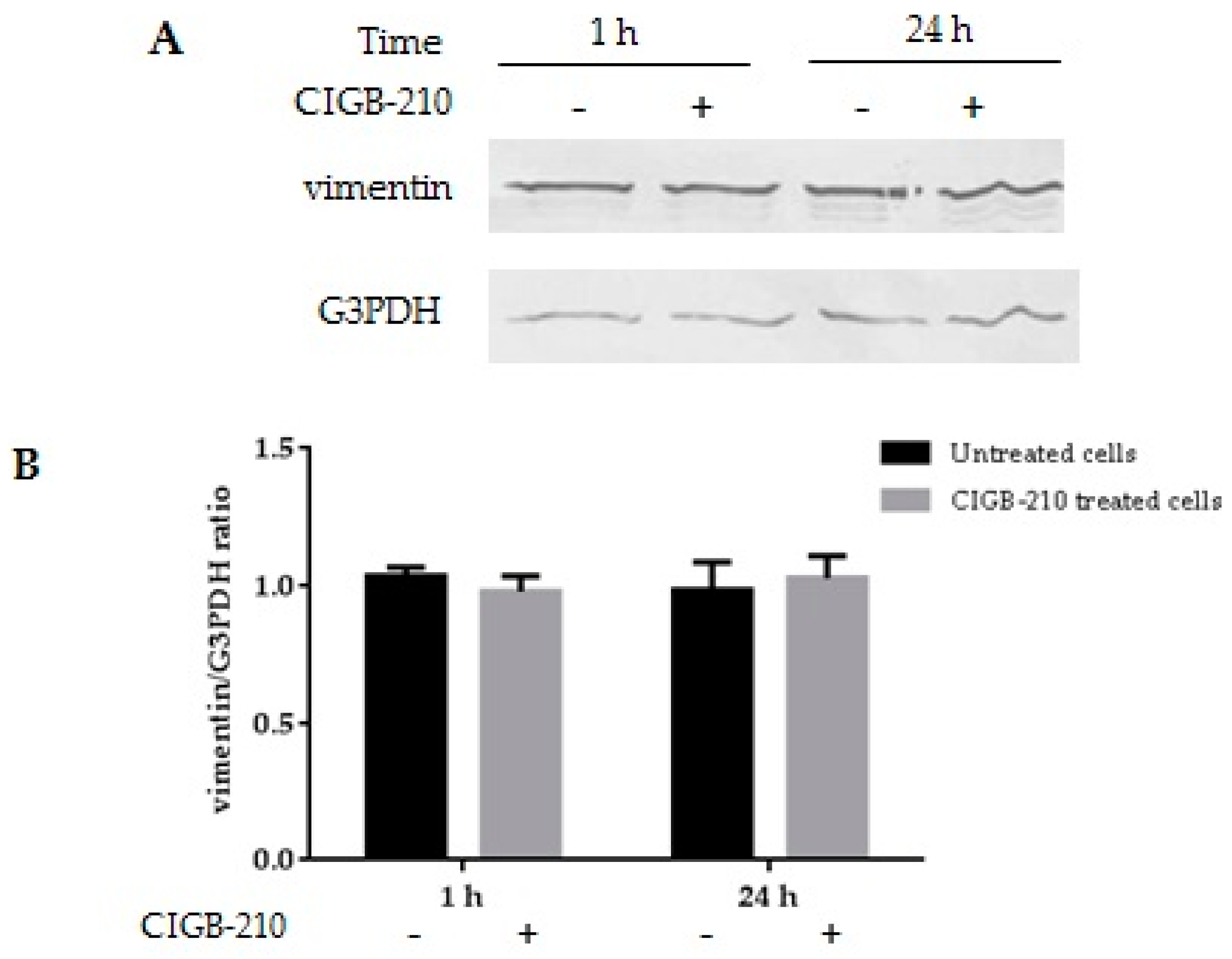
3.9. Effect of CIGB-210 on Vimentin Expression in MT4 Cells
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organization (WHO). The Top 10 Causes of Death. Updated May 2014. Geneva, Switzerland. Available online: http://www.who.int/mediacentre/factsheets/fs310/en/index1.html (accessed on 10 April 2015).
- Joint United Nations Programme on HIV/AIDS (UNAIDS). Global Report: UNAIDS Report on the Global AIDS Epidemic 2013. Geneva, Switzerland. Available online: http://www.who.int/hiv/pub/me/unaids_global_report (accessed on 13 April 2015).
- Joint United Nations Programme on HIV/AIDS (UNAIDS). World AIDS Day 2014 Report. Geneva, Switzerland. Available online: http://www.unaids.org/en/resources/campaigns/World-AIDS-Day-Report-2014/factsheet/html (accessed on 13 April 2015).
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. 2015; Department of Health and Human Services. Available online: http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf (accessed on 15 April 2015). [Google Scholar]
- Lobritz, M.A.; Ratcliff, A.N. HIV-1 entry, inhibitors and resistance. Viruses 2012, 2, 1069–1105. [Google Scholar] [CrossRef] [PubMed]
- Van der Kuyl, A.C.; Bakker, M.; Jurriaans, S.; Back, N.K.T.; Pasternak, A.O.; Cornelissen, M.; Berkhout, B. Translational HIV-1 research: From routine diagnostics to new virology insights in Amsterdam, The Netherlands during 1983–2013. Retrovirology 2013, 10. [Google Scholar] [CrossRef] [PubMed]
- Montaner, J.S.G.; Lima, V.D.; Harrigan, P.R.; Lourenc, L.; Yip, B.; Nosyk, B.; Wood, E.; Kerr, T.; Shannon, K.; Moore, D.; et al. Expansion of HAART coverage is associated with sustained decreases in HIV/AIDS morbidity, mortality and HIV transmission: The “HIV treatment as prevention” experience in a Canadian setting. PLoS ONE 2014, 9, e87872. [Google Scholar] [CrossRef] [PubMed]
- Kitahata, M.M.; Gange, S.J.; Abraham, A.G.; Merriman, B.; Saag, M.S.; Justice, A.C.; Hogg, R.S.; Deeks, S.G.; Eron, J.J.; Brooks, J.T.; et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N. Engl. J. Med. 2009, 360, 1815–1826. [Google Scholar] [CrossRef] [PubMed]
- Joint United Nations Programme on HIV/AIDS (UNAIDS): Global AIDS Response Progress Reporting 2014. Geneva, Switzerland. Available online: http://www.unaids.org/sites/default/files/GARPR_2014_guidelines_0.pdf (accessed on 15 April 2015).
- Arhel, N.; Kirchhoff, F. Host proteins in HIV infection: New therapeutic target. Biochem. Biophys. Acta 2010, 1802, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.R.; Cook, N.; Wong, R.; Hsue, P.; Ridker, P.; Currier, J.; Shurin, S. Stimulating high impact HIV-related cardiovascular research: Recommendations from a multidisciplinary NHLBI Working Group on HIV-related heart, lung, and blood disease. J. Am. Coll. Cardiol. 2015, 24, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Lint, C.V.; Bouchat, S.; Marcello, A. HIV-1 transcription and latency: An update. Retrovirology 2013, 10. [Google Scholar] [CrossRef]
- Dorr, P.; Westby, M.; Dobbs, S.; Griffin, P.; Irvine, B.; Macartney, M.; Mori, J.; Rickett, G.; Smith-Burchnell, C.; Napier, C.; et al. Maraviroc (UK-427,857), a potent, orally bioavailable, and selective small-molecule inhibitor of chemokine receptor CCR5 with broadspectrum anti-human immunodeficiency virus type 1 activity. Antimicrob. Agents Chemother. 2005, 49, 4721–4732. [Google Scholar] [CrossRef] [PubMed]
- Nozza, S.; Galli, L.; Antinori, A.; Chiappetta, S.; Mazzotta, F.; Zaccarelli, M.; Ottou, S.; De Battista, D.; Pogliaghi, M.; Di Pietro, M.; et al. Maraviroc 150 mg daily plus lopinavir/ritonavir, a nucleoside/nucleotide reverse transcriptase inhibitor-sparing regimen for HIV-infected naive patients: 48-week final results of VEMAN study. Clin. Microbiol. Infect. 2014, 21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Du, C.; Feng, Z.; Zhu, J.; Li, Y. Hologram quantitative structure activity relationship, docking, and molecular dynamics studies of inhibitors for CXCR4. Chem. Biol. Drug Des. 2015, 85, 119–136. [Google Scholar] [CrossRef] [PubMed]
- Pace, C.S.; Fordyce, M.W.; Franco, D.; Kao, C.Y.; Seaman, M.S.; Ho, D.D. Anti-CD4 monoclonal antibody ibalizumab exhibits breadth and potency against HIV-1, with natural resistance mediated by the loss of a V5 glycan in envelope. J. Acquir. Immune Defic. Syndr. 2013, 62, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Taltynov, O.; Desimmie, B.A.; Demeulemeester, J.; Christ, F.; Debyzer, Z. Cellular cofactors of lentiviral integrase: From target validation to drug discovery. Mol. Biol. Int. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Moisan, E.; Girard, D. Cell surface expression of intermediate filament proteins vimentin and lamin B1 in human neutrophil spontaneous apoptosis. J. Leukoc. Biol. 2006, 79, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Shoeman, R.L.; Honer, B.; Stollert, T.J.; Kesselmeier, C.; Miedel, M.C.; Traub, P.; Graves, M.C. Human immunodeficiency virus type 1 protease cleaves the intermediate filament proteins vimentin, desmin, and glial fibrillary acidic protein. Proc. Natl. Acad. Sci. USA 1990, 87, 6336–6340. [Google Scholar] [CrossRef] [PubMed]
- Stolp, B.; Fackler, O.T. How HIV Takes advantage of the cytoskeleton in entry and replication. Viruses 2011, 3, 293–311. [Google Scholar] [CrossRef] [PubMed]
- Jolly, C.; Mitar, I.; Sattentau, Q.J. Requirement for an intact T-cell actin and tubulin cytoskeleton for efficient assembly and spread of human immunodeficiency virus type 1. J. Virol. 2007, 81, 5547–5560. [Google Scholar] [CrossRef] [PubMed]
- Sundquist, W.I.; Kräusslich, H.G. HIV-1 assembly, budding, and maturation. Cold Spring Harb. Perspect. Med. 2012. [Google Scholar] [CrossRef]
- Shoeman, R.L.; Hüttermann, C.; Hartig, R.; Traub, P. Amino-terminal polypeptides of vimentin are responsible for the changes in nuclear architecture associated with human immunodeficiency virus type 1 protease activity in tissue culture cells. Mol. Biol. Cell 2001, 12, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Yohannes, E.; Ghosh, S.K.; Jiang, B.; McCormick, T.S.; Weinberg, A.; Hill, E.; Faddoul, F.; Chance, M.R. Proteomic signatures of human oral epithelial cells in HIV-infected subjects. PLoS ONE 2011, 6, e27816. [Google Scholar] [CrossRef] [PubMed]
- Karczewski, M.K.; Strebel, K. Cytoskeleton association and virion incorporation of the human immunodeficiency virus type 1 vif protein. J. Virol. 1996, 70, 494–507. [Google Scholar] [PubMed]
- Fernández-Ortega, C.; Dubed, M.; Ruibal, I.; Vilarrubia, O.L.; Menéndez, J.C.; Navea, L.; Ojeda, M.; Araña, M.J. Inhibition of in vitro HIV infection by dialyzable leukocyte extract. Biotherapy 1996, 9, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ortega, C.; Dubed, M.; Ramos, Y.; Navea, L.; Álvarez, G.; Lovaina, L.; Lopez, L.; Casillas, D.; Rodriguez, L. Non-induced leukocyte extract reduces HIV replication and TNF secretion. Biochem. Biophys. Res. Commun. 2004, 325, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- Perea, S.E.; Reyes, O.; Puchades, Y.; Mendoza, O.; Vispo, N.S.; Torrens, I.; Santos, A.; Silva, R.; Acevedo, B.; Lopez, E.; et al. Antitumor effect of a novel proapoptotic peptide that impairs the phosphorylation by the protein kinase 2 (casein kinase 2). Cancer Res. 2004, 64, 7127–7129. [Google Scholar] [CrossRef] [PubMed]
- Nuutila, J.; Lilius, E.M. Flow cytometric quantitative determination of ingestion by phagocytes needs the distinguishing of overlapping populations of binding and ingesting cells. Cytometry 2005, 65, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Van Bracht, E.; Versteegden, L.R.; Stolle, S.; Verdurmen, W.P.; Woestenenk, R.; Raave, R.; Hafmans, T.; Oosterwijk, E.; Brock, R.; van Kuppevelt, T.H.; et al. Enhanced cellular uptake of albumin-based lyophilisomes when functionalized with cell-penetrating peptide TAT in HeLa cells. PLoS ONE 2014, 9, e110813. [Google Scholar] [CrossRef] [PubMed]
- Goldman, R.; Khuon, S.; Chou, Y.; Opal, P.; Steinert, P. The function of intermediate filaments in cell shape and cytoskeletal integrity. J. Cell Biol. 1996, 134, 971–983. [Google Scholar] [CrossRef] [PubMed]
- Santa-Marta, M.; MatosdeBrito, P.; Godinho-Santos, A.; Goncalves, J. Host factors and HIV-1 replication: Clinical evidence and potential therapeutic approaches. Front. Immunol. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, M. Dissecting HIV-1 through RNA interference. Nat. Rev. 2003, 3, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Garbelli, A.; Radi, M.; Falchi, F.; Beermann, S.; Zanoli, S.; Manetti, F.; Dietrich, U.; Botta, M.; Maga, G. Targeting the human DEAD-Box polypeptide 3 (DDX3) RNA helicase as a novel strategy to inhibit viral replication. Curr. Med. Chem. 2011, 18, 3015–3027. [Google Scholar] [CrossRef] [PubMed]
- McMahon, M.A.; Shen, L.; Siliciano, R. New approaches for quantitating the inhibition of HIV-1 replication by antiviral drugs in vitro and in vivo. Curr. Opin. Infect. Dis. 2009, 22, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Curreli, F.; Zhang, X.; Bhattacharya, S.; Waheed, A.A.; Cooper, A.; Cowburn, D.; Freed, E.O.; Debnath, A.K. Antiviral activity of a-helical stapled peptides designed from the HIV-1 capsid dimerization domain. Retrovirology 2011, 8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Pomerantz, R.J.; Dornadula, G.; Sun, Y. Human immunodeficiency virus type 1 Vif protein is an integral component of an mRNP complex of viral RNA and could be involved in the viral RNA folding and packaging process. J. Virol. 2000, 74, 8252–8261. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.K.; Connelly, R.J.; Pennathur, S.; Dubrovsky, L.; Haffar, O.K.; Bukrinsky, M.I. Anti-idiotypic antibody to the V3 domain of gp120 binds to vimentin: A possible role of intermediate filaments in the early steps of HIV-1 infection cycle. Viral Immunol. 1996, 9, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Khakhulina, T.V.; Vorkunova, G.K.; Manukhina, E.E.; Bukrinskaia, A.G. Intermediate vimentin filaments involved in type I human immunodeficiency virus replication. Dokl. Akad. Nauk. 1999, 368, 706–708. [Google Scholar] [PubMed]
- Stefanovic, S.; Windsor, M.; Nagata, K.I.; Inagaki, M.; Wileman, T. Vimentin rearrangement during African swine fever virus infection involves retrograde transport along microtubules and phosphorylation of vimentin by calcium calmodulin kinase II. J. Virol. 2005, 79, 1766–1775. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, B.; Noad, R.J.; Roy, P. Interaction between Bluetongue virus outer capsid protein VP2 and vimentin is necessary for virus egress. Virol. J. 2007, 4, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Risco, C.; Rodriguez, R.; Lopez-Iglesia, C.; Carrascosa, J.L.; Esteban, M.; Rodriguez, D. Endoplasmic Reticulum Golgi intermediate compartiment membranes and vimentin filaments participate in vaccinia virus assembly. J. Virol. 2002, 76, 1839–1855. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.S.; Hertel, L. Onset of human cytomegalovirus replication in fibroblasts requires the presence of an intact vimentin cytoskeleton. J. Virol. 2009, 83, 7015–7028. [Google Scholar] [CrossRef] [PubMed]
- Ivaska, J.; Pallari, H.; Nevo, J.; Eriksson, J. Novel functions of vimentin in cell adhesion, migration and signalling. Exp. Cell Res. 2007, 313, 2050–2062. [Google Scholar] [CrossRef] [PubMed]





© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Ortega, C.; Ramírez, A.; Casillas, D.; Paneque, T.; Ubieta, R.; Dubed, M.; Navea, L.; Castellanos-Serra, L.; Duarte, C.; Falcon, V.; et al. Identification of Vimentin as a Potential Therapeutic Target against HIV Infection. Viruses 2016, 8, 98. https://doi.org/10.3390/v8060098
Fernández-Ortega C, Ramírez A, Casillas D, Paneque T, Ubieta R, Dubed M, Navea L, Castellanos-Serra L, Duarte C, Falcon V, et al. Identification of Vimentin as a Potential Therapeutic Target against HIV Infection. Viruses. 2016; 8(6):98. https://doi.org/10.3390/v8060098
Chicago/Turabian StyleFernández-Ortega, Celia, Anna Ramírez, Dionne Casillas, Taimi Paneque, Raimundo Ubieta, Marta Dubed, Leonor Navea, Lila Castellanos-Serra, Carlos Duarte, Viviana Falcon, and et al. 2016. "Identification of Vimentin as a Potential Therapeutic Target against HIV Infection" Viruses 8, no. 6: 98. https://doi.org/10.3390/v8060098
APA StyleFernández-Ortega, C., Ramírez, A., Casillas, D., Paneque, T., Ubieta, R., Dubed, M., Navea, L., Castellanos-Serra, L., Duarte, C., Falcon, V., Reyes, O., Garay, H., Silva, E., Noa, E., Ramos, Y., Besada, V., & Betancourt, L. (2016). Identification of Vimentin as a Potential Therapeutic Target against HIV Infection. Viruses, 8(6), 98. https://doi.org/10.3390/v8060098





