Fiber-Modified Adenovirus for Central Nervous System Parkinson’s Disease Gene Therapy
Abstract
:1. Introduction
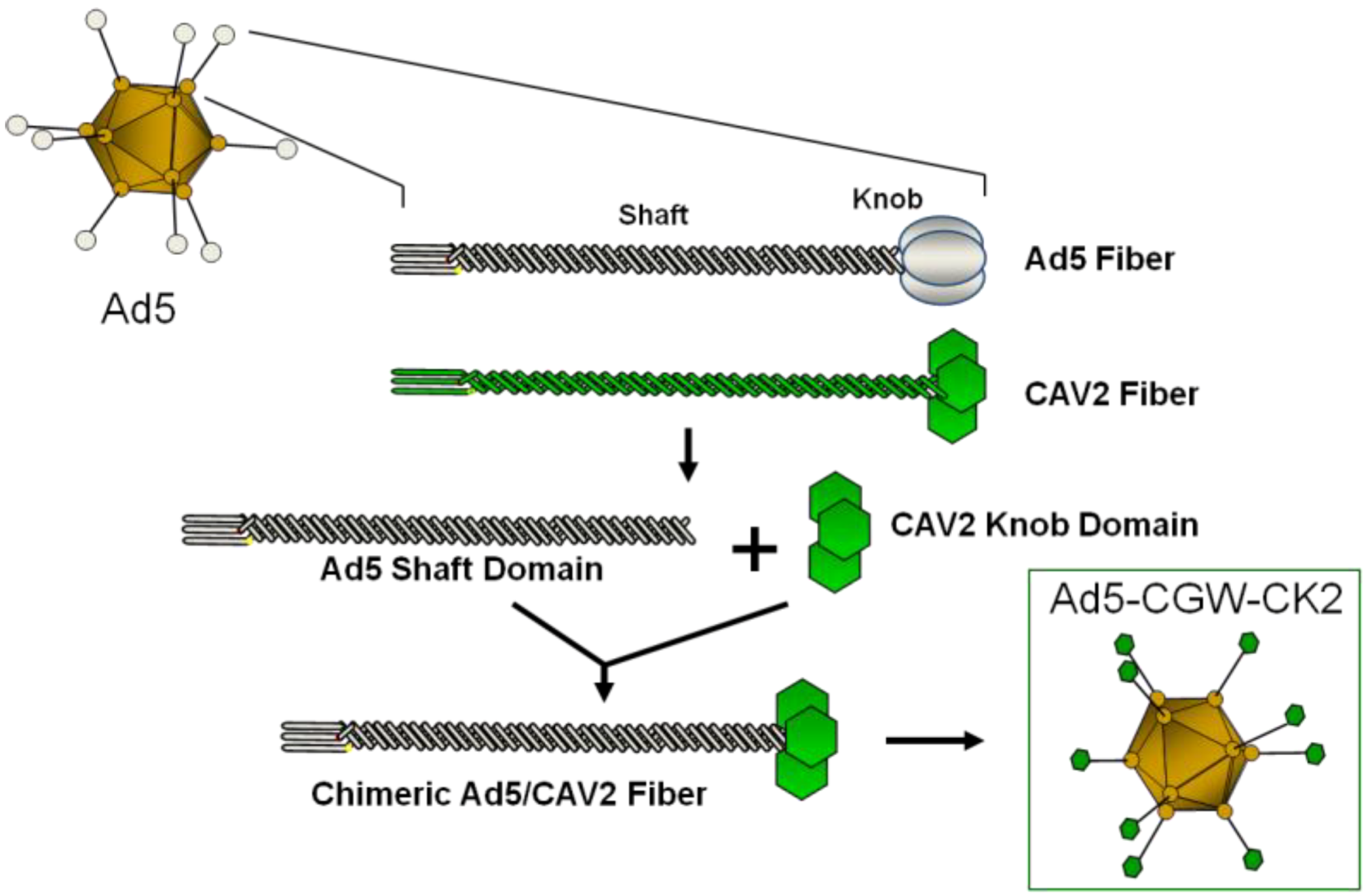
2. Results and Discussion
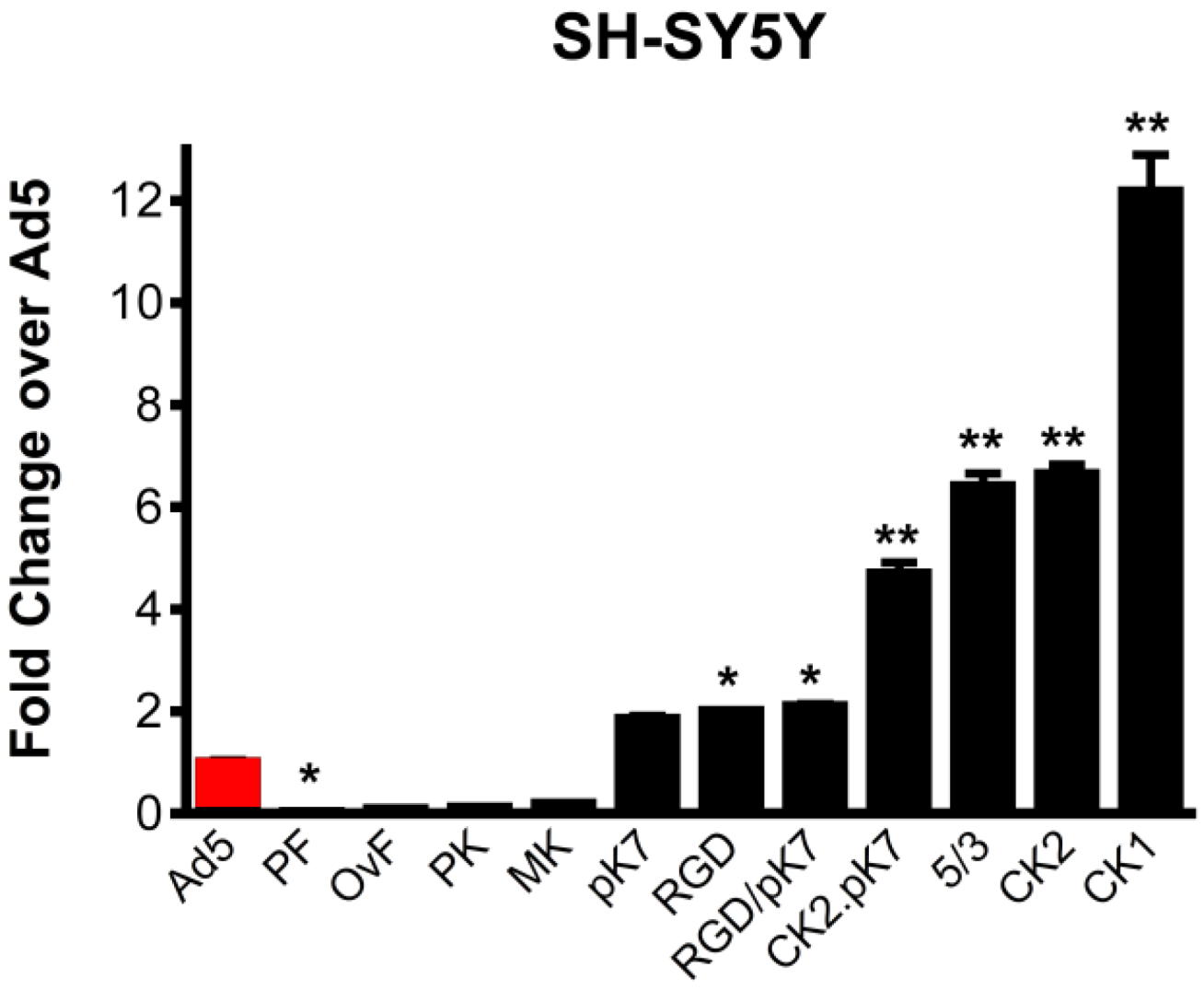
2.1. Gene Delivery in Dopaminergic Cell Lines Using Tropism-Modified Ad-Based Vectors
2.2. Generation of an Unbiased Imaging Cassette and Incorporation into Fiber-Modified Adenovirus

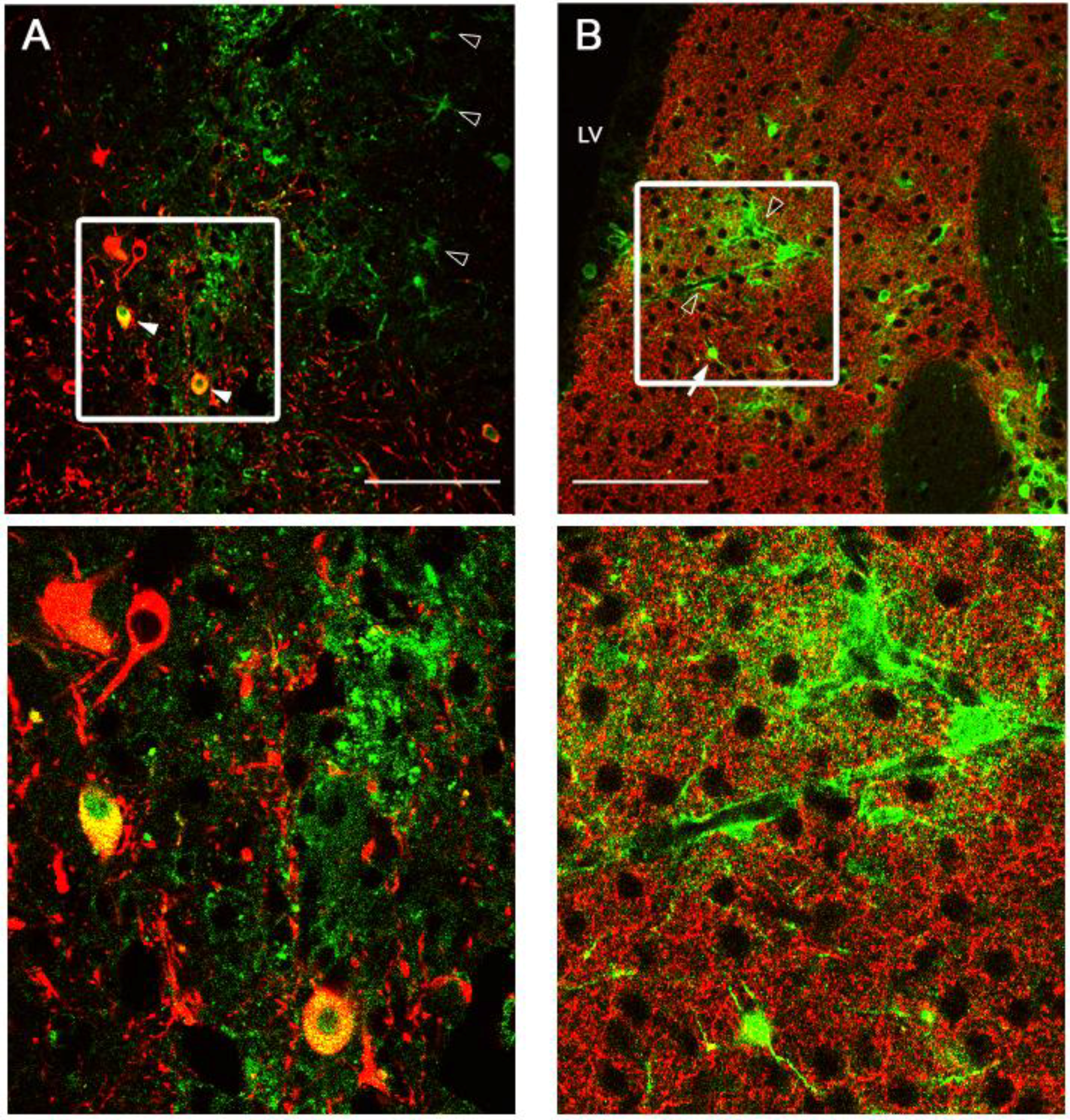

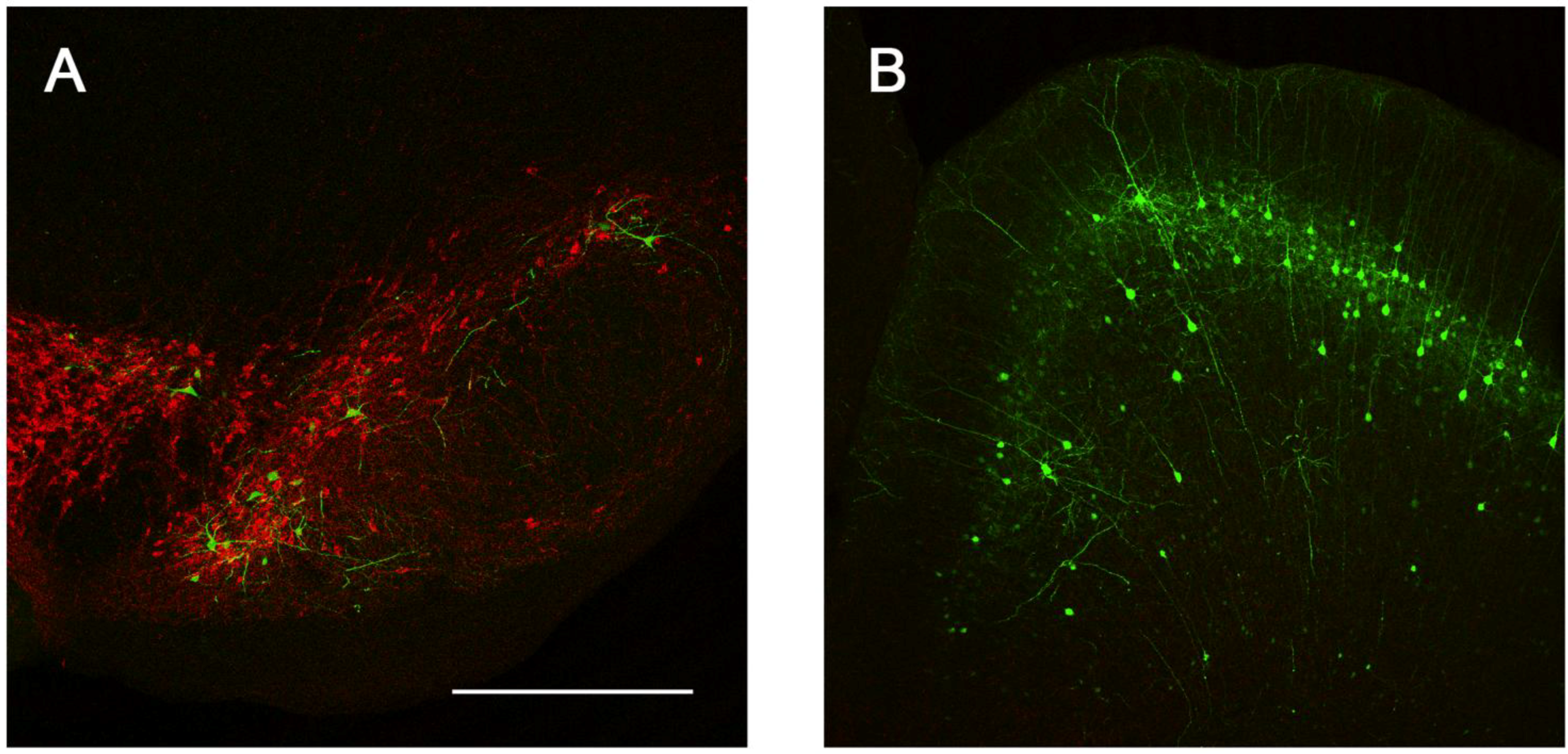
2.3. Intracranial Delivery of Ad5-CGW-CK2 Provides Neuron-Specific Transgene Expression

3. Experimental Section
3.1. In Vitro Fiber-Modified Vector Panel Screen
3.2. Construction of the Transgene Expression Cassette by Polymerase Chain Assembly
 ATGGTGAGCAAGGGC where the underlined sequence is complimentary to the 3’ end of the CAGp, the gray highlighted sequence is a de novo NotI restriction site and the plain-text sequence is complimentary to the 5’ end of GFP. To complete the cassette, we designed a 5’CAGp primer incorporating a KpnI restriction site (AAAA
ATGGTGAGCAAGGGC where the underlined sequence is complimentary to the 3’ end of the CAGp, the gray highlighted sequence is a de novo NotI restriction site and the plain-text sequence is complimentary to the 5’ end of GFP. To complete the cassette, we designed a 5’CAGp primer incorporating a KpnI restriction site (AAAA  ATCGAGGTGAGCCCCACGTT), a 3’ GFP/NheI/5’ WPRE primer (GAGCTGTACAAGTAA
ATCGAGGTGAGCCCCACGTT), a 3’ GFP/NheI/5’ WPRE primer (GAGCTGTACAAGTAA  AATCAACCTCTGGATTACAA) and a 5’ WPRE/XhoI primer (GGAATTTTTTGTGTCTCTCA
AATCAACCTCTGGATTACAA) and a 5’ WPRE/XhoI primer (GGAATTTTTTGTGTCTCTCA  AAAA). Using the appropriate primers and the source template for CAGp, GFP or WPRE-polyA, 3 PCR reactions were performed to derive the three overlapping segments containing the appropriate restriction sites (GC-Rich PCR system, Roche Applied Science, Mannheim, Germany). Using the three overlapping segments, we performed an extension step to create a template of the full-length CGW cassette and, finally, amplified this cassette using the distal 5’ and 3’ primers, producing the complete KpnI/CAGp/NotI/GFP/NheI/WPRE-pA/XhoI construct.
AAAA). Using the appropriate primers and the source template for CAGp, GFP or WPRE-polyA, 3 PCR reactions were performed to derive the three overlapping segments containing the appropriate restriction sites (GC-Rich PCR system, Roche Applied Science, Mannheim, Germany). Using the three overlapping segments, we performed an extension step to create a template of the full-length CGW cassette and, finally, amplified this cassette using the distal 5’ and 3’ primers, producing the complete KpnI/CAGp/NotI/GFP/NheI/WPRE-pA/XhoI construct.3.3. Generation of Recombinant Adenovirus Vectors
3.4. Stereotactic Vector Delivery
3.5. Immunohistochemistry
3.6. Imaging
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Davidson, B.L.; Breakefield, X.O. Viral vectors for gene delivery to the nervous system. Nat. Rev. Neurosci. 2003, 4, 353–364. [Google Scholar]
- Lentz, T.B.; Gray, S.J.; Samulski, R.J. Viral vectors for gene delivery to the central nervous system. Neurobiol. Dis. 2012, 48, 179–188. [Google Scholar]
- Broadstock, M.; Yáñez-Muñoz, R.J. Challenges for Gene Therapy of CNS Disorders and Implications for Parkinson's Disease Therapies. Hum. Gene. Ther. 2012, 4, 340–343. [Google Scholar]
- Nagabhushan Kalburgi, S.; Khan, N.N.; Gray, S.J. Recent gene therapy advancements for neurological diseases. Discov. Med. 2013, 15, 111–119. [Google Scholar]
- Kordower, J.H.; Björklund, A. Trophic factor gene therapy for Parkinson's disease. Mov. Disord. 2013, 28, 96–109. [Google Scholar] [CrossRef]
- Bjorklund, T.; Kordower, J.H. Gene therapy for Parkinson's disease. Mov. Disord. 2010, 25, S161–S173. [Google Scholar] [CrossRef]
- Ramaswamy, S.; Kordower, J.H. Gene therapy for Huntington's disease. Neurobiol. Dis. 2011, 18, 1139–1149. [Google Scholar]
- Foust, K.D.; Nurre, E.; Montgomery, C.L.; Hernandez, A.; Chan, C.M.; Kaspar, B.K. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat. Biotechnol. 2009, 27, 59–65. [Google Scholar]
- Forsayeth, J.R.; Bankiewicz, K.S. AAV9: Over the Fence and Into the Woods. Mol. Ther. 2011, 19, 1006–1007. [Google Scholar]
- Gray, S.J.; Matagne, V.; Bachaboina, L.; Yadav, S.; Ojeda, S.R.; Samulski, R.J. Preclinical Differences of Intravascular AAV9 Delivery to Neurons and Glia: A Comparative Study of Adult Mice and Nonhuman Primates. Mol. Ther. 2011, 19, 1058–1069. [Google Scholar]
- Bartus, R.T.; Herzog, C.D.; Chu, Y.; Wilson, A.; Brown, L.; Siffert, J.; Johnson, E.M.; Olanow, C.W.; Mufson, E.J.; Kordower, J.H. Bioactivity of AAV2-neurturin gene therapy (CERE-120): differences between Parkinson's disease and nonhuman primate brains. Mov. Disord. 2011, 26, 27–36. [Google Scholar] [CrossRef]
- Herzog, C.D.; Brown, L.; Gammon, D.; Kruegel, B.; Lin, R.; Wilson, A.; Bolton, A.; Printz, M.; Gasmi, M.; Bishop, K.M.; Kordower, J.H.; Bartus, R.T. Expression, bioactivity, and safety 1 year after adeno-associated viral vector type 2-mediated delivery of neurturin to the monkey nigrostriatal system support cere-120 for Parkinson's disease. Neurosurgery 2009, 64, 602–612; discussion 612–613. [Google Scholar] [CrossRef]
- Chtarto, A.; Bockstael, O.; Tshibangu, T.; Dewitte, O.; Levivier, M.; Tenenbaum, L. A next step in adeno-associated virus (AAV)-mediated gene therapy for neurological diseases: regulation and targeting. Br. J. Clin. Pharmacol. 2013, 76, 217–232. [Google Scholar]
- Jacobson, S.G.; Cideciyan, A.V.; Ratnakaram, R.; Heon, E.; Schwartz, S.B.; Roman, A.J.; Peden, M.C.; Aleman, T.S.; Boye, S.L.; Sumaroka, A.; et al. Gene Therapy for Leber Congenital Amaurosis Caused by RPE65 Mutations: Safety and Efficacy in 15 Children and Adults Followed Up to 3 Years. Arch. Ophthalmol. 2011, 130, 9–24. [Google Scholar]
- High, K.A.; Aubourg, P. rAAV Human Trial Experience. Methods. Mol. Biol. 2011, 807, 429–457. [Google Scholar]
- Chamberlin, N.L.; Du, B.; de Lacalle, S.; Saper, C.B. Recombinant adeno-associated virus vector: use for transgene expression and anterograde tract tracing in the CNS. Brain Res. 1998, 793, 169–175. [Google Scholar]
- Manfredsson, F.P.; Bloom, D.C.; Mandel, R.J. Regulated protein expression for in vivo gene therapy for neurological disorders: Progress, strategies, and issues. Neurobiol. Dis. 2012, 48, 212–221. [Google Scholar]
- Noureddini, S.C.; Curiel, D.T. Genetic targeting strategies for adenovirus. Mol. Pharm. 2005, 2, 341–347. [Google Scholar]
- Everts, M.; Curiel, D.T. Transductional targeting of adenoviral cancer gene therapy. Curr. Gene. Ther. 2004, 4, 337–346. [Google Scholar]
- Tsuruta, Y.; Pereboeva, L.; Glasgow, J.N.; Rein, D.T.; Kawakami, Y.; Alvarez, R.D.; Rocconi, R.P.; Siegal, G.P.; Dent, P.; Fisher, P.B.; Curiel, D.T. A mosaic fiber adenovirus serotype 5 vector containing reovirus sigma 1 and adenovirus serotype 3 knob fibers increases transduction in an ovarian cancer ex vivo system via a coxsackie and adenovirus receptor-independent pathway. Clin. Cancer Res. 2007, 13, 2777–2783. [Google Scholar] [CrossRef]
- Takayama, K.; Reynolds, P.N.; Short, J.J.; Kawakami, Y.; Adachi, Y.; Glasgow, J.N.; Rots, M.G.; Krasnykh, V.; Douglas, J.T.; Curiel, D.T. A mosaic adenovirus possessing serotype Ad5 and serotype Ad3 knobs exhibits expanded tropism. Virology 2003, 309, 282–293. [Google Scholar]
- Glasgow, J.N.; Mikheeva, G.; Krasnykh, V.; Curiel, D.T. A strategy for adenovirus vector targeting with a secreted single chain antibody. PLoS One 2009, 4, e8355. [Google Scholar] [CrossRef]
- Glasgow, J.N.; Kremer, E.J.; Hemminki, A.; Siegal, G.P.; Douglas, J.T.; Curiel, D.T. An adenovirus vector with a chimeric fiber derived from canine adenovirus type 2 displays novel tropism. Virology 2004, 324, 103–116. [Google Scholar]
- Lewis, T.B.; Glasgow, J.N.; Glandon, A.M.; Curiel, D.T.; Standaert, D.G. Transduction of brain dopamine neurons by adenoviral vectors is modulated by CAR expression: rationale for tropism modified vectors in PD gene therapy. PLoS One 2010, 5, e12672. [Google Scholar]
- Zhu, M.-Y.; Wang, W.-P.; Baldessarini, R.J.; Kim, K.-S. Effects of desipramine treatment on tyrosine hydroxylase gene expression in cultured neuroblastoma cells and rat brain tissue. Brain Res. Mol. Brain Res. 2005, 133, 167–175. [Google Scholar]
- Skog, J.; Mei, Y.F.; Wadell, G. Human adenovirus serotypes 4p and 11p are efficiently expressed in cell lines of neural tumour origin. J. Gen. Virol. 2002, 83, 1299–1309. [Google Scholar]
- Nakayama, M.; Both, G.W.; Banizs, B.; Tsuruta, Y.; Yamamoto, S.; Kawakami, Y.; Douglas, J.T.; Tani, K.; Curiel, D.T.; Glasgow, J.N. An adenovirus serotype 5 vector with fibers derived from ovine atadenovirus demonstrates CAR-independent tropism and unique biodistribution in mice. Virology 2006, 350, 103–115. [Google Scholar]
- Paul, C.P.L.; Everts, M.; Glasgow, J.N.; Dent, P.; Fisher, P.B.; Ulasov, I.V.; Lesniak, M.S.; Stoff-Khalili, M.A.; Roth, J.C.; Preuss, M.A.; et al. Characterization of infectivity of knob-modified adenoviral vectors in glioma. Cancer Biol. Ther. 2008, 7, 786–793. [Google Scholar] [CrossRef]
- Kim, J.W.; Glasgow, J.N.; Nakayama, M.; Ak, F.; Ugai, H.; Curiel, D.T. An adenovirus vector incorporating carbohydrate binding domains utilizes glycans for gene transfer. PLoS One 2013, 8, e55533. [Google Scholar]
- Stoff-Khalili, M.A.; Rivera, A.A.; Glasgow, J.N.; Le, L.P.; Stoff, A.; Everts, M.; Tsuruta, Y.; Kawakami, Y.; Bauerschmitz, G.J.; Mathis, J.M.; Pereboeva, L.; Seigal, G.P.; Dall, P.; Curiel, D.T. A human adenoviral vector with a chimeric fiber from canine adenovirus type 1 results in novel expanded tropism for cancer gene therapy. Gene Ther. 2005, 12, 1696–1706. [Google Scholar]
- Krasnykh, V.N.; Mikheeva, G.V.; Douglas, J.T.; Curiel, D.T. Generation of recombinant adenovirus vectors with modified fibers for altering viral tropism. J. Virol. 1996, 70, 6839–6846. [Google Scholar]
- Kanerva, A.; Mikheeva, G.V.; Krasnykh, V.; Coolidge, C.J.; Lam, J.T.; Mahasreshti, P.J.; Barker, S.D.; Straughn, M.; Barnes, M.N.; Alvarez, R.D.; et al. Targeting adenovirus to the serotype 3 receptor increases gene transfer efficiency to ovarian cancer cells. Clin. Cancer Res. 2002, 8, 275–280. [Google Scholar]
- Wu, H.; Seki, T.; Dmitriev, I.; Uil, T.; Kashentseva, E.; Han, T.; Curiel, D.T. Double modification of adenovirus fiber with RGD and polylysine motifs improves coxsackievirus-adenovirus receptor-independent gene transfer efficiency. Hum. Gene Ther. 2002, 13, 1647–1653. [Google Scholar]
- Le, L.P.; Rivera, A.A.; Glasgow, J.N.; Ternovoi, V.V.; Wu, H.; Wang, M.; Smith, B.F.; Siegal, G.P.; Curiel, D.T. Infectivity enhancement for adenoviral transduction of canine osteosarcoma cells. Gene Ther. 2006, 13, 389–399. [Google Scholar]
- Van Houdt, W.J.; Wu, H.; Glasgow, J.N.; Lamfers, M.L.; Dirven, C.M.; Gillespie, G.Y.; Curiel, D.T.; Haviv, Y.S. Gene delivery into malignant glioma by infectivity-enhanced adenovirus: In vivo versus in vitro models. Neuro-Oncology 2007, 9, 280–290. [Google Scholar] [CrossRef]
- Soudais, C.; Laplace-Builhe, C.; Kissa, K.; Kremer, E.J. Preferential transduction of neurons by canine adenovirus vectors and their efficient retrograde transport in vivo. FASEB J. 2001, 15, 2283–2285. [Google Scholar]
- Soudais, C.; Skander, N.; Kremer, E.J. Long-term in vivo transduction of neurons throughout the rat CNS using novel helper-dependent CAV-2 vectors. FASEB J. 2004, 18, 391–393. [Google Scholar]
- Donello, J.E.; Loeb, J.E.; Hope, T.J. Woodchuck hepatitis virus contains a tripartite posttranscriptional regulatory element. J. Virol. 1998, 72, 5085–5092. [Google Scholar]
- St Martin, J.L.; Klucken, J.; Outeiro, T.F.; Nguyen, P.; Keller-McGandy, C.; Cantuti-Castelvetri, I.; Grammatopoulos, T.N.; Standaert, D.G.; Hyman, B.T.; McLean, P.J. Dopaminergic neuron loss and up-regulation of chaperone protein mRNA induced by targeted over-expression of alpha-synuclein in mouse substantia nigra. J. Neurochem. 2007, 100, 1449–1457. [Google Scholar]
- Stemmer, W.P.; Crameri, A.; Ha, K.D.; Brennan, T.M.; Heyneker, H.L. Single-step assembly of a gene and entire plasmid from large numbers of oligodeoxyribonucleotides. Gene 1995, 164, 49–53. [Google Scholar]
- Maizel, J.V., Jr.; White, D.O.; Scharff, M.D. The polypeptides of adenovirus. I. Evidence for multiple protein components in the virion and a comparison of types 2, 7A, and 12. Virology 1968, 36, 115–125. [Google Scholar] [CrossRef]
- Miyazawa, N.; Leopold, P.L.; Hackett, N.R.; Ferris, B.; Worgall, S.; Falck-Pedersen, E.; Crystal, R.G. Fiber swap between adenovirus subgroups B and C alters intracellular trafficking of adenovirus gene transfer vectors. J. Virol. 1999, 73, 6056–6065. [Google Scholar]
- Miyazawa, N.; Crystal, R.G.; Leopold, P.L. Adenovirus serotype 7 retention in a late endosomal compartment prior to cytosol escape is modulated by fiber protein. J. Virol. 2001, 75, 1387–1400. [Google Scholar]
- Shayakhmetov, D.M.; Li, Z.Y.; Ternovoi, V.; Gaggar, A.; Gharwan, H.; Lieber, A. The interaction between the fiber knob domain and the cellular attachment receptor determines the intracellular trafficking route of adenoviruses. J. Virol. 2003, 77, 3712–3723. [Google Scholar] [CrossRef]
- Kritz, A.B.; Nicol, C.G.; Dishart, K.L.; Nelson, R.; Holbeck, S.; Von Seggern, D.J.; Work, L.M.; McVey, J.H.; Nicklin, S.A.; Baker, A.H. Adenovirus 5 fibers mutated at the putative HSPG-binding site show restricted retargeting with targeting peptides in the HI loop. Mol. Ther. 2007, 15, 741–749. [Google Scholar]
- Chillon, M.; Kremer, E.J. Trafficking and propagation of canine adenovirus vectors lacking a known integrin-interacting motif. Hum. Gene Ther. 2001, 20, 1815–1823. [Google Scholar]
- Soudais, C.; Boutin, S.; Hong, S.S.; Chillon, M.; Danos, O.; Bergelson, J.M.; Boulanger, P.; Kremer, E.J. Canine adenovirus type 2 attachment and internalization: coxsackievirus-adenovirus receptor, alternative receptors, and an RGD-independent pathway. J. Virol. 2000, 74, 10639–10649. [Google Scholar]
- Kremer, E.J.; Boutin, S.; Chillon, M.; Danos, O. Canine adenovirus vectors: An alternative for adenovirus-mediated gene transfer. J. Virol. 2000, 74, 505–512. [Google Scholar]
- Schoehn, G.; Bakkouri, E.; Fabry, C.M.S.; Billet, O.; Estrozi, L.F.; Le, L.; Curiel, D.T.; Kajava, A.V.; Ruigrok, R.W.H.; Kremer, E.J. Three-dimensional structure of canine adenovirus serotype 2 capsid. 2008, 82, 3192–3203. [Google Scholar]
- Castle, M.J.; Perlson, E.; Holzbaur, E.L.; Wolfe, J.H. Long-distance Axonal Transport of AAV9 Is Driven by Dynein and Kinesin-2 and Is Trafficked in a Highly Motile Rab7-positive Compartment. Mol. Ther. 2014, 22, 554–566. [Google Scholar]
- Barcia, C.; Jimenez-Dalmaroni, M.; Kroeger, K.M.; Puntel, M.; Rapaport, A.J.; Larocque, D.; King, G.D.; Johnson, S.A.; Liu, C.; Xiong, W.; et al. One-year expression from high-capacity adenoviral vectors in the brains of animals with pre-existing anti-adenoviral immunity: clinical implications. Mol. Ther. 2007, 15, 2154–2163. [Google Scholar]
- Parr, M.J.; Wen, P.Y.; Schaub, M.; Khoury, S.J.; Sayegh, M.H.; Fine, H.A. Immune parameters affecting adenoviral vector gene therapy in the brain. J. Neurovirol. 1998, 4, 194–203. [Google Scholar]
- Lowenstein, P.R.; Castro, M.G. Inflammation and adaptive immune responses to adenoviral vectors injected into the brain: Peculiarities, mechanisms, and consequences. Gene Ther. 2003, 10, 946–954. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lewis, T.B.; Glasgow, J.N.; Harms, A.S.; Standaert, D.G.; Curiel, D.T. Fiber-Modified Adenovirus for Central Nervous System Parkinson’s Disease Gene Therapy. Viruses 2014, 6, 3293-3310. https://doi.org/10.3390/v6083293
Lewis TB, Glasgow JN, Harms AS, Standaert DG, Curiel DT. Fiber-Modified Adenovirus for Central Nervous System Parkinson’s Disease Gene Therapy. Viruses. 2014; 6(8):3293-3310. https://doi.org/10.3390/v6083293
Chicago/Turabian StyleLewis, Travis B., Joel N. Glasgow, Ashley S. Harms, David G. Standaert, and David T. Curiel. 2014. "Fiber-Modified Adenovirus for Central Nervous System Parkinson’s Disease Gene Therapy" Viruses 6, no. 8: 3293-3310. https://doi.org/10.3390/v6083293
APA StyleLewis, T. B., Glasgow, J. N., Harms, A. S., Standaert, D. G., & Curiel, D. T. (2014). Fiber-Modified Adenovirus for Central Nervous System Parkinson’s Disease Gene Therapy. Viruses, 6(8), 3293-3310. https://doi.org/10.3390/v6083293



