Exposure to West Nile Virus Increases Bacterial Diversity and Immune Gene Expression in Culex pipiens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mosquito Blood Feeding and Testing
2.2. Bacterial Sequencing and Analysis
2.3. Immune Transcript Quantification
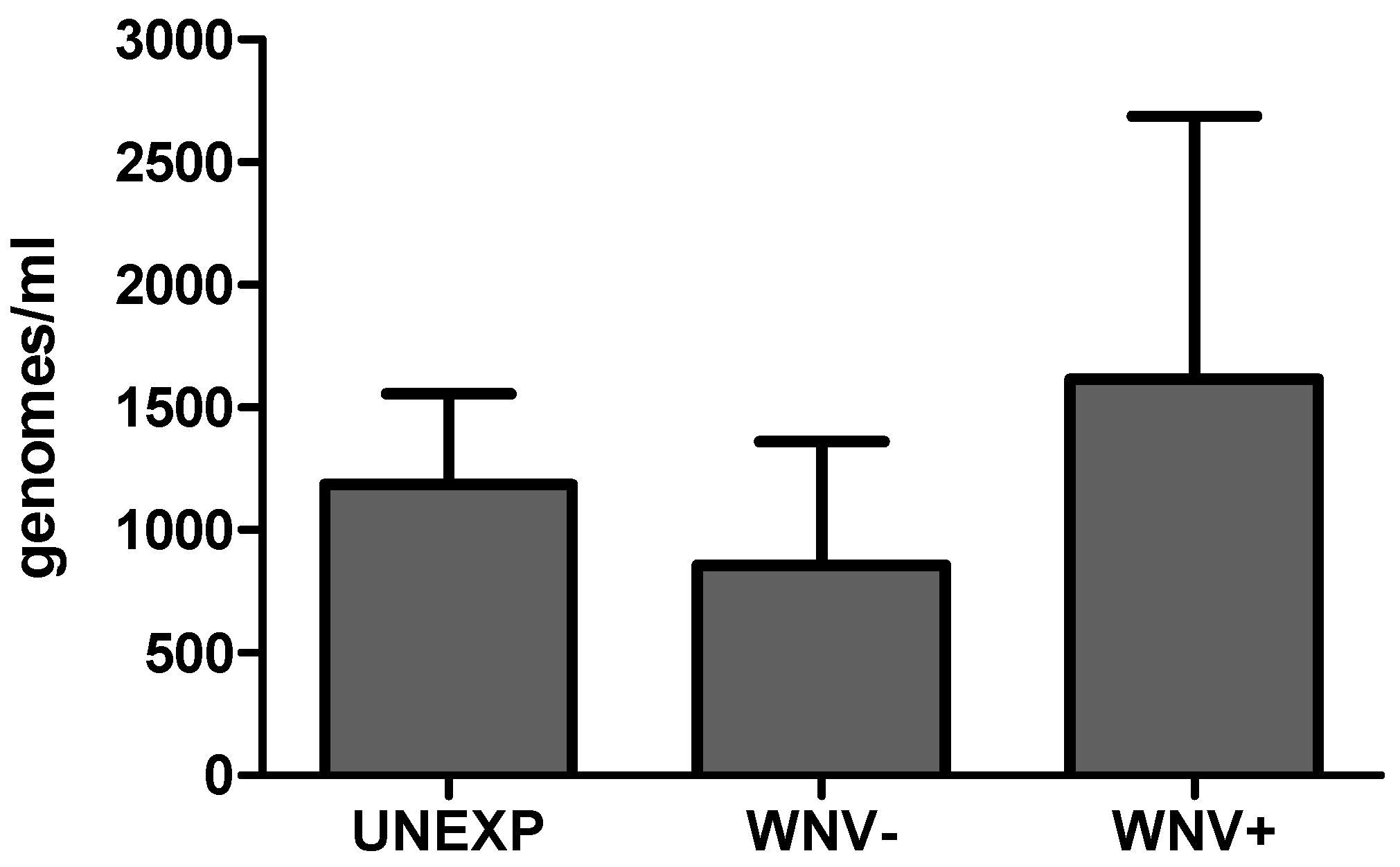
2.4. Total Microbial Load Determination
3. Results
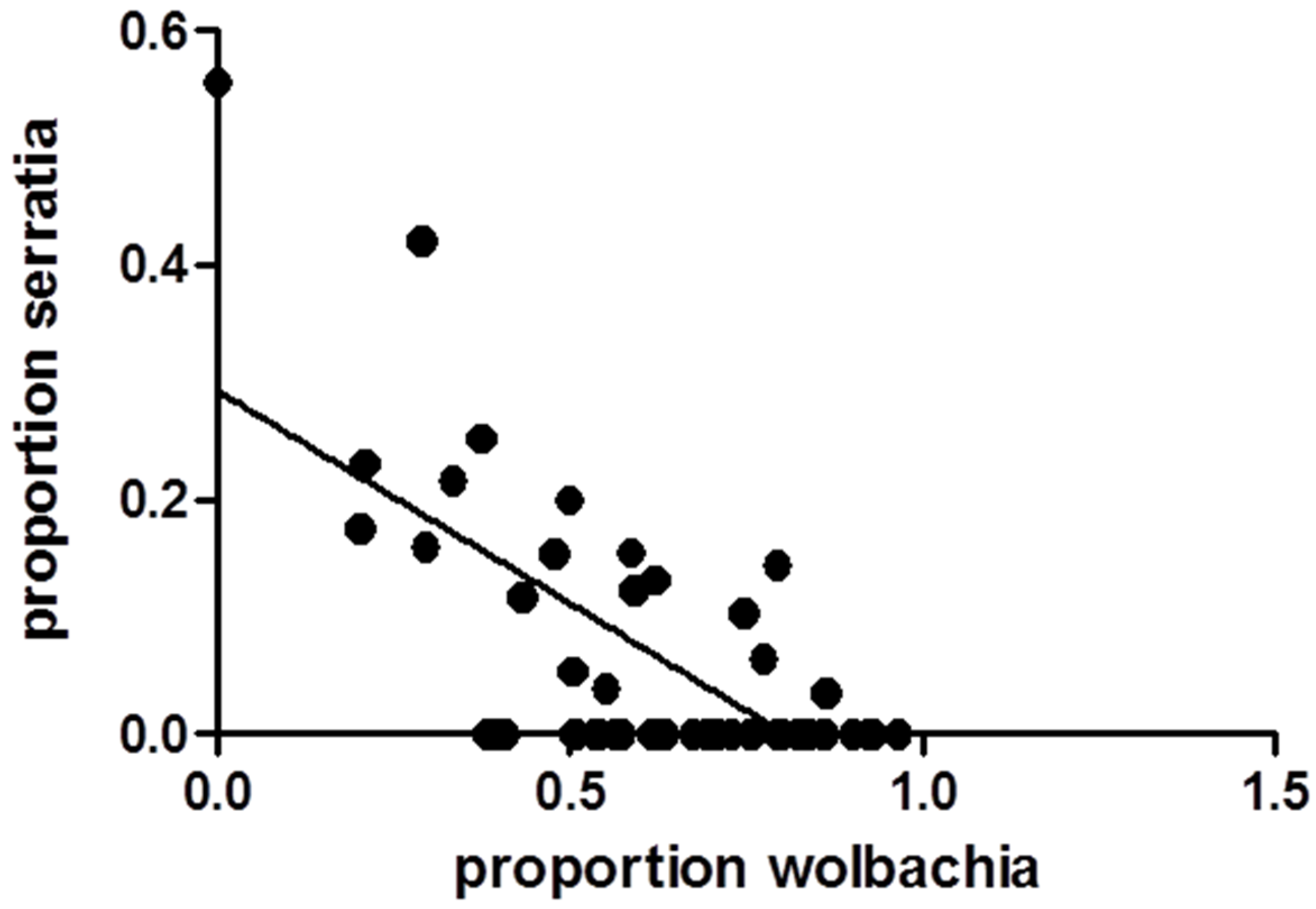
3.1. Relationships between WNV Exposure and Microbial Signatures in Cx. pipiens
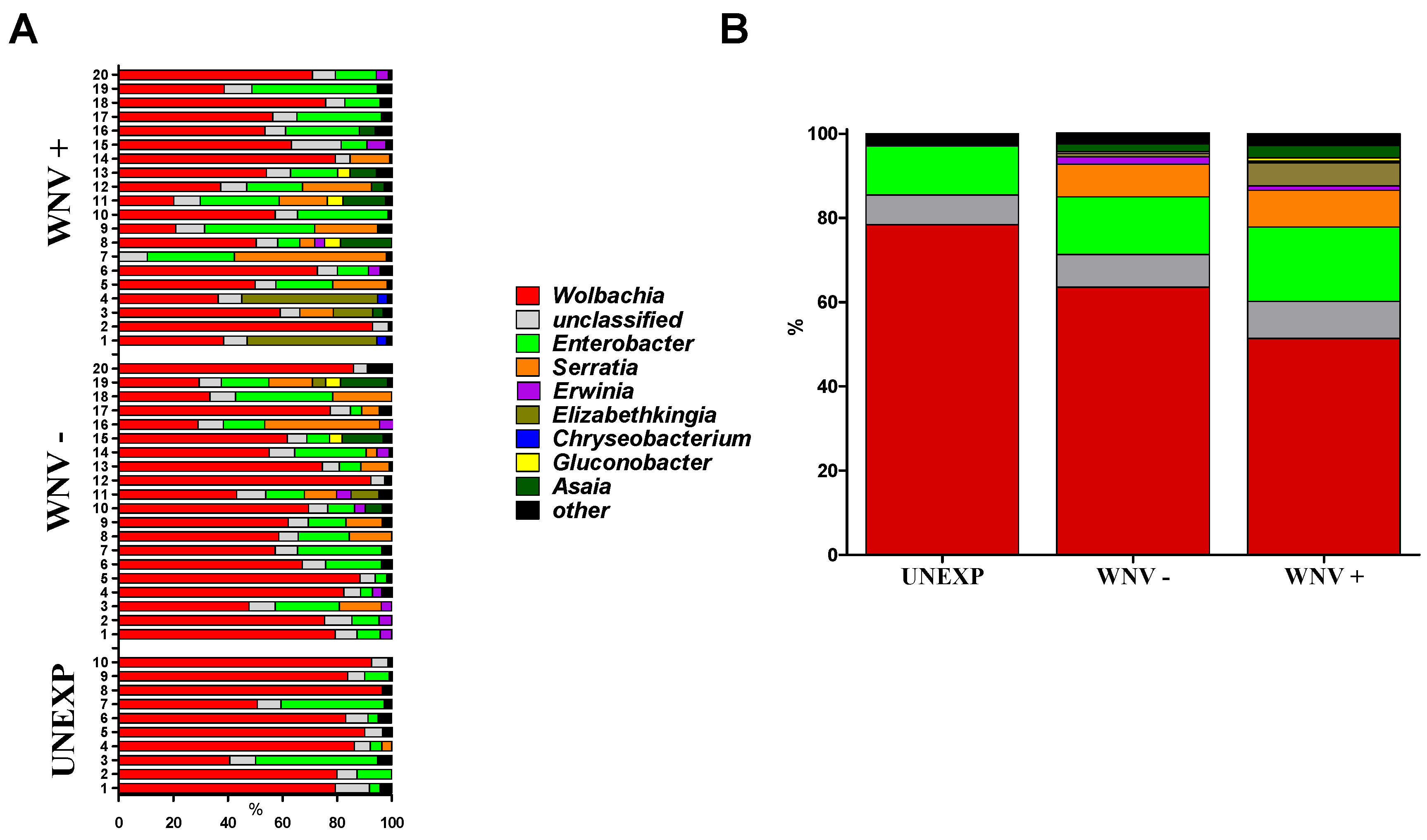
| Group | Families/Sample | Genera/Sample | Species/Sample | 1-D 1 | Sn 2 |
|---|---|---|---|---|---|
| UNEXP | 54 | 85 | 83 | 0.37 | 0.052 |
| WNV− | 66 | 109 | 118 | 0.55 | 0.080 |
| WNV+ | 60 | 98 | 105 | 0.68 | 0.102 |
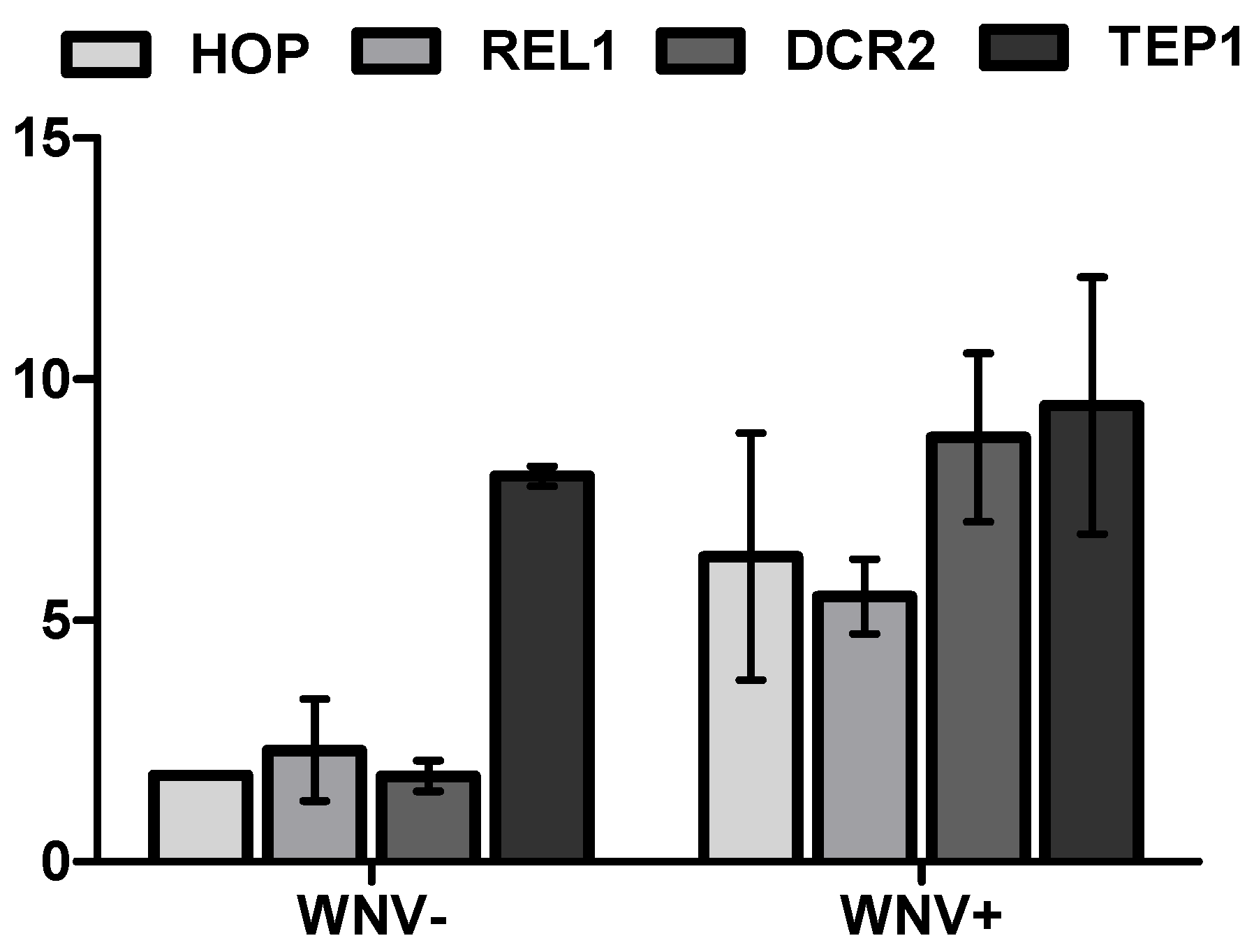
3.2. Mosquito Immune Gene Expression Following WNV Exposure and Infection
4. Discussion
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ciota, A.T.; Chin, P.A.; Kramer, L.D. The effect of hybridization of Culex pipiens complex mosquitoes on transmission of West Nile virus. Parasit. Vectors 2013, 6. [Google Scholar] [CrossRef]
- Nelms, B.M.; Fechter-Leggett, E.; Carroll, B.D.; Macedo, P.; Kluh, S.; Reisen, W.K. Experimental and natural vertical transmission of West Nile virus by California Culex (Diptera: Culicidae) mosquitoes. J. Med. Entomol. 2013, 50, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Dennison, N.J.; Jupatanakul, N.; Dimopoulos, G. The mosquito microbiota influences vector competence for human pathogens. Curr. Opin. Insect. Sci. 2014, 3, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Minard, G.; Mavingui, P.; Moro, C.V. Diversity and function of bacterial microbiota in the mosquito holobiont. Parasit. Vectors 2013, 6, e146. [Google Scholar] [CrossRef] [PubMed]
- Ricci, I.; Mosca, M.; Valzano, M.; Damiani, C.; Scuppa, P.; Rossi, P.; Crotti, E.; Cappelli, A.; Ulissi, U.; Capone, A.; et al. Different mosquito species host Wickerhamomyces anomalus (Pichia anomala): Perspectives on vector-borne diseases symbiotic control. Antonie Leeuwenhoek 2011, 99, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Kim, S.H.; Lee, H.Y.; Bai, J.Y.; Nam, Y.D.; Bae, J.W.; Lee, D.G.; Shin, S.C.; Ha, E.M.; Lee, W.J. Innate immune homeostasis by the homeobox gene caudal and commensal-gut mutualism in Drosophila. Science 2008, 319, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Duguma, D.; Rugman-Jones, P.; Kaufman, M.G.; Hall, M.W.; Neufeld, J.D.; Stouthamer, R.; Walton, W.E. Bacterial communities associated with culex mosquito larvae and two emergent aquatic plants of bioremediation importance. PLoS ONE 2013, 8, e72522. [Google Scholar] [CrossRef] [PubMed]
- Rejmankova, E.; Harbin-Ireland, A.; Lege, M. Bacterial abundance in larval habitats of four species of Anopheles (Diptera: Culicidae) in Belize, Central America. J. Vector Ecol. 2000, 25, 229–239. [Google Scholar] [PubMed]
- Volf, P.; Kiewegova, A.; Nemec, A. Bacterial colonisation in the gut of Phlebotomus duboseqi (Diptera: Psychodidae): Transtadial passage and the role of female diet. Folia Parasit. (Praha) 2002, 49, 73–77. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, S.; Kaufman, M.G.; Maknojia, S.; Bagdasarian, M.; Walker, E.D. Bacterial community structure in tree hole habitats of Ochlerotatus triseriatus: Influences of larval feeding. J. Am. Mosq. Control Assoc. 2008, 24, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Yee, D.A.; Allgood, D.; Kneitel, J.M.; Kuehn, K.A. Constitutive differences between natural and artificial container mosquito habitats: Vector communities, resources, microorganisms, and habitat parameters. J. Med. Entomol. 2012, 49, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.H.; Goncalves, R.L.; Lara, F.A.; Dias, F.A.; Gandara, A.C.; Menna-Barreto, R.F.; Edwards, M.C.; Laurindo, F.R.; Silva-Neto, M.A.; Sorgine, M.H.; et al. Blood meal-derived heme decreases ROS levels in the midgut of Aedes aegypti and allows proliferation of intestinal microbiota. PLoS Pathog. 2011, 7, e1001320. [Google Scholar] [CrossRef] [PubMed]
- Broderick, N.A.; Buchon, N.; Lemaitre, B. Microbiota-induced changes in drosophila melanogaster host gene expression and gut morphology. mBio 2014, 5, e01117-14. [Google Scholar] [CrossRef] [PubMed]
- Blair, C.D. Mosquito RNAi is the major innate immune pathway controlling arbovirus infection and transmission. Future Microbiol. 2011, 6, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Arjona, A.; Wang, P.; Montgomery, R.R.; Fikrig, E. Innate immune control of West Nile virus infection. Cell Microbiol. 2011, 13, 1648–1658. [Google Scholar] [CrossRef] [PubMed]
- Ciota, A.T.; Styer, L.M.; Meola, M.A.; Kramer, L.D. The costs of infection and resistance as determinants of West Nile virus susceptibility in Culex mosquitoes. BMC Ecol. 2011, 11, e23. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.L.; Sharma, A.; Shouche, Y.; Severson, D.W. Dynamics of midgut microflora and dengue virus impact on life history traits in Aedes aegypti. Acta Trop. 2014, 140, 151–157. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Styer, L.M.; Meola, M.A.; Kramer, L.D. West Nile virus infection decreases fecundity of Culex tarsalis females. J. Med. Entomol. 2007, 44, 1074–1085. [Google Scholar] [CrossRef] [PubMed]
- McMeniman, C.J.; O’Neill, S.L. A virulent Wolbachia infection decreases the viability of the dengue vector Aedes aegypti during periods of embryonic quiescence. PLoS Negl. Trop. Dis. 2010, 4, e748. [Google Scholar] [CrossRef] [PubMed]
- Putnam, J.L.; Scott, T.W. Blood-feeding behavior of dengue-2 virus-infected Aedes aegypti. Am. J. Trop. Med. Hyg. 1995, 52, 225–227. [Google Scholar] [PubMed]
- Walker, T.; Johnson, P.H.; Moreira, L.A.; Iturbe-Ormaetxe, I.; Frentiu, F.D.; McMeniman, C.J.; Leong, Y.S.; Dong, Y.; Axford, J.; Kriesner, P.; et al. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature 2011, 476, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Weiss, B.; Aksoy, S. Microbiome influences on insect host vector competence. Trends Parasitol. 2011, 27, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Ebel, G.D.; Carricaburu, J.; Young, D.; Bernard, K.A.; Kramer, L.D. Genetic and phenotypic variation of West Nile virus in New York, 2000–2003. Am. J. Trop. Med. Hyg. 2004, 71, 493–500. [Google Scholar] [PubMed]
- Shi, P.-Y.; Kauffman, E.B.; Ren, P.; Felton, A.; Tai, J.H.; Dupuis, A.P., II; Jones, S.A.; Ngo, K.A.; Nicholas, D.C.; Maffei, J.G.; et al. High throughput detection of West Nile virus RNA. J. Clin. Microbiol. 2001, 39, 1264–1271. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. 1), 4516–4522. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Aguilar, R.; Xi, Z.; Warr, E.; Mongin, E.; Dimopoulos, G. Anopheles gambiae immune responses to human and rodent Plasmodium parasite species. PLoS Pathog. 2006, 2, e52. [Google Scholar] [CrossRef] [PubMed]
- Carvalho-Leandro, D.; Ayres, C.F.; Guedes, D.R.; Suesdek, L.; Melo-Santos, M.A.; Oliveira, C.F.; Cordeiro, M.T.; Regis, L.N.; Marques, E.T.; Gil, L.H.; et al. Immune transcript variations among Aedes aegypti populations with distinct susceptibility to dengue virus serotype 2. Acta Trop. 2012, 124, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Garver, L.S.; Dong, Y.; Dimopoulos, G. Caspar controls resistance to Plasmodium falciparum in diverse anopheline species. PLoS Pathog. 2009, 5, e1000335. [Google Scholar] [CrossRef] [PubMed]
- Goic, B.; Vodovar, N.; Mondotte, J.A.; Monot, C.; Frangeul, L.; Blanc, H.; Gausson, V.; Vera-Otarola, J.; Cristofari, G.; Saleh, M.C. RNA-mediated interference and reverse transcription control the persistence of RNA viruses in the insect model Drosophila. Nat. Immunol. 2013, 14, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Fujimoto, C.; Haruki, Y.; Maeda, T.; Kokeguchi, S.; Petelin, M.; Arai, H.; Tanimoto, I.; Nishimura, F.; Takashiba, S. Quantitative real-time PCR using TaqMan and SYBR Green for Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis, Prevotella intermedia, tetQ gene and total bacteria. FEMS Immunol. Med. Microbiol. 2003, 39, 81–86. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- McLean, R.G.; Ubico, S.R.; Docherty, D.E.; Hansen, W.R.; Sileo, L.; McNamara, T.S. West Nile virus transmission and ecology in birds. Ann. N. Y. Acad. Sci. 2001, 951, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Azambuja, P.; Garcia, E.S.; Ratcliffe, N.A. Gut microbiota and parasite transmission by insect vectors. Trends Parasitol. 2005, 21, 568–572. [Google Scholar] [CrossRef] [PubMed]
- Brownlie, J.C.; Johnson, K.N. Symbiont-mediated protection in insect hosts. Trends Microbiol. 2009, 17, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Cirimotich, C.M.; Ramirez, J.L.; Dimopoulos, G. Native microbiota shape insect vector competence for human pathogens. Cell Host Microbe 2011, 10, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Gaio Ade, O.; Gusmao, D.S.; Santos, A.V.; Berbert-Molina, M.A.; Pimenta, P.F.; Lemos, F.J. Contribution of midgut bacteria to blood digestion and egg production in Aedes aegypti (diptera: Culicidae) (L.). Parasit. Vectors 2011, 4, e105. [Google Scholar] [CrossRef] [PubMed]
- Bian, G.; Xu, Y.; Lu, P.; Xie, Y.; Xi, Z. The endosymbiotic bacterium Wolbachia induces resistance to dengue virus in Aedes aegypti. PLoS Pathog. 2010, 6, e1000833. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.A.; Montgomery, B.L.; Popovici, J.; Iturbe-Ormaetxe, I.; Johnson, P.H.; Muzzi, F.; Greenfield, M.; Durkan, M.; Leong, Y.S.; Dong, Y.; et al. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature 2011, 476, 454–457. [Google Scholar] [CrossRef] [PubMed]
- Moreira, L.A.; Iturbe-Ormaetxe, I.; Jeffery, J.A.; Lu, G.; Pyke, A.T.; Hedges, L.M.; Rocha, B.C.; Hall-Mendelin, S.; Day, A.; Riegler, M.; et al. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell 2009, 139, 1268–1278. [Google Scholar] [CrossRef] [PubMed]
- Glaser, R.L.; Meola, M.A. The native Wolbachia endosymbionts of Drosophila melanogaster and Culex quinquefasciatus increase host resistance to West Nile virus infection. PLoS ONE 2010, 5, e11977. [Google Scholar] [CrossRef] [PubMed]
- Micieli, M.V.; Glaser, R.L. Somatic Wolbachia (Rickettsiales: Rickettsiaceae) levels in Culex quinquefasciatus and Culex pipiens (Diptera: Culicidae) and resistance to West Nile virus infection. J. Med. Entomol. 2014, 51, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Hedges, L.M.; Brownlie, J.C.; O’Neill, S.L.; Johnson, K.N. Wolbachia and virus protection in insects. Science 2008, 322, 702. [Google Scholar] [CrossRef] [PubMed]
- Mousson, L.; Martin, E.; Zouache, K.; Madec, Y.; Mavingui, P.; Failloux, A.B. Wolbachia modulates Chikungunya replication in Aedes albopictus. Mol. Ecol. 2010, 19, 1953–1964. [Google Scholar] [CrossRef] [PubMed]
- Van den Hurk, A.F.; Hall-Mendelin, S.; Pyke, A.T.; Frentiu, F.D.; McElroy, K.; Day, A.; Higgs, S.; O’Neill, S.L. Impact of Wolbachia on infection with chikungunya and yellow fever viruses in the mosquito vector Aedes aegypti. PLoS Negl. Trop. Dis. 2012, 6, e1892. [Google Scholar] [CrossRef] [PubMed]
- Chandel, K.; Mendki, M.J.; Parikh, R.Y.; Kulkarni, G.; Tikar, S.N.; Sukumaran, D.; Prakash, S.; Parashar, B.D.; Shouche, Y.S.; Veer, V. Midgut microbial community of Culex quinquefasciatus mosquito populations from India. PLoS ONE 2013, 8, e80453. [Google Scholar] [CrossRef] [PubMed]
- Demaio, J.; Pumpuni, C.B.; Kent, M.; Beier, J.C. The midgut bacterial flora of wild Aedes triseriatus, Culex pipiens, and Psorophora columbiae mosquitoes. Am. J. Trop. Med. Hyg. 1996, 54, 219–223. [Google Scholar] [PubMed]
- Pidiyar, V.J.; Jangid, K.; Patole, M.S.; Shouche, Y.S. Studies on cultured and uncultured microbiota of wild culex quinquefasciatus mosquito midgut based on 16s ribosomal RNA gene analysis. Am. J. Trop. Med. Hyg. 2004, 70, 597–603. [Google Scholar] [PubMed]
- Lindh, J.M.; Terenius, O.; Faye, I. 16S rRNA gene-based identification of midgut bacteria from field-caught Anopheles gambiae sensu lato and A. funestus mosquitoes reveals new species related to known insect symbionts. Appl. Environ. Microbiol. 2005, 71, 7217–7223. [Google Scholar] [CrossRef] [PubMed]
- Rani, A.; Sharma, A.; Rajagopal, R.; Adak, T.; Bhatnagar, R.K. Bacterial diversity analysis of larvae and adult midgut microflora using culture-dependent and culture-independent methods in lab-reared and field-collected Anopheles stephensi-an Asian malarial vector. BMC Microbiol. 2009, 9, e96. [Google Scholar] [CrossRef] [PubMed]
- Zouache, K.; Michelland, R.J.; Failloux, A.B.; Grundmann, G.L.; Mavingui, P. Chikungunya virus impacts the diversity of symbiotic bacteria in mosquito vector. Mol. Ecol. 2012, 21, 2297–2309. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Lu, G.; Torres, S.; Edmonds, J.H.; Kay, B.H.; Khromykh, A.A.; Asgari, S. Effect of wolbachia on replication of west nile virus in a mosquito cell line and adult mosquitoes. J. Virol. 2013, 87, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.; Longdon, B.; Bauer, S.; Chan, Y.S.; Miller, W.J.; Bourtzis, K.; Teixeira, L.; Jiggins, F.M. Symbionts commonly provide broad spectrum resistance to viruses in insects: A comparative analysis of Wolbachia strains. PLoS Pathog. 2014, 10, e1004369. [Google Scholar] [CrossRef] [PubMed]
- Dodson, B.L.; Hughes, G.L.; Paul, O.; Matacchiero, A.C.; Kramer, L.D.; Rasgon, J.L. Wolbachia enhances West Nile virus (WNV) infection in the mosquito Culex tarsalis. PLoS Negl. Trop. Dis. 2014, 8, e2965. [Google Scholar] [CrossRef] [PubMed]
- Kambris, Z.; Cook, P.E.; Phuc, H.K.; Sinkins, S.P. Immune activation by life-shortening Wolbachia and reduced filarial competence in mosquitoes. Science 2009, 326, 134–136. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Zhou, G.; Wu, J.; Bian, G.; Lu, P.; Raikhel, A.S.; Xi, Z. Wolbachia induces reactive oxygen species (ROS)-dependent activation of the Toll pathway to control dengue virus in the mosquito Aedes aegypti. Proc. Natl. Acad. Sci. USA 2012, 109, E23–E31. [Google Scholar] [CrossRef] [PubMed]
- Rances, E.; Ye, Y.H.; Woolfit, M.; McGraw, E.A.; O’Neill, S.L. The relative importance of innate immune priming in Wolbachia-mediated dengue interference. PLoS Pathog. 2012, 8, e1002548. [Google Scholar] [CrossRef] [PubMed]
- Caragata, E.P.; Rances, E.; O’Neill, S.L.; McGraw, E.A. Competition for amino acids between Wolbachia and the mosquito host, Aedes aegypti. Microb. Ecol. 2014, 67, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Fragkoudis, R.; ttarzadeh-Yazdi, G.; Nash, A.A.; Fazakerley, J.K.; Kohl, A. Advances in dissecting mosquito innate immune responses to arbovirus infection. J. Gen. Virol. 2009, 90, 2061–2072. [Google Scholar] [CrossRef] [PubMed]
- Paradkar, P.N.; Trinidad, L.; Voysey, R.; Duchemin, J.B.; Walker, P.J. Secreted Vago restricts West Nile virus infection in Culex mosquito cells by activating the Jak-STAT pathway. Proc. Natl. Acad. Sci. USA 2012, 109, 18915–18920. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, J.L.; Souza-Neto, J.; Torres, C.R.; Rovira, J.; Ortiz, A.; Pascale, J.M.; Dimopoulos, G. Reciprocal tripartite interactions between the Aedes aegypti midgut microbiota, innate immune system and dengue virus influences vector competence. PLoS. Negl. Trop. Dis. 2012, 6, e1561. [Google Scholar] [CrossRef] [PubMed]
- Xi, Z.; Ramirez, J.L.; Dimopoulos, G. The Aedes aegypti toll pathway controls dengue virus infection. PLoS Pathog. 2008, 4, e1000098. [Google Scholar] [CrossRef] [PubMed]
- Avadhanula, V.; Weasner, B.P.; Hardy, G.G.; Kumar, J.P.; Hardy, R.W. A novel system for the launch of alphavirus RNA synthesis reveals a role for the Imd pathway in arthropod antiviral response. PLoS Pathog. 2009, 5, e1000582. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Jan, E.; Sarnow, P.; Schneider, D. The Imd pathway is involved in antiviral immune responses in Drosophila. PLoS ONE 2009, 4, e7436. [Google Scholar] [CrossRef] [PubMed]
- Ferrandon, D.; Imler, J.L.; Hetru, C.; Hoffmann, J.A. The Drosophila systemic immune response: Sensing and signalling during bacterial and fungal infections. Nat. Rev. Immunol. 2007, 7, 862–874. [Google Scholar] [CrossRef] [PubMed]
- Nehme, N.T.; Quintin, J.; Cho, J.H.; Lee, J.; Lafarge, M.C.; Kocks, C.; Ferrandon, D. Relative roles of the cellular and humoral responses in the Drosophila host defense against three gram-positive bacterial infections. PLoS ONE 2011, 6, e14743. [Google Scholar] [CrossRef] [PubMed]
- Chandler, J.A.; Liu, R.M.; Bennett, S.N. RNA shotgun metagenomic sequencing of northern California (USA) mosquitoes uncovers viruses, bacteria, and fungi. Front. Microbiol. 2015, 6, e185. [Google Scholar] [CrossRef] [PubMed]
- Ciota, A.T.; Matacchiero, A.C.; Kilpatrick, A.M.; Kramer, L.D. The effect of temperature on life history traits of Culex mosquitoes. J. Med. Entomol. 2013, 51, 55–62. [Google Scholar] [CrossRef]
- McKean, K.A.; Nunney, L. Sexual selection and immune function in Drosophila melanogaster. Evolution 2008, 62, 386–400. [Google Scholar] [CrossRef] [PubMed]
- McKean, K.A.; Yourth, C.P.; Lazzaro, B.P.; Clark, A.G. The evolutionary costs of immunological maintenance and deployment. BMC. Evol. Biol. 2008, 8, e76. [Google Scholar] [CrossRef] [PubMed]
- Apte-Deshpande, A.; Paingankar, M.; Gokhale, M.D.; Deobagkar, D.N. Serratia odorifera a midgut inhabitant of Aedes aegypti mosquito enhances its susceptibility to dengue-2 virus. PLoS ONE 2012, 7, e40401. [Google Scholar] [CrossRef] [PubMed]
- Apte-Deshpande, A.D.; Paingankar, M.S.; Gokhale, M.D.; Deobagkar, D.N. Serratia odorifera mediated enhancement in susceptibility of Aedes aegypti for chikungunya virus. Indian J. Med. Res. 2014, 139, 762–768. [Google Scholar] [PubMed]
- Bando, H.; Okado, K.; Guelbeogo, W.M.; Badolo, A.; Aonuma, H.; Nelson, B.; Fukumoto, S.; Xuan, X.; Sagnon, N.; Kanuka, H. Intra-specific diversity of Serratia marcescens in Anopheles mosquito midgut defines Plasmodium transmission capacity. Sci. Rep. 2013, 3, 1641. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Ceron, L.; Santillan, F.; Rodriguez, M.H.; Mendez, D.; Hernandez-Avila, J.E. Bacteria in midguts of field-collected Anopheles albimanus block Plasmodium vivax sporogonic development. J. Med. Entomol. 2003, 40, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Seitz, H.M.; Maier, W.A.; Rottok, M.; Becker-Feldmann, H. Concomitant infections of Anopheles stephensi with Plasmodium berghei and Serratia marcescens: Additive detrimental effects. Zentralbl. Bakteriol. Mikrobiol. Hyg. A 1987, 266, 155–166. [Google Scholar] [CrossRef]
- Okamoto, N. Norrie disease. Ryoikibetsu Shokogun Shirizu 1998, 19 Pt 2, 627–629. [Google Scholar]
- Someya, Y. Caliciviruses. Uirusu 2000, 50, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Goto, S.; Anbutsu, H.; Fukatsu, T. Asymmetrical interactions between Wolbachia and Spiroplasma endosymbionts coexisting in the same insect host. Appl. Environ. Microbiol. 2006, 72, 4805–4810. [Google Scholar] [CrossRef] [PubMed]
- Kondo, N.; Shimada, M.; Fukatsu, T. Infection density of Wolbachia endosymbiont affected by co-infection and host genotype. Biol. Lett. 2005, 1, 488–491. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente, J.; Garcia-Garcia, J.C.; Blouin, E.F.; Kocan, K.M. Characterization of the functional domain of major surface protein 1a involved in adhesion of the rickettsia Anaplasma marginale to host cells. Vet. Microbiol. 2003, 91, 265–283. [Google Scholar] [CrossRef]
- Macaluso, K.R.; Sonenshine, D.E.; Ceraul, S.M.; Azad, A.F. Rickettsial infection in Dermacentor variabilis (Acari: Ixodidae) inhibits transovarial transmission of a second Rickettsia. J. Med. Entomol. 2002, 39, 809–813. [Google Scholar] [CrossRef] [PubMed]
- Hughes, G.L.; Dodson, B.L.; Johnson, R.M.; Murdock, C.C.; Tsujimoto, H.; Suzuki, Y.; Patt, A.A.; Cui, L.; Nossa, C.W.; Barry, R.M.; et al. Native microbiome impedes vertical transmission of Wolbachia in Anopheles mosquitoes. Proc. Natl. Acad. Sci. USA 2014, 111, 12498–12503. [Google Scholar] [CrossRef] [PubMed]
- Nunez-Valdez, M.E.; Mahanty, H.K. The amb2 locus from Serratia entomophila confers anti-feeding effect on larvae of Costelytra zealandica (Coleoptera: Scarabaeidae). Gene 1996, 172, 75–79. [Google Scholar] [CrossRef]
- Grimont, P.A.D.; Jackson, T.A.; Ageron, E.; Noonan, M.J. Serratia entomophila sp. nov. Associated with Amber Disease in the New Zealand Grass Grub Costelytra zealandica. Int. J.Syst. Bacteriol. 1988, 38, 1–6. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zink, S.D.; Van Slyke, G.A.; Palumbo, M.J.; Kramer, L.D.; Ciota, A.T. Exposure to West Nile Virus Increases Bacterial Diversity and Immune Gene Expression in Culex pipiens. Viruses 2015, 7, 5619-5631. https://doi.org/10.3390/v7102886
Zink SD, Van Slyke GA, Palumbo MJ, Kramer LD, Ciota AT. Exposure to West Nile Virus Increases Bacterial Diversity and Immune Gene Expression in Culex pipiens. Viruses. 2015; 7(10):5619-5631. https://doi.org/10.3390/v7102886
Chicago/Turabian StyleZink, Steven D., Greta A. Van Slyke, Michael J. Palumbo, Laura D. Kramer, and Alexander T. Ciota. 2015. "Exposure to West Nile Virus Increases Bacterial Diversity and Immune Gene Expression in Culex pipiens" Viruses 7, no. 10: 5619-5631. https://doi.org/10.3390/v7102886
APA StyleZink, S. D., Van Slyke, G. A., Palumbo, M. J., Kramer, L. D., & Ciota, A. T. (2015). Exposure to West Nile Virus Increases Bacterial Diversity and Immune Gene Expression in Culex pipiens. Viruses, 7(10), 5619-5631. https://doi.org/10.3390/v7102886





