Glycosylation of KSHV Encoded vGPCR Functions in Its Signaling and Tumorigenicity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells and Plasmids
2.2. Endo H and PNGase F Digestion
2.3. Immunoblotting
2.4. Luciferase Reporter Assay
2.5. Immunofluorescence Microscopy
2.6. Apoptosis Assay
2.7. Tumor Formation Assays in Nude Mice
3. Results
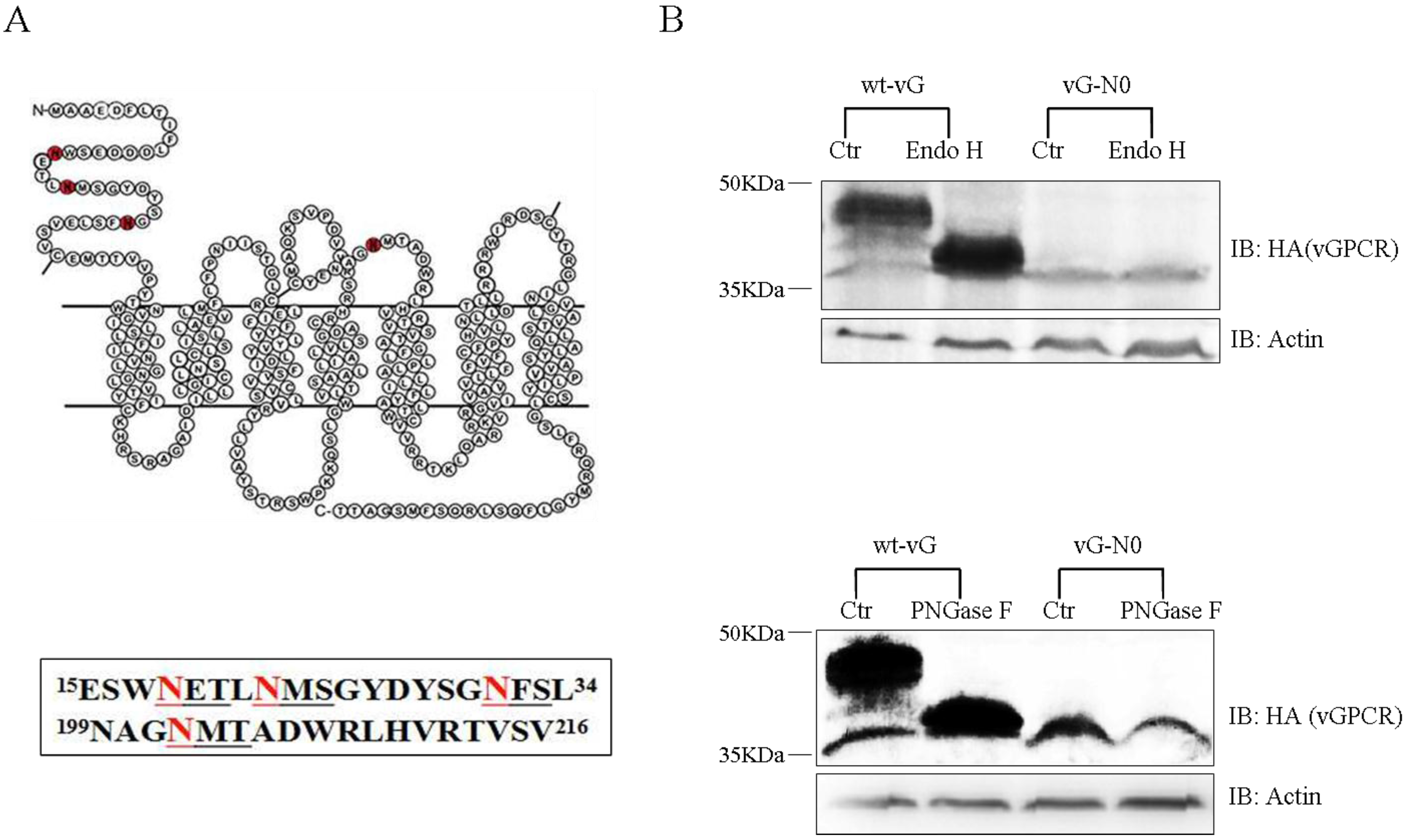
3.1. vGPCR Undergoes N-linked Glycosylation within Its Extracellular Sequences
3.2. vGPCR Possesses Four Sites of N-Linked Glycosylation
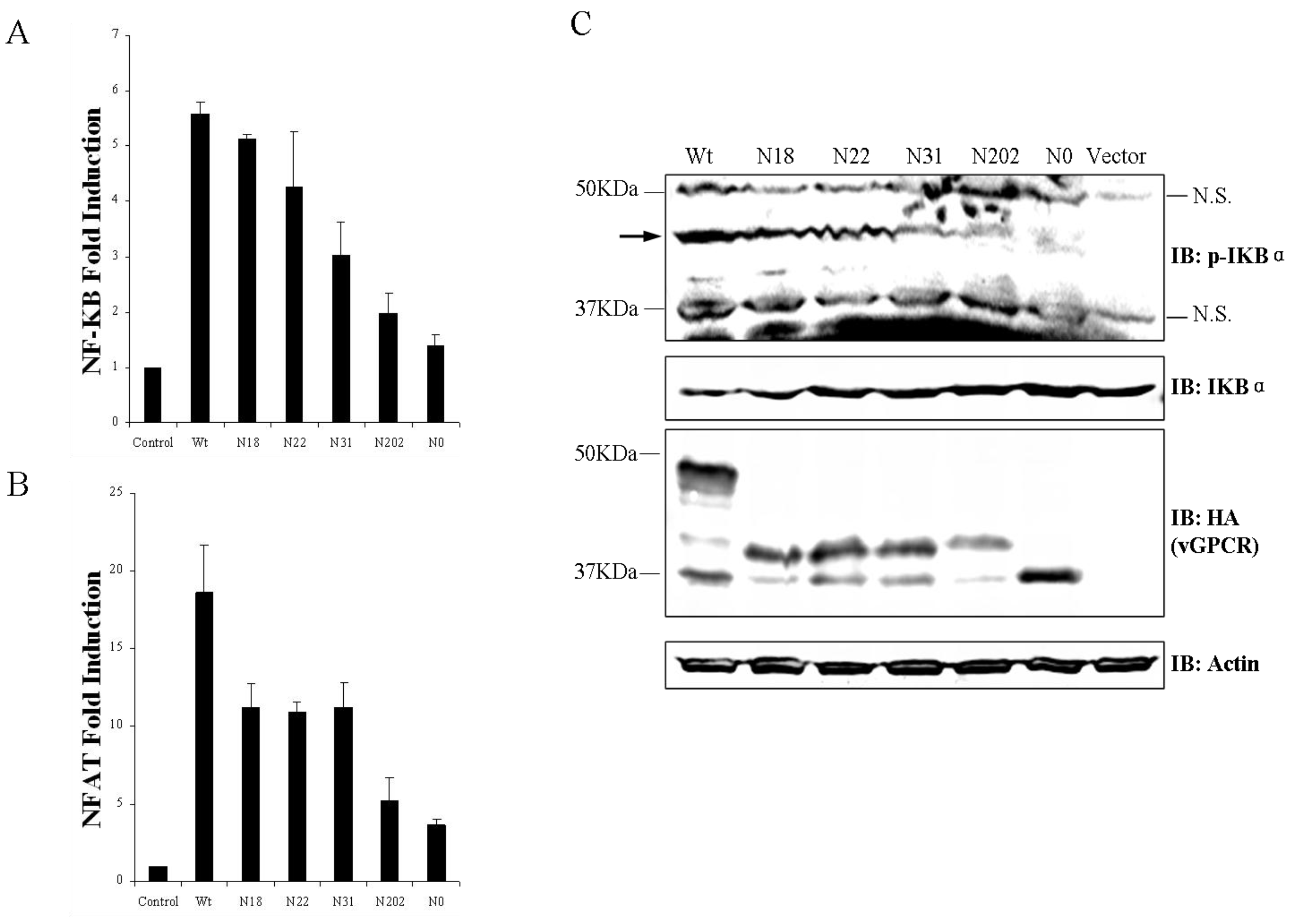
3.3. Glycosylation of vGPCR Impacts Its Signal Transduction

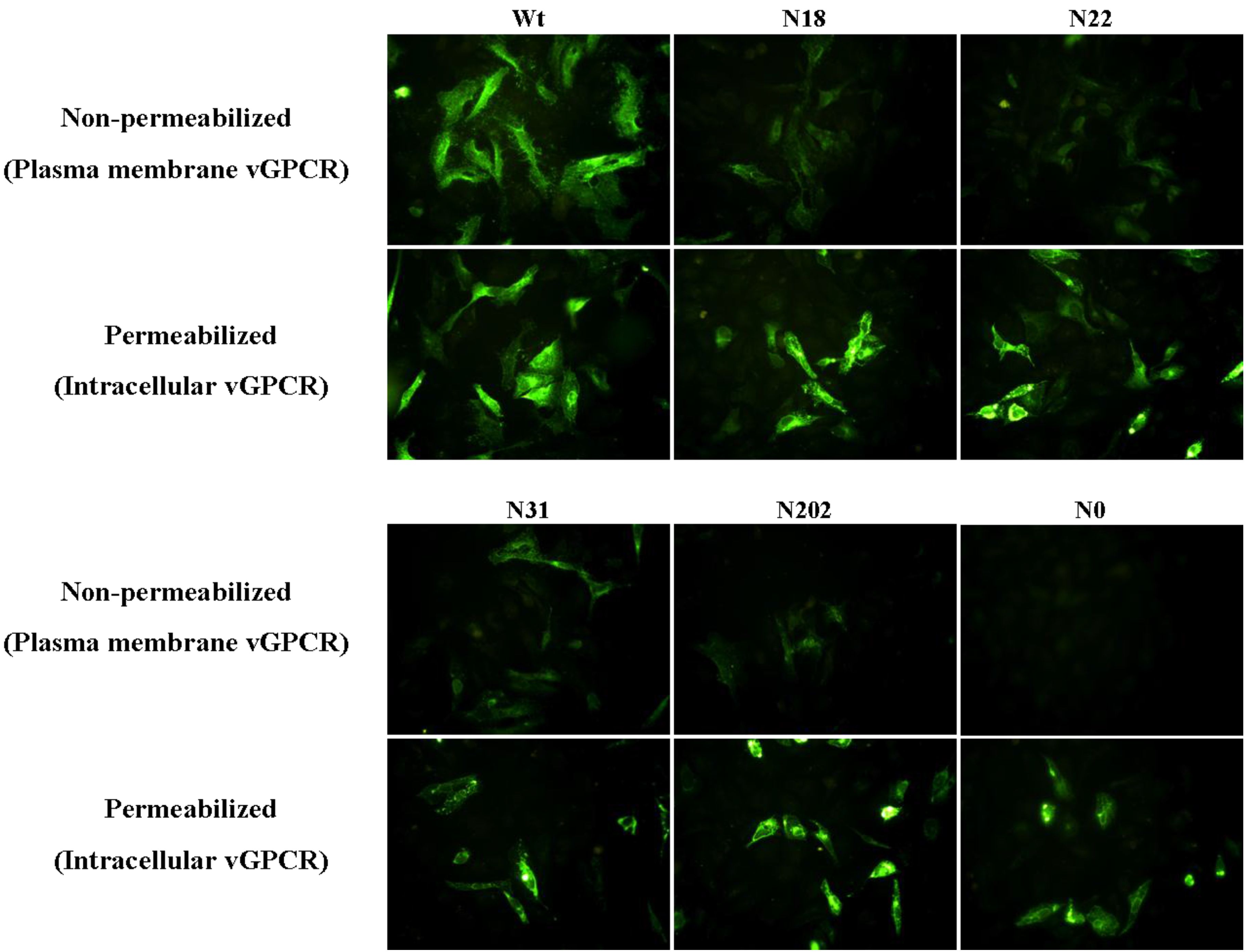
3.4 Glycosylation of vGPCR Plays an Important Role in Its Membrane Traffic
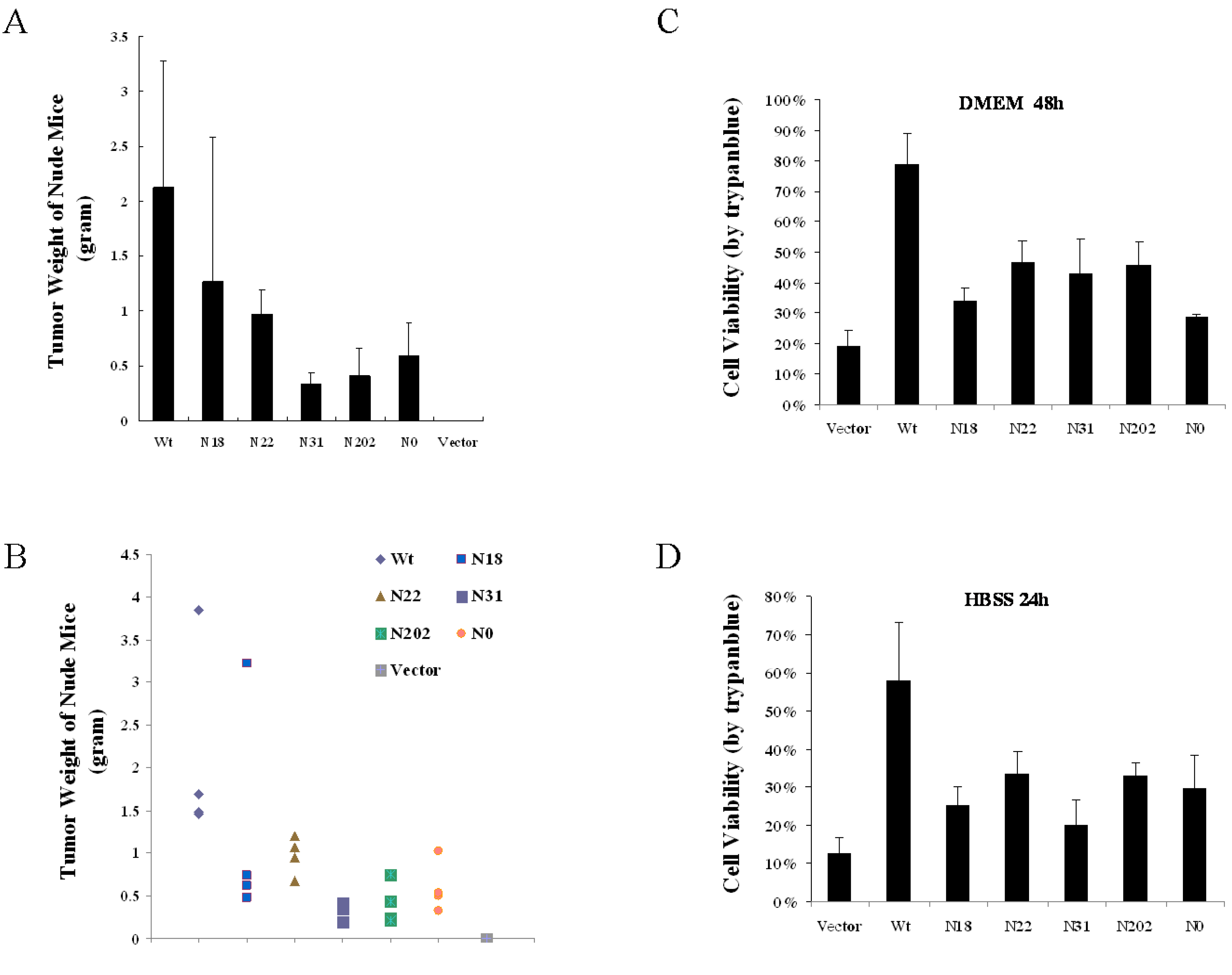
3.5. Glycosylation of vGPCR Impacts Its Tumorigenicity in Nude Mice
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chang, Y.; Cesarman, E.; Pessin, M.S.; Lee, F.; Culpepper, J.; Knowles, D.M.; Moore, P.S. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science 1994, 266, 1865–1869. [Google Scholar]
- Cesarman, E.; Chang, Y.; Moore, P.S.; Said, J.W.; Knowles, D.M. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N. Engl. J. Med. 1995, 332, 1186–1191. [Google Scholar]
- Bais, C.; Santomasso, B.; Coso, O.; Arvanitakis, L.; Raaka, E.G.; Gutkind, J.S.; Asch, A.S.; Cesarman, E.; Gershengorn, M.C.; Mesri, E.A. G-protein-coupled receptor of Kaposi’s sarcoma-associated herpesvirus is a viral oncogene and angiogenesis activator. Nature 1998, 391, 86–89. [Google Scholar]
- Jham, B.C.; Montaner, S. The Kaposi’s sarcoma-associated herpesvirus G protein-coupled receptor: Lessons on dysregulated angiogenesis from a viral oncogene. J. Cell. Biochem. 2010, 110, 1–9. [Google Scholar]
- Cannon, M.; Philpott, N.J.; Cesarman, E. The Kaposi’s sarcoma-associated herpesvirus G protein-coupled receptor has broad signaling effects in primary effusion lymphoma cells. J. Virol. 2003, 77, 57–67. [Google Scholar]
- Emuss, V.; Lagos, D.; Pizzey, A.; Gratrix, F.; Henderson, S.R.; Boshoff, C. KSHV manipulates notch signaling by DLL4 and JAG1 to alter cell cycle genes in lymphatic endothelia. PLoS Pathog. 2009, 5, e1000616. [Google Scholar]
- Martin, D.; Galisteo, R.; Molinolo, A.A.; Wetzer, R.; Hirsch, E.; Gutkind, J.S. PI3Kγ mediates Kaposi’s sarcoma-associated herpesvirus vGPCR-induced sarcomagenesis. Cancer Cell 2011, 19, 805–813. [Google Scholar]
- Montaner, S.; Sodhi, A.; Molinolo, A.; Bugge, T.H.; Sawai, E.T.; He, Y.; Li, Y.; Ray, P.E.; Gutkind, J.S. Endothelial infection with KSHV genes in vivo reveals that vGPCR initiates Kaposi’s sarcomagenesis and can promote the tumorigenic potential of viral latent genes. Cancer Cell 2003, 3, 23–36. [Google Scholar]
- Sodhi, A.; Montaner, S.; Patel, V.; Gomez-Roman, J.J.; Li, Y.; Sausville, E.A.; Sawai, E.T.; Gutkind, J.S. Akt plays a central role in sarcomagenesis induced by Kaposi’s sarcoma herpesvirusencoded G protein-coupled receptor. Proc. Natl. Acad. Sci. USA 2004, 101, 4821–4826. [Google Scholar]
- Sodhi, A.; Chaisuparat, R.; Hu, J.; Ramsdell, A.K.; Manning, B.D.; Sausville, E.A.; Sawai, E.T.; Molinolo, A.; Gutkind, J.S.; Montaner, S. The TSC2/mTOR pathway drives endothelial cell transformation induced by the Kaposi’s sarcoma-associated herpesvirus G protein-coupled receptor. Cancer Cell 2006, 10, 133–143. [Google Scholar]
- Montaner, S.; Sodhi, A.; Servitja, J.M.; Ramsdell, A.K.; Barac, A.; Sawai, E.T.; Gutkind, J.S. The small GTPase Rac1 links the Kaposi sarcoma-associated herpesvirus vGPCR to cytokine secretion and paracrine neoplasia. Blood 2004, 104, 2903–2911. [Google Scholar]
- Martin, D.; Galisteo, R.; Ji, Y.; Montaner, S.; Gutkind, J.S. An NF-κB gene expression signature contributes to Kaposi’s sarcoma virus vGPCR-induced direct and paracrine neoplasia. Oncogene 2008, 27, 1844–1852. [Google Scholar]
- Feng, H.; Dong, X.; Negaard, A.; Feng, P. Kaposi’s sarcoma-associated herpesvirus K7 induces viral G protein-coupled receptor degradation and reduces its tumorigenicity. PLoS Pathog. 2008, 4, e1000157. [Google Scholar]
- Feng, H.; Sun, Z.; Farzan, M.R.; Feng, P. Sulfotyrosines of the Kaposi’s sarcoma-associated herpesvirus G protein-coupled receptor promote tumorigenesis through autocrine activation. J. Virol. 2010, 84, 3351–3361. [Google Scholar]
- Wu, H.; Fu, Y.; Xiao, J.; Zhou, M.; Zhou, W.; Feng, H. The unsulfated extracellular N-terminus of vGPCR reduces the tumorigenicity of hGRO-α in nude mice. Sci. China Life Sci. 2013, 56, 26–31. [Google Scholar]
- Watanabe, I.; Zhu, J.; Recio-Pinto, E.; Thornhill, W.B. Glycosylation affects the protein stability and cell surface expression of Kv1.4 but Not Kv1.1 potassium channels. A pore region determinant dictates the effect of glycosylation on trafficking. J. Biol. Chem. 2004, 279, 8879–8885. [Google Scholar]
- Cunningham, M.R.; McIntosh, K.A.; Pediani, J.D.; Robben, J.; Cooke, A.E.; Nilsson, M.; Gould, G.W.; Mundell, S.; Milligan, G.; Plevin, R. Novel role for proteinase-activated receptor 2 (PAR2) in membrane trafficking of proteinase-activated receptor 4 (PAR4). J. Biol. Chem. 2012, 287, 16656–16669. [Google Scholar]
- Cordat, E.; Reithmeier, R.A. Structure; function; and trafficking of SLC4 and SLC26 anion transporters. Curr. Top. Membr. 2014, 73, 1–67. [Google Scholar]
- Bais, C.; van Geelen, A.; Eroles, P.; Mutlu, A.; Chiozzini, C.; Dias, S.; Silverstein, R.L.; Rafii, S.; Mersi, E.A. Kaposi’s sarcoma associated herpesvirus G protein-coupled receptor immortalizes human endothelial cells by activation of the VEGF receptor-2/ KDR. Cancer Cell 2003, 3, 131–143. [Google Scholar]
- Hu, J.; Jham, B.C.; Ma, T.; Friedman, E.R.; Ferreira, L.; Wright, J.M.; Accurso, B.; Allen, C.M.; Basile, J.R.; Montaner, S. Angiopoietin-like-4: A novel molecular hallmark in oral Kaposi’s sarcoma. Oral Oncol. 2011, 47, 371–375. [Google Scholar]
- Moenner, M.; Pluquet, O.; Bouchecareilh, M.; Chevet, E. Integrated endoplasmic reticulum stress responses in cancer. Cancer Res. 2007, 67, 10631–10634. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.; Liu, L.; Xiao, J.; Chi, M.; Qu, Y.; Feng, H. Glycosylation of KSHV Encoded vGPCR Functions in Its Signaling and Tumorigenicity. Viruses 2015, 7, 1627-1641. https://doi.org/10.3390/v7041627
Wu H, Liu L, Xiao J, Chi M, Qu Y, Feng H. Glycosylation of KSHV Encoded vGPCR Functions in Its Signaling and Tumorigenicity. Viruses. 2015; 7(4):1627-1641. https://doi.org/10.3390/v7041627
Chicago/Turabian StyleWu, Hui, Liqun Liu, Jun Xiao, Mengdie Chi, Yixiao Qu, and Hao Feng. 2015. "Glycosylation of KSHV Encoded vGPCR Functions in Its Signaling and Tumorigenicity" Viruses 7, no. 4: 1627-1641. https://doi.org/10.3390/v7041627




