Simian Foamy Virus in Non-Human Primates and Cross-Species Transmission to Humans in Gabon: An Emerging Zoonotic Disease in Central Africa?
Abstract
:1. Introduction
2. SFV Infection in Mandrills


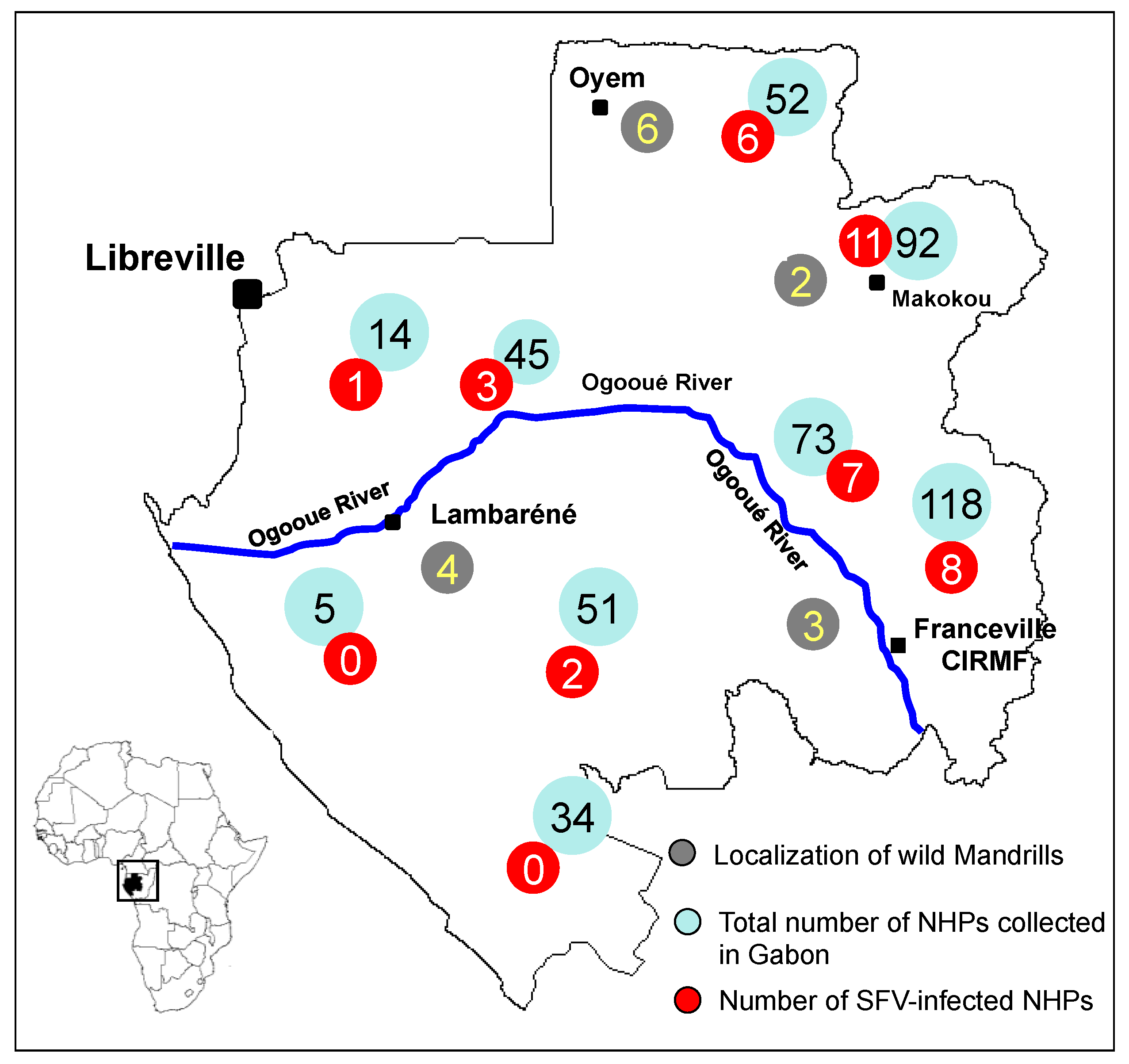
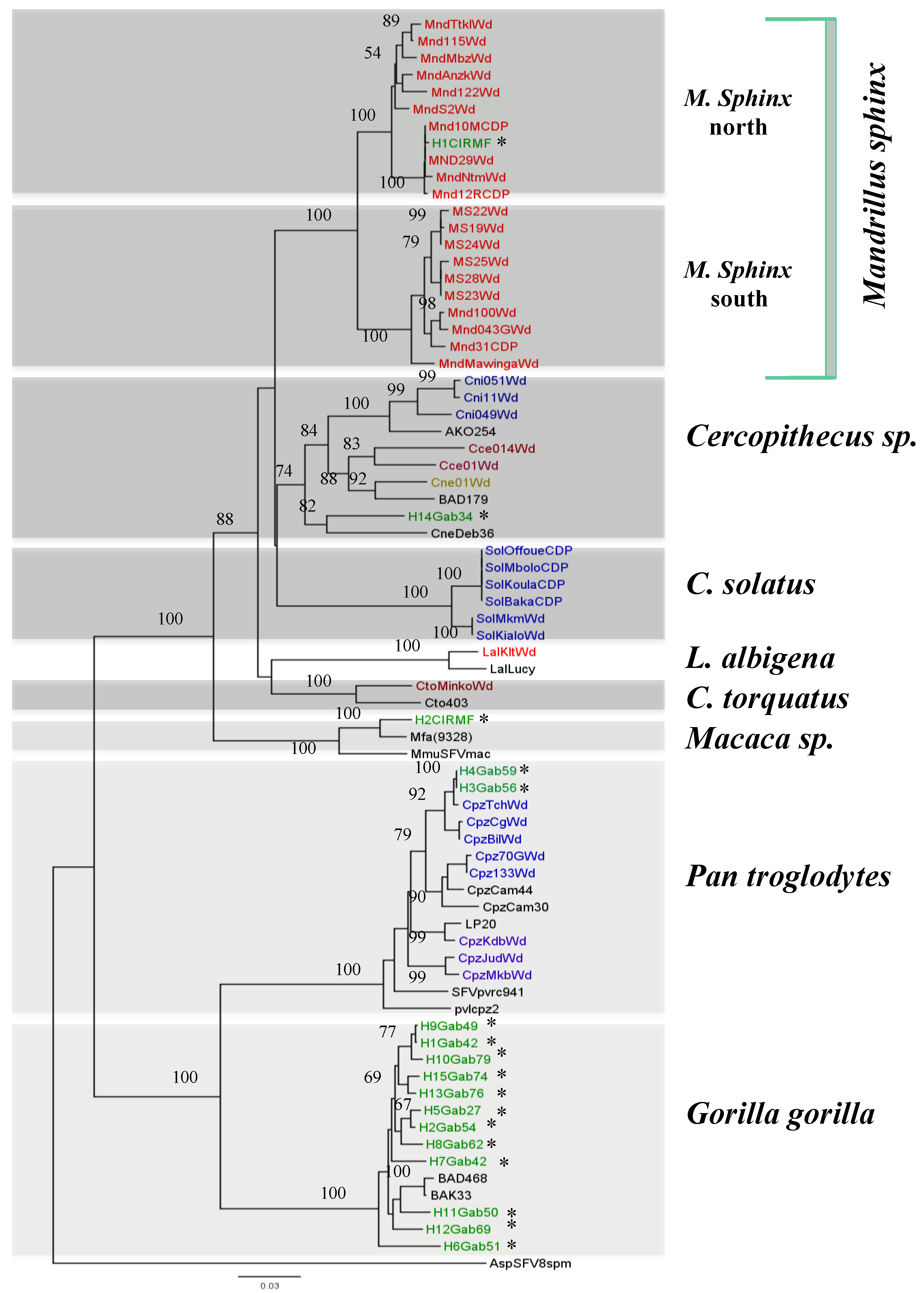
3. SFV Infection in Wild-Born Non-Human Primates
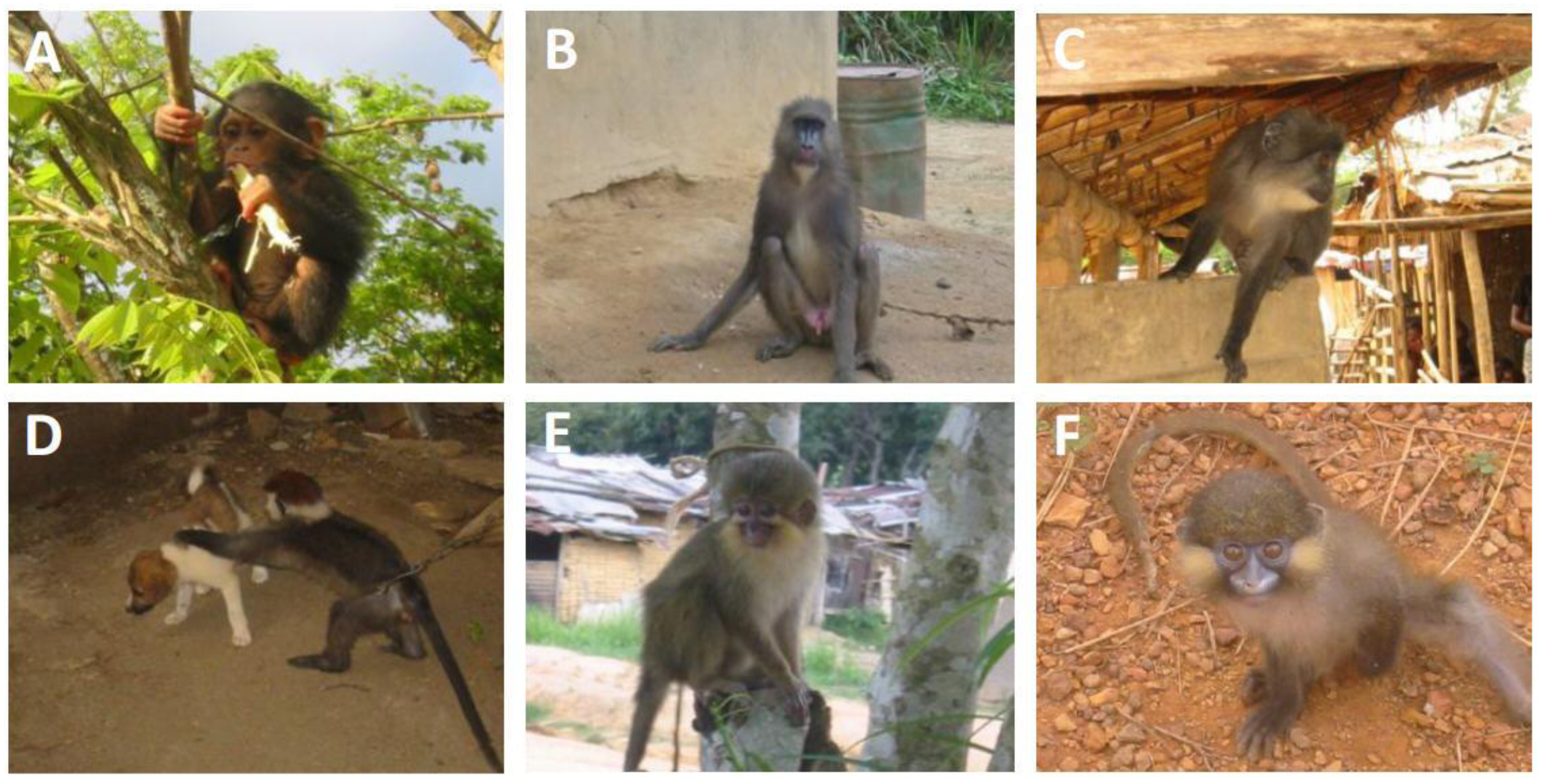

| Species | Common name | SFV | |
|---|---|---|---|
| Serology | PCR | ||
| Cercopithecus neglectus | De Brazza guenon | NA | + |
| Cercopithecus solatus | Sun-tailed monkey | + | + |
| Cercopithecus cephus | Red-eared guenon | NA | + |
| Cercopithecus nictitans | Greater white-nosed monkey | NA | + |
| Mandrillus sphinx | Mandrill | + | + |
| Pan troglodytes troglodytes | Central African chimpanzee | + | + |
| Cercocebus torquatus | Red-capped mangabey | + | + |
| Lophocebus albigena | Grey-cheeked mangabey | + | + |
4. SFV Cross-Species Transmission to Humans in Gabon

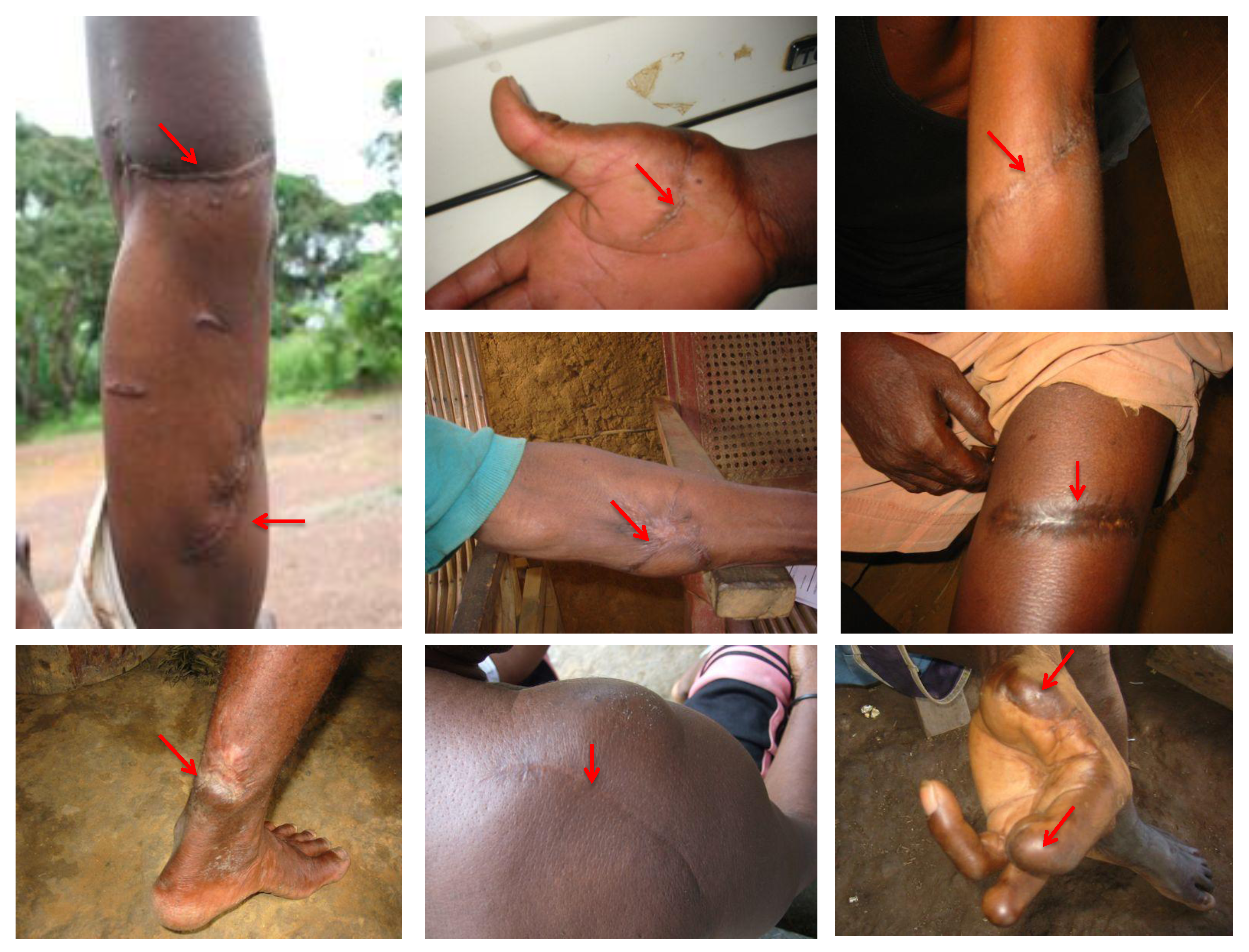
5. Conclusion and Remaining Questions
Conflict of Interest
References and Notes
- Hooks, J.J.; Gibbs, C.J., Jr. The foamy viruses. Bacteriol. Rev. 1975, 39, 169–185. [Google Scholar]
- Blewett, E.L.; Black, D.H.; Lerche, N.W.; White, G.; Eberle, R. Simian foamy virus infections in a baboon breeding colony. Virology 2000, 278, 183–193. [Google Scholar] [CrossRef]
- Hussain, A.I.; Shanmugam, V.; Bhullar, V.B.; Beer, B.E.; Vallet, D.; Gautier-Hion, A.; Wolfe, N.D.; Karesh, W.B.; Kilbourn, A.M.; Tooze, Z.; et al. Screening for simian foamy virus infection by using a combined antigen Western blot assay: Evidence for a wide distribution among Old World primates and identification of four new divergent viruses. Virology 2003, 309, 248–257. [Google Scholar] [CrossRef]
- McClure, M.O.; Bieniasz, P.D.; Schulz, T.F.; Chrystie, I.L.; Simpson, G.; Aguzzi, A.; Hoad, J.G.; Cunningham, A.; Kirkwood, J.; Weiss, R.A. Isolation of a new foamy retrovirus from orangutans. J. Virol. 1994, 68, 7124–7130. [Google Scholar]
- Saib, A. Non-primate foamy viruses. Curr. Top. Microbiol. Immunol. 2003, 277, 197–211. [Google Scholar] [CrossRef]
- Tobaly-Tapiero, J.; Bittoun, P.; Neves, M.; Guillemin, M.C.; Lecellier, C.H.; Puvion-Dutilleul, F.; Gicquel, B.; Zientara, S.; Giron, M.L.; de The, H.; et al. Isolation and characterization of an equine foamy virus. J. Virol. 2000, 74, 4064–4073. [Google Scholar] [CrossRef]
- Murray, S.M.; Linial, M.L. Foamy virus infection in primates. J. Med. Primatol. 2006, 35, 225–235. [Google Scholar] [CrossRef]
- Calattini, S.; Wanert, F.; Thierry, B.; Schmitt, C.; Bassot, S.; Saib, A.; Herrenschmidt, N.; Gessain, A. Modes of transmission and genetic diversity of Foamy Viruses in a Macaca Tonkeana colony. Retrovirology 2006, 3, 23. [Google Scholar] [CrossRef]
- Broussard, S.R.; Comuzzie, A.G.; Leighton, K.L.; Leland, M.M.; Whitehead, E.M.; Allan, J.S. Characterization of new simian foamy viruses from African nonhuman primates. Virology 1997, 237, 349–359. [Google Scholar] [CrossRef]
- Calattini, S.; Nerrienet, E.; Mauclere, P.; Georges-Courbot, M.C.; Saib, A.; Gessain, A. Natural simian foamy virus infection in wild-caught gorillas, mandrills and drills from Cameroon and Gabon. J. Gen. Virol. 2004, 85, 3313–3317. [Google Scholar] [CrossRef]
- Jones-Engel, L.; Steinkraus, K.A.; Murray, S.M.; Engel, G.A.; Grant, R.; Aggimarangsee, N.; Lee, B.P.; May, C.; Schillaci, M.A.; Somgird, C.; et al. Sensitive assays for simian foamy viruses reveal a high prevalence of infection in commensal, free-ranging Asian monkeys. J. Virol. 2007, 81, 7330–7337. [Google Scholar] [CrossRef]
- Liu, W.; Worobey, M.; Li, Y.; Keele, B.F.; Bibollet-Ruche, F.; Guo, Y.; Goepfert, P.A.; Santiago, M.L.; Ndjango, J.B.; Neel, C.; et al. Molecular ecology and natural history of simian foamy virus infection in wild-living chimpanzees. PLoS Pathog. 2008, 4, e1000097. [Google Scholar] [CrossRef]
- Brooks, J.I.; Merks, H.W.; Fournier, J.; Boneva, R.S.; Sandstrom, P.A. Characterization of blood-borne transmission of simian foamy virus. Transfusion 2007, 47, 162–170. [Google Scholar] [CrossRef]
- Heneine, W.; Switzer, W.M.; Sandstrom, P.; Brown, J.; Vedapuri, S.; Schable, C.A.; Khan, A.S.; Lerche, N.W.; Schweizer, M.; Neumann-Haefelin, D.; et al. Identification of a human population infected with simian foamy viruses. Nat. Med. 1998, 4, 403–407. [Google Scholar] [CrossRef]
- Sandstrom, P.A.; Phan, K.O.; Switzer, W.M.; Fredeking, T.; Chapman, L.; Heneine, W.; Folks, T.M. Simian foamy virus infection among zoo keepers. Lancet 2000, 355, 551–552. [Google Scholar]
- Schweizer, M.; Falcone, V.; Gange, J.; Turek, R.; Neumann-Haefelin, D. Simian foamy virus isolated from an accidentally infected human individual. J. Virol. 1997, 71, 4821–4824. [Google Scholar]
- Switzer, W.M.; Bhullar, V.; Shanmugam, V.; Cong, M.E.; Parekh, B.; Lerche, N.W.; Yee, J.L.; Ely, J.J.; Boneva, R.; Chapman, L.E.; et al. Frequent simian foamy virus infection in persons occupationally exposed to nonhuman primates. J. Virol. 2004, 78, 2780–2789. [Google Scholar] [CrossRef]
- Wolfe, N.D.; Switzer, W.M.; Carr, J.K.; Bhullar, V.B.; Shanmugam, V.; Tamoufe, U.; Prosser, A.T.; Torimiro, J.N.; Wright, A.; Mpoudi-Ngole, E.; et al. Naturally acquired simian retrovirus infections in central African hunters. Lancet 2004, 363, 932–937. [Google Scholar] [CrossRef]
- Calattini, S.; Betsem, E.B.; Froment, A.; Mauclere, P.; Tortevoye, P.; Schmitt, C.; Njouom, R.; Saib, A.; Gessain, A. Simian foamy virus transmission from apes to humans, rural Cameroon. Emerg. Infect. Dis. 2007, 13, 1314–1320. [Google Scholar] [CrossRef]
- Mouinga-Ondeme, A.; Betsem, E.; Caron, M.; Makuwa, M.; Salle, B.; Renault, N.; Saib, A.; Telfer, P.; Marx, P.; Gessain, A.; et al. Two distinct variants of simian foamy virus in naturally infected mandrills (Mandrillus sphinx) and cross-species transmission to humans. Retrovirology 2010, 7, 105. [Google Scholar] [CrossRef]
- Calattini, S.; Betsem, E.; Bassot, S.; Chevalier, S.A.; Tortevoye, P.; Njouom, R.; Mahieux, R.; Froment, A.; Gessain, A. Multiple retroviral infection by HTLV type 1, 2, 3 and simian foamy virus in a family of Pygmies from Cameroon. Virology 2011, 410, 48–55. [Google Scholar] [CrossRef]
- Mouinga-Ondeme, A.; Caron, M.; Nkoghe, D.; Telfer, P.; Marx, P.; Saib, A.; Leroy, E.; Gonzalez, J.P.; Gessain, A.; Kazanji, M. Cross-species transmission of simian foamy virus to humans in rural Gabon, Central Africa. J. Virol. 2012, 86, 1255–1260. [Google Scholar]
- Jones-Engel, L.; Engel, G.A.; Schillaci, M.A.; Rompis, A.; Putra, A.; Suaryana, K.G.; Fuentes, A.; Beer, B.; Hicks, S.; White, R.; et al. Primate-to-human retroviral transmission in Asia. Emerg. Infect. Dis. 2005, 11, 1028–1035. [Google Scholar] [CrossRef]
- Murray, S.M.; Picker, L.J.; Axthelm, M.K.; Linial, M.L. Expanded tissue targets for foamy virus replication with simian immunodeficiency virus-induced immunosuppression. J. Virol. 2006, 80, 663–670. [Google Scholar] [CrossRef]
- Khan, A.S. Simian foamy virus infection in humans: Prevalence and management. Expert Rev. Anti Infect. Ther. 2009, 7, 569–580. [Google Scholar] [CrossRef]
- Gessain, A.; Calattini, S. Emergence of simian foamy viruses in humans: Facts and unanswered questions. Future Virol. 2008, 3, 71–81. [Google Scholar] [CrossRef]
- Boneva, R.S.; Switzer, W.M.; Spira, T.J.; Bhullar, V.B.; Shanmugam, V.; Cong, M.E.; Lam, L.; Heneine, W.; Folks, T.M.; Chapman, L.E. Clinical and virological characterization of persistent human infection with simian foamy viruses. AIDS Res. Hum. Retrovir. 2007, 23, 1330–1337. [Google Scholar] [CrossRef]
- Linial, M. Why aren’t foamy viruses pathogenic? Trends Microbiol. 2000, 8, 284–289. [Google Scholar] [CrossRef]
- Meiering, C.D.; Linial, M.L. Historical perspective of foamy virus epidemiology and infection. Clin. Microbiol. Rev. 2001, 14, 165–176. [Google Scholar]
- Boneva, R.S.; Grindon, A.J.; Orton, S.L.; Switzer, W.M.; Shanmugam, V.; Hussain, A.I.; Bhullar, V.B.; Chamberland, M.E.; Heneine, W.; Folks, T.M.; et al. Simian foamy virus infection in a blood donor. Transfusion 2002, 42, 886–891. [Google Scholar] [CrossRef]
- Swanstrom, R.; Wills, J.W. Synthesis, assembly, and processing of viral proteins. In Retroviruses; Coffin, J.M., Hughes, S.H., Varmus, H.E., Eds.; Laboratory Press: Cold Spring Harbor, NY, USA, 1997. [Google Scholar]
- Delelis, O.; Lehmann-Che, J.; Saib, A. Foamy viruses—A world apart. Curr. Opin. Microbiol. 2004, 7, 400–406. [Google Scholar]
- Lecellier, C.H.; Saib, A. Foamy viruses: Between retroviruses and pararetroviruses. Virology 2000, 271, 1–8. [Google Scholar] [CrossRef]
- Schweizer, M.; Schleer, H.; Pietrek, M.; Liegibel, J.; Falcone, V.; Neumann-Haefelin, D. Genetic stability of foamy viruses: Long-term study in an African green monkey population. J. Virol. 1999, 73, 9256–9265. [Google Scholar]
- Schweizer, M.; Neumann-Haefelin, D. Phylogenetic analysis of primate foamy viruses by comparison of pol sequences. Virology 1995, 207, 577–582. [Google Scholar] [CrossRef]
- Gartner, K.; Wiktorowicz, T.; Park, J.; Mergia, A.; Rethwilm, A.; Scheller, C. Accuracy estimation of foamy virus genome copying. Retrovirology 2009, 6, 32. [Google Scholar] [CrossRef]
- Switzer, W.M.; Salemi, M.; Shanmugam, V.; Gao, F.; Cong, M.E.; Kuiken, C.; Bhullar, V.; Beer, B.E.; Vallet, D.; Gautier-Hion, A.; et al. Ancient co-speciation of simian foamy viruses and primates. Nature 2005, 434, 376–380. [Google Scholar] [CrossRef]
- Verschoor, E.J.; Langenhuijzen, S.; Bontjer, I.; Fagrouch, Z.; Niphuis, H.; Warren, K.S.; Eulenberger, K.; Heeney, J.L. The phylogeography of orangutan foamy viruses supports the theory of ancient repopulation of Sumatra. J. Virol. 2004, 78, 12712–12716. [Google Scholar]
- Heneine, W.; Schweizer, M.; Sandstrom, P.; Folks, T. Human infection with foamy viruses. Curr. Top Microbiol. Immunol. 2003, 277, 181–196. [Google Scholar]
- Falcone, V.; Schweizer, M.; Neumann-Haefelin, D. Replication of primate foamy viruses in natural and experimental hosts. Curr. Top Microbiol. Immunol. 2003, 277, 161–180. [Google Scholar] [CrossRef]
- Schweizer, M.; Turek, R.; Hahn, H.; Schliephake, A.; Netzer, K.O.; Eder, G.; Reinhardt, M.; Rethwilm, A.; Neumann-Haefelin, D. Markers of foamy virus infections in monkeys, apes, and accidentally infected humans: Appropriate testing fails to confirm suspected foamy virus prevalence in humans. AIDS Res. Hum. Retrovir. 1995, 11, 161–170. [Google Scholar] [CrossRef]
- Souquiere, S.; Bibollet-Ruche, F.; Robertson, D.L.; Makuwa, M.; Apetrei, C.; Onanga, R.; Kornfeld, C.; Plantier, J.C.; Gao, F.; Abernethy, K.; et al. Wild Mandrillus sphinx are carriers of two types of lentivirus. J. Virol. 2001, 75, 7086–7096. [Google Scholar] [CrossRef]
- Georges-Courbot, M.C.; Moisson, P.; Leroy, E.; Pingard, A.M.; Nerrienet, E.; Dubreuil, G.; Wickings, E.J.; Debels, F.; Bedjabaga, I.; Poaty-Mavoungou, V.; et al. Occurrence and frequency of transmission of naturally occurring simian retroviral infections (SIV, STLV, and SRV) at the CIRMF Primate Center, Gabon. J. Med. Primatol. 1996, 25, 313–326. [Google Scholar]
- Onanga, R.; Kornfeld, C.; Pandrea, I.; Estaquier, J.; Souquiere, S.; Rouquet, P.; Mavoungou, V.P.; Bourry, O.; M’Boup, S.; Barre-Sinoussi, F.; et al. High levels of viral replication contrast with only transient changes in CD4(+) and CD8(+) cell numbers during the early phase of experimental infection with simian immunodeficiency virus SIVmnd-1 in Mandrillus sphinx. J. Virol. 2002, 76, 10256–10263. [Google Scholar] [CrossRef]
- Pandrea, I.; Onanga, R.; Souquiere, S.; Mouinga-Ondeme, A.; Bourry, O.; Makuwa, M.; Rouquet, P.; Silvestri, G.; Simon, F.; Roques, P.; et al. Paucity of CD4+ CCR5+ T cells may prevent transmission of simian immunodeficiency virus in natural nonhuman primate hosts by breast-feeding. J. Virol. 2008, 82, 5501–5509. [Google Scholar] [CrossRef]
- Nerrienet, E.; Amouretti, X.; Muller-Trutwin, M.C.; Poaty-Mavoungou, V.; Bedjebaga, I.; Nguyen, H.T.; Dubreuil, G.; Corbet, S.; Wickings, E.J.; Barre-Sinoussi, F.; et al. Phylogenetic analysis of SIV and STLV type I in mandrills (Mandrillus sphinx): Indications that intracolony transmissions are predominantly the result of male-to-male aggressive contacts. AIDS Res. Hum. Retrovir. 1998, 14, 785–796. [Google Scholar] [CrossRef]
- Makuwa, M.; Souquiere, S.; Clifford, S.L.; Telfer, P.T.; Salle, B.; Bourry, O.; Onanga, R.; Mouinga-Ondeme, A.; Wickings, E.J.; Abernethy, K.A.; et al. Two distinct STLV-1 subtypes infecting Mandrillus sphinx follow the geographic distribution of their hosts. AIDS Res. Hum. Retrovir. 2004, 20, 1137–1143. [Google Scholar] [CrossRef]
- Souquiere, S.; Onanga, R.; Makuwa, M.; Pandrea, I.; Ngari, P.; Rouquet, P.; Bourry, O.; Kazanji, M.; Apetrei, C.; Simon, F.; et al. Simian immunodeficiency virus types 1 and 2 (SIV mnd 1 and 2) have different pathogenic potentials in rhesus macaques upon experimental cross-species transmission. J. Gen. Virol. 2009, 90, 488–499. [Google Scholar] [CrossRef]
- Mahieux, R.; Chappey, C.; Georges-Courbot, M.C.; Dubreuil, G.; Mauclere, P.; Georges, A.; Gessain, A. Simian T-cell lymphotropic virus type 1 from Mandrillus sphinx as a simian counterpart of human T-cell lymphotropic virus type 1 subtype D. J. Virol. 1998, 72, 10316–10322. [Google Scholar]
- Switzer, W.M.; Garcia, A.D.; Yang, C.; Wright, A.; Kalish, M.L.; Folks, T.M.; Heneine, W. Coinfection with HIV-1 and simian foamy virus in West Central Africans. J. Infect. Dis. 2008, 197, 1389–1393. [Google Scholar] [CrossRef]
- Laidre, M.E. Informative breath: Olfactory cues sought during social foraging among Old World monkeys (Mandrillus sphinx, M. Leucophaeus, and Papio anubis). J. Comp. Psychol. 2009, 123, 34–44. [Google Scholar] [CrossRef]
- Telfer, P.T.; Souquiere, S.; Clifford, S.L.; Abernethy, K.A.; Bruford, M.W.; Disotell, T.R.; Sterner, K.N.; Roques, P.; Marx, P.A.; Wickings, E.J. Molecular evidence for deep phylogenetic divergence in Mandrillus sphinx. Mol. Ecol. 2003, 12, 2019–2024. [Google Scholar] [CrossRef]
- Thumer, L.; Rethwilm, A.; Holmes, E.C.; Bodem, J. The complete nucleotide sequence of a New World simian foamy virus. Virology 2007, 369, 191–197. [Google Scholar] [CrossRef]
- Jones-Engel, L.; May, C.C.; Engel, G.A.; Steinkraus, K.A.; Schillaci, M.A.; Fuentes, A.; Rompis, A.; Chalise, M.K.; Aggimarangsee, N.; Feeroz, M.M.; et al. Diverse contexts of zoonotic transmission of simian foamy viruses in Asia. Emerg. Infect. Dis. 2008, 14, 1200–1208. [Google Scholar] [CrossRef]
- Leendertz, F.H.; Zirkel, F.; Couacy-Hymann, E.; Ellerbrok, H.; Morozov, V.A.; Pauli, G.; Hedemann, C.; Formenty, P.; Jensen, S.A.; Boesch, C.; et al. Interspecies transmission of simian foamy virus in a natural predator-prey system. J. Virol. 2008, 82, 7741–7744. [Google Scholar] [CrossRef]
- Apetrei, C.; Marx, P.A. Simian retroviral infections in human beings. Lancet 2004, 364, 137–138; author reply 139–140. [Google Scholar] [CrossRef]
- Cayabyab, M.; Karlsson, G.B.; Etemad-Moghadam, B.A.; Hofmann, W.; Steenbeke, T.; Halloran, M.; Fanton, J.W.; Axthelm, M.K.; Letvin, N.L.; Sodroski, J.G. Changes in human immunodeficiency virus type 1 envelope glycoproteins responsible for the pathogenicity of a multiply passaged simian-human immunodeficiency virus (SHIV-HXBc2). J. Virol. 1999, 73, 976–984. [Google Scholar]
- Feeroz, M.; Soliven, K.; Small, C.; Engel, G.; Pacheco, M.; Yee, J.; Wang, X.; Hasan, K.; Oh, G.; Levine, K.; et al. Population dynamics of rhesus macaques and associated foamy virus in Bangladesh. Emerg. Microbes Infect. 2013, 2, e29. [Google Scholar] [CrossRef]
- Caron, M.; Lekana-Douki, S.E.; Makuwa, M.; Obiang-Ndong, G.P.; Biba, O.; Nkoghe, D.; Kazanji, M. Prevalence, genetic diversity and antiretroviral drugs resistance-associated mutations among untreated HIV-1-infected pregnant women in Gabon, central Africa. BMC infect. Dis. 2012, 12, 64. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mouinga-Ondémé, A.; Kazanji, M. Simian Foamy Virus in Non-Human Primates and Cross-Species Transmission to Humans in Gabon: An Emerging Zoonotic Disease in Central Africa? Viruses 2013, 5, 1536-1552. https://doi.org/10.3390/v5061536
Mouinga-Ondémé A, Kazanji M. Simian Foamy Virus in Non-Human Primates and Cross-Species Transmission to Humans in Gabon: An Emerging Zoonotic Disease in Central Africa? Viruses. 2013; 5(6):1536-1552. https://doi.org/10.3390/v5061536
Chicago/Turabian StyleMouinga-Ondémé, Augustin, and Mirdad Kazanji. 2013. "Simian Foamy Virus in Non-Human Primates and Cross-Species Transmission to Humans in Gabon: An Emerging Zoonotic Disease in Central Africa?" Viruses 5, no. 6: 1536-1552. https://doi.org/10.3390/v5061536





