Validation of the Filovirus Plaque Assay for Use in Preclinical Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Virus
2.2. Cells
2.3. Reagents, Cell Culture Media, Supplements and Stains
2.4. GLP Compliance
2.5. Filovirus Plaque Assay
2.6. Plaque Counting and Titer Calculation
2.7. Variables under Test for the Determination of Assay Robustness
2.8. Statistical Tests
3. Results
3.1. Confirmation of Accuracy
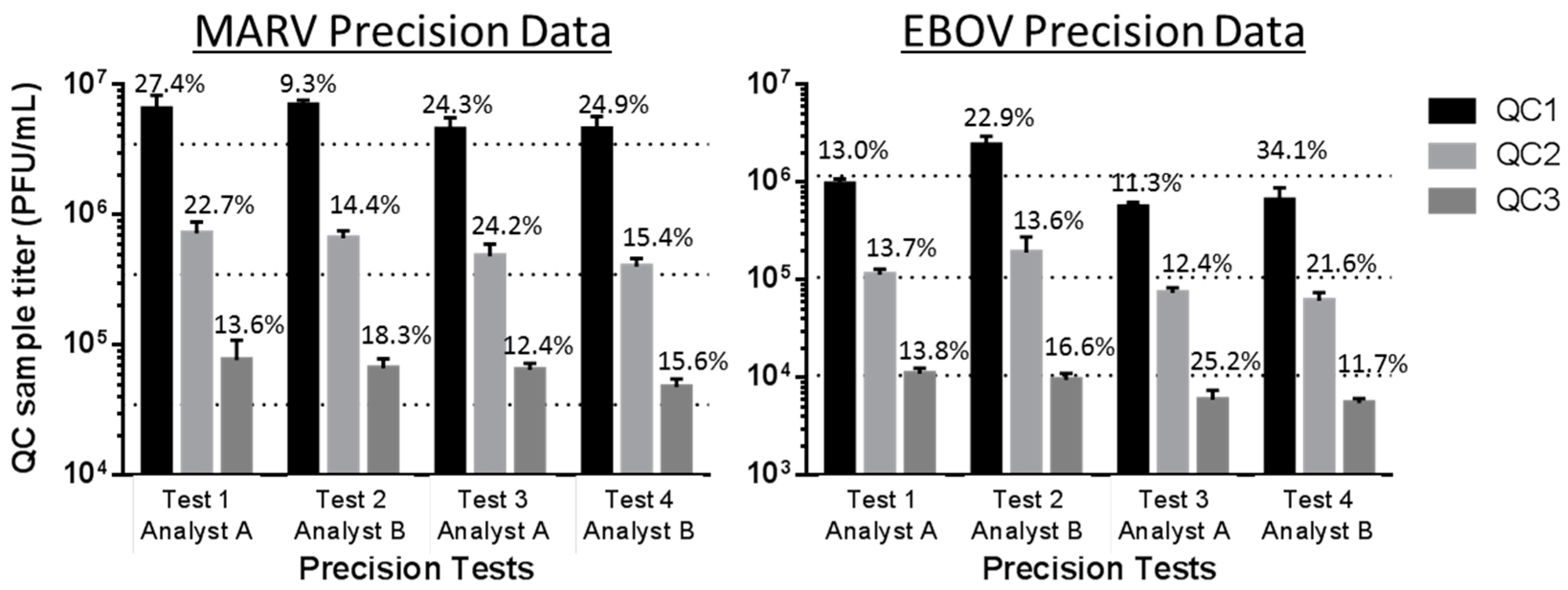
3.2. Confirmation of Precision
3.3. Confirmation of Linearity
3.4. Robustness in the Assay
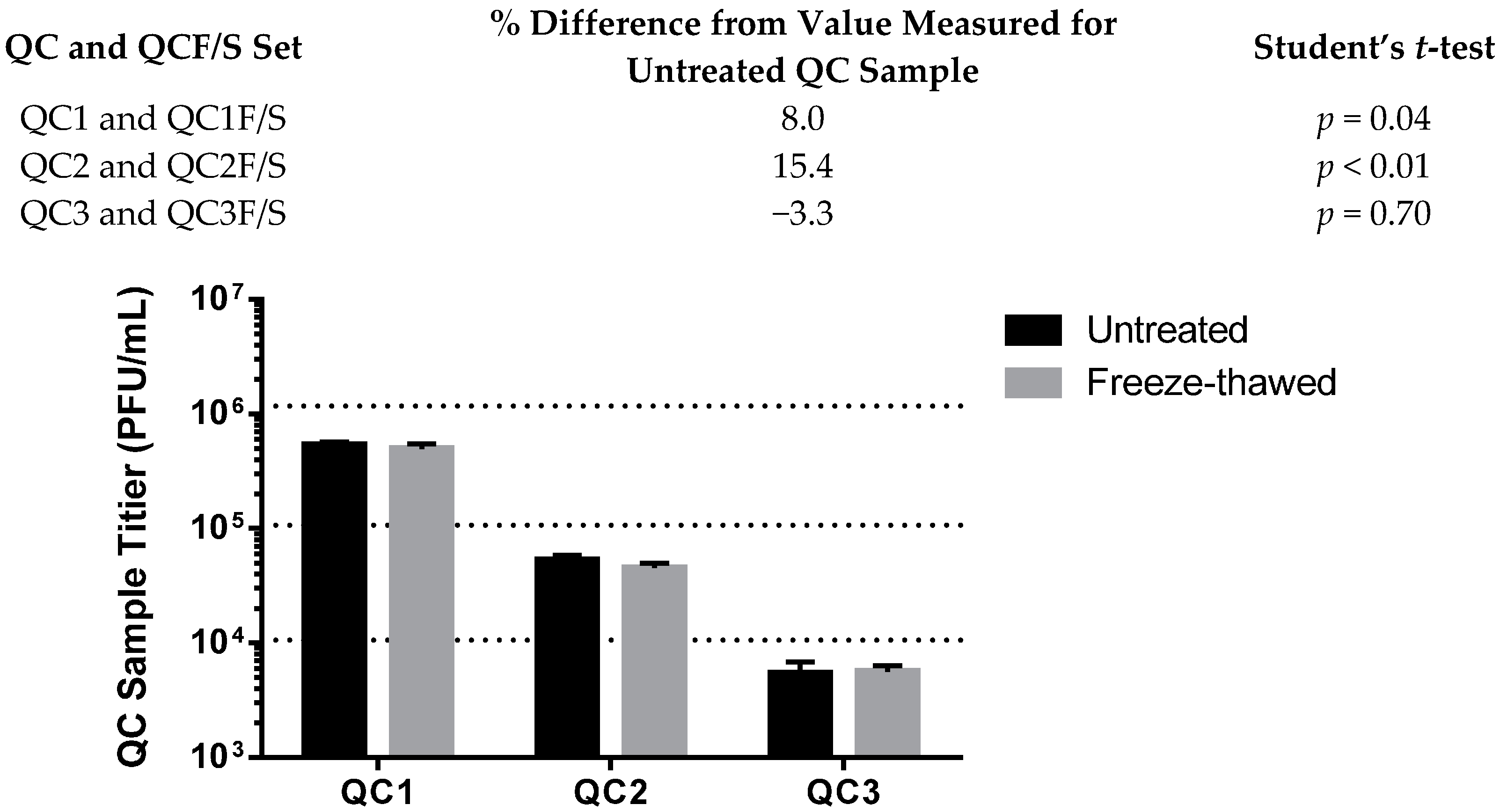
3.4.1. Stability
3.4.2. Change of Inoculum Volume and Tissue Culture Vessel
3.4.3. Optimal Day to Stain and Count Plaques
3.4.4. Cell Seeding and Time of Cell Use
3.4.5. Cell Passage Number
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kugelman, J.R.; Lee, M.S.; Rossi, C.A.; McCarthy, S.E.; Radoshitzky, S.R.; Dye, J.M.; Hensley, L.E.; Honko, A.; Kuhn, J.H.; Jahrling, P.B.; et al. Ebola virus genome plasticity as a marker of its passaging history: A comparison of in vitro passaging to non-human primate infection. PLoS ONE 2012, 7, e50316. [Google Scholar] [CrossRef] [PubMed]
- Rossi, C.A.; Kearney, B.J.; Olschner, S.P.; Williams, P.L.; Robinson, C.G.; Heinrich, M.L.; Zovanyi, A.M.; Ingram, M.F.; Norwood, D.A.; Schoepp, R.J. Evaluation of ViroCyt(R) Virus Counter for rapid filovirus quantitation. Viruses 2015, 7, 857–872. [Google Scholar] [CrossRef] [PubMed]
- Smither, S.J.; Lear-Rooney, C.; Biggins, J.; Pettitt, J.; Lever, M.S.; Olinger, G.G., Jr. Comparison of the plaque assay and 50% tissue culture infectious dose assay as methods for measuring filovirus infectivity. J. Virol. Methods 2013, 193, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Alfson, K.J.; Avena, L.E.; Beadles, M.W.; Staples, H.; Nunneley, J.W.; Ticer, A.; Dick, E.J., Jr.; Owston, M.A.; Reed, C.; Patterson, J.L.; et al. Particle-to-PFU ratio of Ebola virus influences disease course and survival in Cynomolgus macaques. J. Virol. 2015, 89, 6773–6781. [Google Scholar] [CrossRef] [PubMed]
- Cooper, P.D. The plaque assay of animal viruses. Adv. Virus Res. 1961, 8, 319–378. [Google Scholar] [PubMed]
- Product Development Under the Animal Rule: Guidance for Industry; US Depart of Health and Human Services FDA, Center for Drug Evaluation, Editor: Silver Spring, MD, USA, October 2015.
- Guidance for Industry—Bioanalytical Method Validation; US Depart of Health and Human Services FDA, Center for Drug Evaluation, Editor: Silver Spring, MD, USA, 2001.
- Kugelman, J.R.; Rossi, C.A.; Wiley, M.R.; Ladner, J.T.; Nagle, E.R.; Pfeffer, B.P.; Garcia, K.; Prieto, K.; Wada, J.; Kuhn, J.H.; et al. Informing the historical record of experimental nonhuman primate infections with Ebola virus: Genomic characterization of USAMRIID Ebola virus/H.sapiens-tc/COD/1995/Kikwit-9510621 challenge stock “R4368” and its replacement “R4415”. PLoS ONE 2016, 11, e0150919. [Google Scholar] [CrossRef] [PubMed]
- Shurtleff, A.C.; Biggins, J.E.; Keeney, A.E.; Zumbrun, E.E.; Bloomfield, H.A.; Kuehne, A.; Audet, J.L.; Alfson, K.J.; Griffiths, A.; Olinger, G.G.; et al. Standardization of the filovirus plaque assay for use in preclinical studies. Viruses 2012, 4, 3511–3530. [Google Scholar] [CrossRef] [PubMed]
- Sutton, S. Accuracy of plate counts. J. Valid. Technol. 2011, 17, 42–46. [Google Scholar]
- Kolesnikova, L.; Bugany, H.; Klenk, H.D.; Becker, S. VP40, the matrix protein of Marburg virus, is associated with membranes of the late endosomal compartment. J. Virol. 2002, 76, 1825–1838. [Google Scholar] [CrossRef] [PubMed]
- Wright, S. The Theoretical variance within and among subdivisions of a population that is in a steady state. Genetics 1952, 37, 312–321. [Google Scholar] [PubMed]
- Gupta, S.; Devanarayan, V.; Finco, D.; Gunn, G.R., 3rd; Kirshner, S.; Richards, S.; Rup, B.; Song, A.; Subramanyam, M. Recommendations for the validation of cell-based assays used for the detection of neutralizing antibody immune responses elicited against biological therapeutics. J. Pharm. Biomed. Anal. 2011, 55, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Pharmacopeia, U.S. Chapter 1223 validation of alternative microbiological methods. USP 2007, 39, 1616–1630. [Google Scholar]
- Shrout, P.E.; Fleiss, J.L. Intraclass correlations: uses in assessing rater reliability. Psychol. Bull. 1979, 86, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Sagripanti, J.L.; Lytle, C.D. Sensitivity to ultraviolet radiation of Lassa, vaccinia, and Ebola viruses dried on surfaces. Arch. Virol. 2011, 156, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Sagripanti, J.L.; Rom, A.M.; Holland, L.E. Persistence in darkness of virulent alphaviruses, Ebola virus, and Lassa virus deposited on solid surfaces. Arch. Virol. 2010, 155, 2035–2039. [Google Scholar] [CrossRef] [PubMed]
- Schuit, M.; Miller, D.M.; Reddick-Elick, M.S.; Wlazlowski, C.B.; Filone, C.M.; Herzog, A.; Colf, L.A.; Wahl-Jensen, V.; Hevey, M.; Noah, J.W. Differences in the comparative stability of Ebola virus makona-C05 and yambuku-mayinga in blood. PLoS ONE 2016, 11, e0148476. [Google Scholar] [CrossRef] [PubMed]
- Chepurnov, A.A.; Chuev Iu, P.; P'Iankov, O.V.; Efimova, I.V. The effect of some physical and chemical factors on inactivation of the Ebola virus. Vopr. Virusol. 1995, 40, 74–76. [Google Scholar] [PubMed]
- Hirschberg, R.; Ward, L.A.; Kilgore, N.; Kurnat, R.; Schiltz, H.; Albrecht, M.T.; Christopher, G.W.; Nuzum, E. Challenges, progress, and opportunities: Proceedings of the filovirus medical countermeasures workshop. Viruses 2014, 6, 2673–2697. [Google Scholar] [CrossRef] [PubMed]
- Warren, T.K.; Trefry, J.C.; Marko, S.T.; Chance, T.B.; Wells, J.B.; Pratt, W.D.; Johnson, J.C.; Mucker, E.M.; Norris, S.L.; Chappell, M.; et al. Euthanasia assessment in Ebola virus infected nonhuman primates. Viruses 2014, 6, 4666–4682. [Google Scholar] [CrossRef] [PubMed]
- Warren, T.K.; Warfield, K.L.; Wells, J.; Swenson, D.L.; Donner, K.S.; Van Tongeren, S.A.; Garza, N.L.; Dong, L.; Mourich, D.V.; Crumley, S.; et al. Advanced antisense therapies for postexposure protection against lethal filovirus infections. Nat. Med. 2010, 16, 991–994. [Google Scholar] [CrossRef] [PubMed]
- Volchkova, V.A.; Dolnik, O.; Martinez, M.J.; Reynard, O.; Volchkov, V.E. Genomic RNA editing and its impact on Ebola virus adaptation during serial passages in cell culture and infection of guinea pigs. J. Infect. Dis. 2011, 204, S941–S946. [Google Scholar] [CrossRef] [PubMed]
- Trefry, J.C.; Wollen, S.E.; Nasar, F.; Shamblin, J.D.; Kern, S.J.; Bearss, J.J.; Jefferson, M.A.; Chance, T.B.; Kugelman, J.R.; Ladner, J.T.; et al. Ebola virus infections in nonhuman primates are temporally influenced by glycoprotein poly-U editing site populations in the exposure material. Viruses 2015, 7, 6739–6754. [Google Scholar] [CrossRef] [PubMed]
- Geisbert, T.W.; Strong, J.E.; Feldmann, H. Considerations in the use of nonhuman primate models of Ebola Virus and Marburg virus infection. J. Infect. Dis. 2015, 212, S91–S97. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, J.H.; Andersen, K.G.; Baize, S.; Bao, Y.; Bavari, S.; Berthet, N.; Blinkova, O.; Brister, J.R.; Clawson, A.N.; Fair, J.; et al. Nomenclature- and database-compatible names for the two Ebola virus variants that emerged in guinea and the democratic republic of the Congo in 2014. Viruses 2014, 6, 4760–4799. [Google Scholar] [CrossRef] [PubMed]
- Marzi, A.; Feldmann, F.; Hanley, P.W.; Scott, D.P.; Gunther, S.; Feldmann, H. Delayed disease progression in cynomolgus macaques infected with Ebola Virus makona strain. Emerg. Infect. Dis. 2015, 21, 1777–1783. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, J.H.; Dodd, L.E.; Wahl-Jensen, V.; Radoshitzky, S.R.; Bavari, S.; Jahrling, P.B. Evaluation of perceived threat differences posed by filovirus variants. Biosecur. Bioterror. 2011, 9, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Heald, A.E.; Charleston, J.S.; Iversen, P.L.; Warren, T.K.; Saoud, J.B.; Al-Ibrahim, M.; Wells, J.; Warfield, K.L.; Swenson, D.L.; Welch, L.S.; et al. AVI-7288 for Marburg virus in nonhuman primates and humans. N. Engl. J. Med. 2015, 373, 339–348. [Google Scholar] [CrossRef] [PubMed]


| Sample | Nominal Titer 1 | Low End of Range (PFU/mL) (this number is −0.5 log10) | High End of Range (PFU/mL) (this number is +0.5 log10) | % Difference of Low End from Nominal Titer | % Difference of High End from Nominal Titer |
|---|---|---|---|---|---|
| QC1 | 1.17 × 106 | 3.70 × 105 | 3.70 × 106 | −68.4% | +216% |
| EBOV QC1 average observed titer (n = 12) was 6.18 × 105 PFU/mL (CV = 3.9%). This is −47.2% different from nominal, and the value passes. | |||||
| QC2 | 1.07 × 105 | 3.38 × 104 | 3.38 × 105 | −68.4% | +216% |
| EBOV QC2 average observed titer (n = 12) was 6.99 × 104 PFU/mL (CV = 16.2%). This is a −34.7% difference from nominal, and the value passes. | |||||
| QC3 | 1.06 × 104 | 3.35 × 103 | 3.35 × 104 | −68.4% | +216% |
| EBOV QC3 average observed titer (n = 12) was 5.86 × 103 PFU/mL (CV = 11.0%). This is a −44.7% difference from nominal, and the value passes. | |||||
| Sample | Nominal Titer 1 | Low End of Range (PFU/mL) (this number is −0.5 log10) | High End of Range (PFU/mL) (this number is +0.5 log10) | % Difference of Low End from Nominal Titer | % Difference of High End from Nominal Titer |
|---|---|---|---|---|---|
| QC1 | 3.5 × 106 | 1.096 × 106 | 1.096 × 107 | −68.7% | +213% |
| MARV QC1 average observed titer (n = 12) was 2.88 × 106 PFU/mL (CV = 34.9%). This is −17.7% different from nominal, and the value passes. | |||||
| QC2 | 3.5 × 105 | 1.096 × 105 | 1.096 × 106 | −68.7% | +213% |
| MARV QC2 average observed titer (n = 12) was 3.27 × 105 PFU/mL (CV = 23.6%). This is a -6.4% difference from nominal, and the value passes. | |||||
| QC3 | 3.5 × 104 | 1.096 × 104 | 1.096 × 105 | −68.7% | +213% |
| MARV QC3 average observed titer (n = 12) was 4.95 × 104 PFU/mL (CV = 20.1%). This is a 41.4% difference from nominal, and the value passes. | |||||
| Test | Number of EBOV Assay Runs within Nominal Titer Range for Each QC (% Difference of Average Observed Titer from Nominal) | Number of MARV Assay Runs within Nominal Titer Range for Each QC (% Difference of Average Observed Titer from Nominal) |
|---|---|---|
| Precision 1 | QC1 12 of 12 (−18.1%) | QC1 12 of 12 (86.2%) |
| QC2 11 of 12 (6.3%) | QC2 12 of 12 (105.8%) | |
| QC3 12 of 12 (3.3%) | QC3 10 of 12 (175.7%) | |
| Precision 2 | QC1 12 of 12 (105.5%) | QC1 12 of 12 (98.8%) |
| QC2 12 of 12 (111.4%) | QC2 12 of 12 (89.4%) | |
| QC3 12 of 12 (−10.5%) | QC3 12 of 12 (90.1%) | |
| Precision 3 | QC1 11 of 12 (−52.1%) | QC1 12 of 12 (29.2%) |
| QC2 12 of 12 (−30.7%) | QC2 12 of 12 (37.6%) | |
| QC3 12 of 12 (−43.9%) | QC3 12 of 12 (84.9%) | |
| Precision 4 | QC1 12 of 12 (−43.9%) | QC1 12 of 12 (30.6%) |
| QC2 12 of 12 (−42.7%) | QC2 12 of 12 (14.8%) | |
| QC3 11 of 12 (−48.1%) | QC3 11 of 12 (36.3%) | |
| Change of inoculum volume | QC1 N/A due to experimental design | QC1 N/A due to experimental design |
| QC2 N/A due to experimental design | QC2 N/A due to experimental design | |
| QC3 12 of 12 (−38.3%) | QC3 12 of 12 (2.9%) | |
| Stability | QC1 12 of 12 (−54.3%) | QC1 12 of 12 (109.9%) |
| QC2 12 of 12 (−50.8%) | QC2 12 of 12 (83.0%) | |
| QC3 11 of 12 (−48.1%) | QC3 11 of 12 (95.4%) | |
| Cell Seeding Time (24 h) | QC1 12 of 12 (−17.3%) | QC1 12 of 12 (94.3%) |
| QC2 12 of 12 (−2.1%) | QC2 12 of 12 (67.0%) | |
| QC3 12 of 12 (−12.0%) | QC3 12 of 12 (68.1%) | |
| Cell Seeding Time (48 h) | QC1 12 of 12 (9.0%) | Not performed * |
| QC2 12 of 12 (22.0%) | ||
| QC3 12 of 12 (18.4%) | ||
| Day to Stain | QC1 12 of 12 (−52.4%) | QC1 12 of 12 (141.6%) |
| QC2 12 of 12 (−28.5%) | QC2 12 of 12 (39.9%) | |
| QC3 12 of 12 (−40.0%) | QC3 12 of 12 (29.2%) |
| Test Counted by both Analysts | % Variability in EBOV Plaque Counts between Two Analysts | % Variability in MARV Plaque Counts between Two Analysts | ||||
|---|---|---|---|---|---|---|
| QC1 | QC2 | QC3 | QC1 | QC2 | QC3 | |
| Test 1 (run by Analyst A) | 0.6% | 2.9% | 9.3% | 17.8% | 15.7% | 13.5% |
| Test 2 (run by Analyst B) | 6.0% | 3.4% | 1.9% | 28.2% | 27.4% | 26.8% |
| Test 3 (run by Analyst A) | 6.5% | 7.2% | 4.2% | 9.5% | 7.3% | 4.8% |
| Test 4 (run by Analyst B) | 3.6% | 6.5% | 1.3% | 15.1% | 8.3% | 8.6% |
| EBOV | MARV | ||||||
|---|---|---|---|---|---|---|---|
| Precision Test/Analyst | Intra-class Correlation | 95% Confidence Limits | Precision Test/Analyst | Intra-class Correlation | 95% Confidence Limits | ||
| Lower | Upper | Lower | Upper | ||||
| Test 1 | 0.99013 | 0.98809 | 0.99182 | Test 1 | 0.92346 | 0.90471 | 0.93819 |
| Test 2 | 0.99490 | 0.99384 | 0.99578 | Test 2 | 0.92959 | 0.89137 | 0.95150 |
| Test 3 | 0.96788 | 0.96130 | 0.97334 | Test 3 | 0.96623 | 0.95935 | 0.97197 |
| Test 4 | 0.96507 | 0.95796 | 0.97100 | Test 4 | 0.96050 | 0.95105 | 0.96798 |
| # U Wells in 144 Wells Plated (% of Total Wells) | QC1 | QC2 | QC3 |
|---|---|---|---|
| Stained Day 7, counted Day 8 | 5 (3.5%) | 0 (0%) | 1 (0.7%) |
| Stained Day 7, counted Day 9 | 4 (2.8%) | 0 (0%) | 2 (1.4%) |
| Stained Day 8, counted Day 9 | 6 (4.2%) | 0 (0%) | 2 (1.4%) |
| Stained Day 8, counted Day 10 | 66 (45.8%) | 79 (54.9%) * | 56 (38.9%) * |
| # U Wells in 144 Wells Plated (% of Total Wells) | QC1 | QC2 | QC3 |
| Stained Day 7, counted Day 8 | 24 (16.7%) | 1 (0.7%) | 4 (2.8%) |
| Stained Day 7, counted Day 9 | 67 (46.5%) | 5 (3.5%) | 4 (2.8%) |
| Stained Day 8, counted Day 9 | 58 (40.3%) | 1 (0.7%) | 23 (16.0%) |
| Stained Day 8, counted Day 10 | 96 (66.7%) | 56 (38.9%) | 41 (28.5%) * |
| Virus | First Plate Set | Second Plate Set | ||
|---|---|---|---|---|
| Counted Day 8 (Stained Day 7) | Counted Day 9 (Stained Day 7) | Counted Day 9 (Stained Day 8) | Counted Day 10 (Stained Day 8) | |
| EBOV | QC1 = 5.57 × 105 | QC1 = 6.95 × 105 22% titer increase | QC1 = 5.63 × 105 | QC1 = 6.71 × 105 17.5% titer increase |
| QC2 = 7.65 × 104 | QC2 = 9.05 × 104 16.8% titer increase | QC2 = 8.13 × 104 | QC2 = dataset failed * | |
| QC3 = 6.25 × 103 | QC3 = 7.63 × 103 19.9% titer increase | QC3 = 8.74 × 103 | QC3 = dataset failed * | |
| MARV | QC1 = 8.46 × 106 | QC1 = 9.79 × 106 14.5% titer increase | QC1 = 8.95 × 106 | QC1 = 1.02 × 107 13.2% titer increase |
| QC2 = 4.90 × 105 | QC2 = 5.50 × 105 11.5% titer increase | QC2 = 6.06 × 105 | QC2 = 7.22 × 105 17.5% titer increase | |
| QC3 = 4.52 × 104 | QC3 = 5.02 × 104 10.4% titer increase | QC3 = 4.40 × 104 | QC3 = dataset failed * | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shurtleff, A.C.; Bloomfield, H.A.; Mort, S.; Orr, S.A.; Audet, B.; Whitaker, T.; Richards, M.J.; Bavari, S. Validation of the Filovirus Plaque Assay for Use in Preclinical Studies. Viruses 2016, 8, 113. https://doi.org/10.3390/v8040113
Shurtleff AC, Bloomfield HA, Mort S, Orr SA, Audet B, Whitaker T, Richards MJ, Bavari S. Validation of the Filovirus Plaque Assay for Use in Preclinical Studies. Viruses. 2016; 8(4):113. https://doi.org/10.3390/v8040113
Chicago/Turabian StyleShurtleff, Amy C., Holly A. Bloomfield, Shannon Mort, Steven A. Orr, Brian Audet, Thomas Whitaker, Michelle J. Richards, and Sina Bavari. 2016. "Validation of the Filovirus Plaque Assay for Use in Preclinical Studies" Viruses 8, no. 4: 113. https://doi.org/10.3390/v8040113





