West Nile Virus Antibody Prevalence in Horses of Ukraine
Abstract
:1. Introduction
2. Results and Discussion
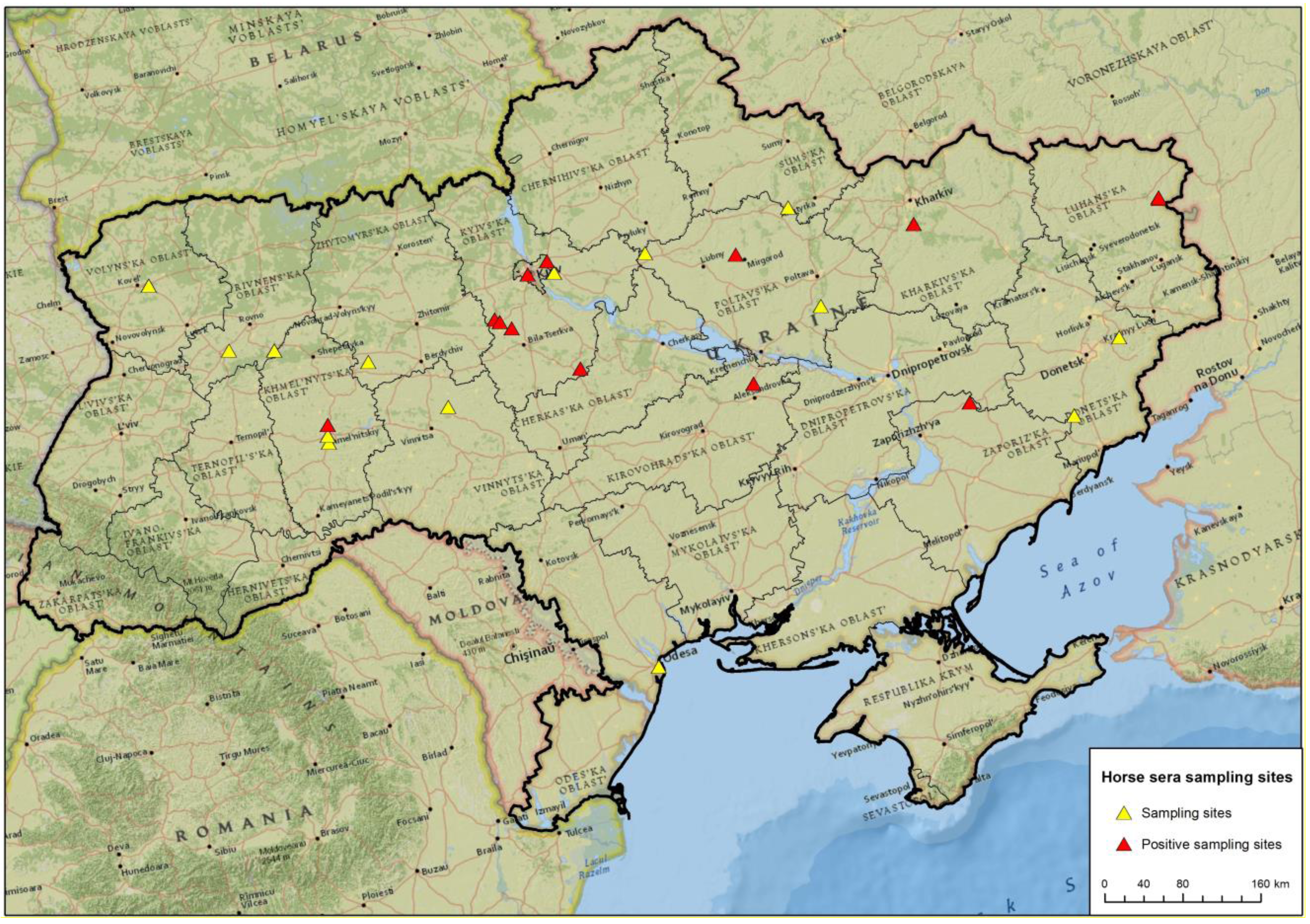
| Ukraine | Region | Investigated sera | by WNV-ELISA | only WNV-NT |
| 1 | Donetska | 11 | 11 | - |
| 2 | Kharkivska | 21 | 21 | - |
| 3 | Khmelnytska | 39 | 38 | 1 |
| 4 | Kyivska | 118 | 113 | 5 |
| 5 | Kirovohradska | 59 | 52 | 7 |
| 6 | Luhanska | 20 | 20 | - |
| 7 | Odeska | 1 | 1 | - |
| 8 | Poltavska | 6 | 6 | - |
| 9 | Rivnenska | 13 | 11 | 2 |
| 10 | Sumska | 2 | 2 | - |
| 11 | Vinnytska | 2 | 2 | - |
| 12 | Volynska | 10 | 10 | - |
| 13 | Zaporizka | 6 | 6 | - |
| 14 | Zhytomyrska | 2 | 2 | - |
| Total | 310 | 295 | 15 |
2.1. WNV competition Enzyme-linked immunosorbent assay (ELISA).
| Ukraine | Region | by ELISA | comp. ELISA negative | comp. ELISA reactive (doubtful or positive) | ELISA prevalence |
| 1 | Donetska | 11 | 11 | - | - |
| 2 | Kharkivska | 21 | 16 | 5 | 23.80 % |
| 3 | Khmelnytska | 38 | 37 | 1 | 2.63 % |
| 4 | Kyivska | 113 | 94 | 19 | 16.81 % |
| 5 | Kirovohradska | 52 | 39 | 13 | 25.00 % |
| 6 | Luhanska | 20 | 16 | 4 | 20.00 % |
| 7 | Odeska | 1 | 1 | - | - |
| 8 | Poltavska | 6 | 4 | 2 | 33.33 % |
| 9 | Rivnenska | 11 | 11 | - | - |
| 10 | Sumska | 2 | 2 | - | - |
| 11 | Vinnytska | 2 | 2 | - | - |
| 12 | Volynska | 10 | 10 | - | - |
| 13 | Zaporizka | 6 | 1 | 5 | 83.33 % |
| 14 | Zhytomyrska | 2 | 2 | - | - |
| Total | 295 | 246 | 49 | 16.61 % |

2.2. Virus neutralization assays (NT)
2.3. Indirect WNV IgM ELISA
| Laboratory sample no. | comp. WNV-ELISA S/N% | WNV-ELISA results | NT-ND50 (WNV lineage 2) | WNV-NT results | NT-ND50 (TBEV-Neudoerfl) | TBEV-NT results |
|---|---|---|---|---|---|---|
| ELISA and WNV-NT positive (n=42) | ||||||
| 33 | 14.19% | positive | 10 | weak positive | < 10 | negative |
| 44 | 12.28% | positive | 15 | weak positive | < 10 | negative |
| 53 | 6.18% | positive | 60 | positive | < 10 | negative |
| 54 | 4.98% | positive | 480 | positive | < 10 | negative |
| 58 | 6.35% | positive | 640 | positive | < 10 | negative |
| 59 | 5.09% | positive | 320 | positive | < 10 | negative |
| 64 | 4.73% | positive | 640 | positive | < 10 | negative |
| 68 | 4.94% | positive | 160 | positive | < 10 | negative |
| 69 | 4.87% | positive | 640 | positive | < 10 | negative |
| 71 | 4.98% | positive | 240 | positive | < 10 | negative |
| 72 | 4.83% | positive | 160 | positive | < 10 | negative |
| 75 | 5.90% | positive | 30 | positive | < 10 | negative |
| 88 | 5.53% | positive | 40 | positive | < 10 | negative |
| 96 | 5.97% | positive | 40 | positive | < 10 | negative |
| 97 | 4.91% | positive | 160 | positive | < 10 | negative |
| 105 | 5.77% | positive | 60 | positive | < 10 | negative |
| 107 | 4.73% | positive | 160 | positive | < 10 | negative |
| 108 | 4.67% | positive | 160 | positive | < 10 | negative |
| 123 | 7.86% | positive | 80 | positive | < 10 | negative |
| 124 | 4.81% | positive | 80 | positive | < 10 | negative |
| 128 | 4.15% | positive | 160 | positive | < 10 | negative |
| 131 | 3.88% | positive | 80 | positive | < 10 | negative |
| 146 | 8.79% | positive | 15 | weak positive | < 10 | negative |
| 149 | 4.31% | positive | 20 | positive | < 10 | negative |
| 158 | 4.04% | positive | 80 | positive | < 10 | negative |
| 160 | 3.78% | positive | 320 | positive | < 10 | negative |
| 166 | 4.55% | positive | 120 | positive | < 10 | negative |
| 187 | 4.63% | positive | 180 | positive | < 10 | negative |
| 208 | 6.09% | positive | 10 | weak positive | < 10 | negative |
| 217 | 7.84% | positive | 30 | positive | < 10 | negative |
| 228 | 4.44% | positive | 120 | positive | < 10 | negative |
| 232 | 10.92% | positive | 10 | weak positive | < 10 | negative |
| 288 | 4.48% | positive | 320 | positive | < 10 | negative |
| 289 | 9.16% | positive | 60 | positive | < 10 | negative |
| 292 | 5.09% | positive | 40 | positive | < 10 | negative |
| 297 | 5.35% | positive | 480 | positive | < 10 | negative |
| 312 | 5.12% | positive | 60 | positive | < 10 | negative |
| 313 | 4.92% | positive | 160 | positive | < 10 | negative |
| 330 | 7.98% | positive | 80 | positive | < 10 | negative |
| 336 | 4.18% | positive | 120 | positive | < 10 | negative |
| 337 | 4.44% | positive | 160 | positive | < 10 | negative |
| 343 | 7.08% | positive | 10 | weak positive | < 10 | negative |
| ELISA positive and WNV-NT negative (n=7) | ||||||
| 98 | 34.70% | positive | < 10 | negative | < 10 | negative |
| 106 | 26.64% | positive | < 10 | negative | < 10 | negative |
| 183 | 14.07% | positive | < 10 | negative | < 10 | negative |
| 230 | 23.58% | positive | < 10 | negative | < 10 | negative |
| 250 | 30.98% | positive | < 10 | negative | < 10 | negative |
| 295 | 25.88% | positive | < 10 | negative | < 10 | negative |
| 328 | 39.16% | positive | < 10 | negative | < 10 | negative |
| ELISA negative and WNV-NT positive (n=4) | ||||||
| 209 | 91.31% | negative | 30 | positive | < 10 | negative |
| 210 | 86.89% | negative | 120 | positive | < 10 | negative |
| 212 | 90.21% | negative | 10 | weak positive | < 10 | negative |
| 214 | 93.03% | negative | 320 | positive | < 10 | negative |
| Low volume ➔ no ELISA, WNV-NT positive (n=3) | ||||||
| 126 | n.d. | * | 80 | positive | < 10 | negative |
| 127 | n.d. | * | 60 | positive | < 10 | negative |
| 162 | n.d. | * | 60 | positive | < 10 | negative |
| Low volume ➔ no ELISA, WNV-NT negative (n=11+1 not evaluable) | ||||||
| 27 | n.d. | * | < 10 | negative | < 10 | negative |
| 76 | n.d. | * | < 10 | negative | < 10 | negative |
| 100 | n.d. | * | not evaluable, because of serum toxicity for cells | |||
| 125 | n.d. | * | < 10 | negative | < 10 | negative |
| 129 | n.d. | * | < 10 | negative | < 10 | negative |
| 130 | n.d. | * | < 10 | negative | < 10 | negative |
| 138 | n.d. | * | < 10 | negative | < 10 | negative |
| 139 | n.d. | * | < 10 | negative | < 10 | negative |
| 163 | n.d. | * | < 10 | negative | < 10 | negative |
| 179 | n.d. | * | < 10 | negative | < 10 | negative |
| 180 | n.d. | * | < 10 | negative | < 10 | negative |
| 186 | n.d. | * | < 10 | negative | < 10 | negative |
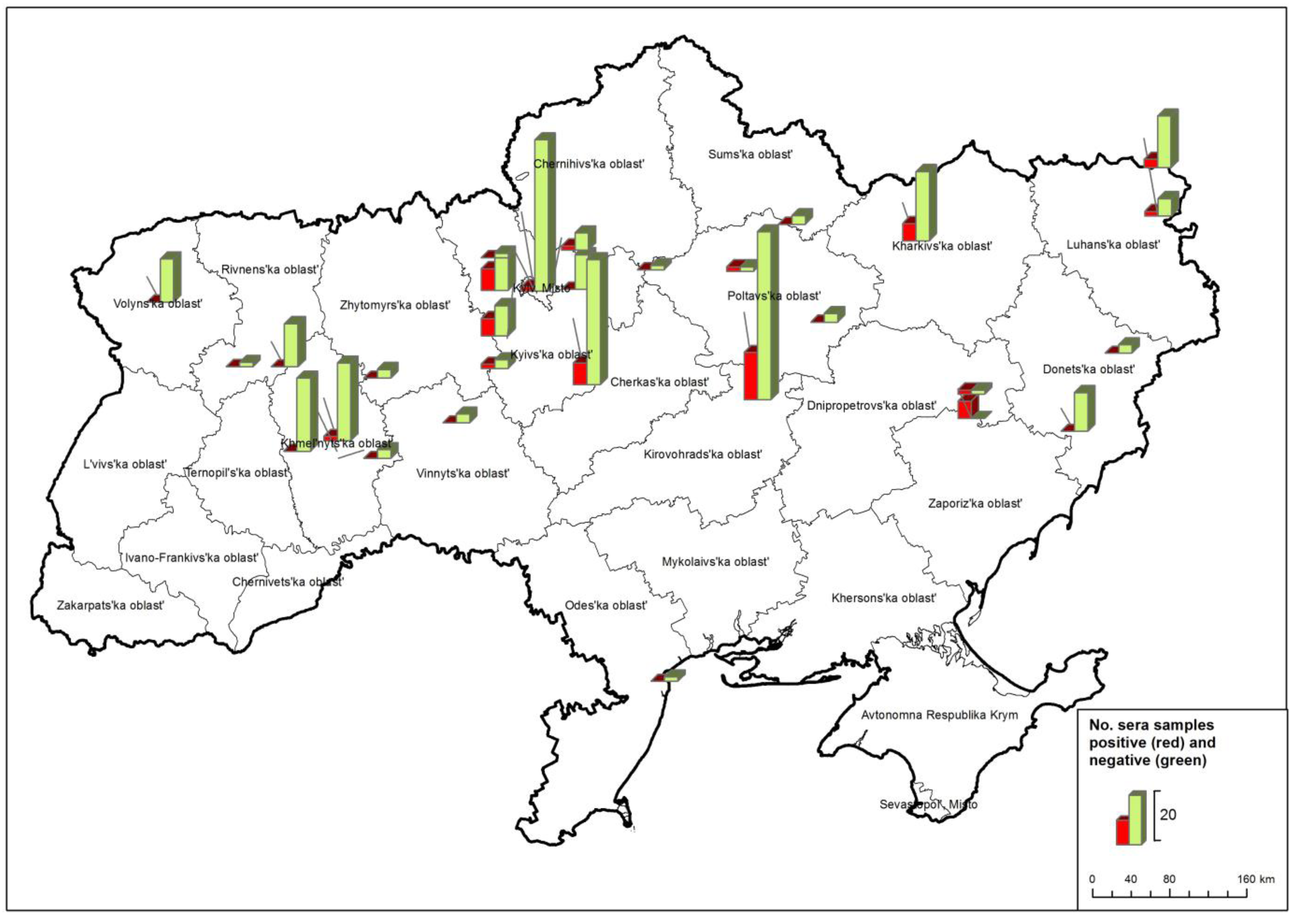
| Ukraine | region | comp. ELISA positive and WNV-NT positive | WNV-NT ND50 titres |
|---|---|---|---|
| 1 | Donetska | - | - |
| 2 | Kharkivska | 4 | 10 - 160 |
| 3 | Khmelnytska | 1 | 10 |
| 4 | Kyivska | 17 | 10 - 640 |
| 5 | Kirovohradska | 11 | 30 - 160 |
| 6 | Luhanska | 3 | 15 - 120 |
| 7 | Odeska | - | - |
| 8 | Poltavska | 1 | 40 |
| 9 | Rivnenska | - | - |
| 10 | Sumska | - | - |
| 11 | Vinnytska | - | - |
| 12 | Volynska | - | - |
| 13 | Zaporizka | 5 | 60 - 480 |
| 14 | Zhytomyrska | - | - |
| 42 |
3. Experimental Materials and Methods
4. Conclusions
Acknowledgments
Conflict of Interest
References and Notes
- Calistri, P.; Giovannini, A.; Hubalek, Z.; Ionescu, A.; Monaco, F.; Savini, G.; Lelli, R. Epidemiology of West Nile in europe and in the Mediterranean basin. Open Virol. J. 2010, 4, 29–37. [Google Scholar]
- Weissenbock, H.; Hubalek, Z.; Bakonyi, T.; Nowotny, N. Zoonotic mosquito–borne flaviviruses: worldwide presence of agents with proven pathogenicity and potential candidates of future emerging diseases. Vet. Microbiol. 2010, 140, 271–280. [Google Scholar] [CrossRef]
- van der Meulen, K.M.; Pensaert, M.B.; Nauwynck, H.J. West Nile virus in the vertebrate world. Arch. Virol. 2005, 150, 637–657. [Google Scholar] [CrossRef]
- Turell, M.J. Members of the Culex pipiens complex as vectors of viruses. J. Am. Mosq. Control Assoc. 2012, 28, 123–126. [Google Scholar] [CrossRef]
- Garcia–Bocanegra, I.; Jaen–Tellez, J.A.; Napp, S.; Arenas–Montes, A.; Fernandez–Morente, M.; Fernandez–Molera, V.; Arenas, A. West Nile fever outbreak in horses and humans, Spain, 2010. Emerg. Infect. Dis. 2011, 17, 2397–2399. [Google Scholar] [CrossRef]
- Tsai, T.F.; Popovici, F.; Cernescu, C.; Campbell, G.L.; Nedelcu, N.I. West Nile encephalitis epidemic in southeastern Romania. Lancet 1998, 352, 767–771. [Google Scholar] [CrossRef]
- Hubalek, Z. Mosquito–borne viruses in Europe. Parasitol. Res. 2008, 103 Suppl 1, S29–43. [Google Scholar] [CrossRef]
- Lvov, D.K.; Butenko, A.M.; Gromashevsky, V.L.; Kovtunov, A.I.; Prilipov, A.G.; Kinney, R.; Aristova, V.A.; Dzharkenov, A.F.; Samokhvalov, E.I.; Savage, H.M.; et al. West Nile virus and other zoonotic viruses in Russia: examples of emerging–reemerging situations. Arch. Virol. Suppl. 2004, 85–96. [Google Scholar]
- Murgue, B.; Murri, S.; Zientara, S.; Durand, B.; Durand, J.P.; Zeller, H. West Nile outbreak in horses in southern France, 2000: the return after 35 years. Emerg. Infect. Dis. 2001, 7, 692–696. [Google Scholar]
- Pfeffer, M.; Dobler, G. Emergence of zoonotic arboviruses by animal trade and migration. Parasit. Vectors 2010, 3, 35. [Google Scholar] [CrossRef]
- De Filette, M.; Ulbert, S.; Diamond, M.; Sanders, N.N. Recent progress in West Nile virus diagnosis and vaccination. Vet. Res. 2012, 43, 16. [Google Scholar] [CrossRef]
- Lvov, D.K.; Butenko, A.M.; Gromashevsky, V.L.; Larichev, V.P.; Gaidamovich, S.Y.; Vyshemirsky, O.I.; Zhukov, A.N.; Lazorenko, V.V.; Salko, V.N.; Kovtunov, A.I.; et al. Isolation of two strains of West Nile virus during an outbreak in southern Russia, 1999. Emerg. Infect. Dis. 2000, 6, 373–376. [Google Scholar] [CrossRef]
- Komar, N. West Nile virus: epidemiology and ecology in North America. Adv Virus Res 2003, 61, 185–234. [Google Scholar] [CrossRef]
- Papa, A. West Nile virus infections in Greece: an update. Expert Rev. Anti Infect. Ther. 2012, 10, 743–750. [Google Scholar] [CrossRef]
- Hubalek, Z.; Ludvikova, E.; Jahn, P.; Treml, F.; Rudolf, I.; Svobodova, P.; Sikutova, S.; Betasova, L.; Bires, J.; Mojzis, M.; et al. West Nile Virus Equine Serosurvey in the Czech and Slovak Republics. Vector Borne Zoonotic Dis. 2013. [Epub ahead of print]. [Google Scholar]
- Promedmail: Archive Number: 20120921.1304610. Available online: http://www.promedmail.org (accessed on 13 August 2013).
- Monaco, F.; Savini, G.; Calistri, P.; Polci, A.; Pinoni, C.; Bruno, R.; Lelli, R. 2009 West Nile disease epidemic in Italy: first evidence of overwintering in Western Europe? Res. Vet. Sci. 2011, 91, 321–326. [Google Scholar] [CrossRef]
- Savini, G.; Puggioni, G.; Di Gennaro, A.; Di Francesco, G.; Rocchigiani, A.M.; Polci, A.; Marini, V.; Pinoni, C.; Rolesu, S.; Marruchella, G.; et al. West Nile virus lineage 2 in Sardinian wild birds in 2012: a further threat to public health. Epidemiol. Infect. 2013, 1–4. [Google Scholar]
- Sidenko, V.P.; Stepankovskaia, L.D.; Solomko, R.M.; Poliakov, E.M.; Grekov, V.S.; Mosketi, K.B.; Scharanova, O.K.; Alekseenko, O.A.; Woljanskaia, E.A.; Fiadina, D.D. [Results of a study of West Nile fever in the South of the European part of the USSR]. Zh. Mikrobiol. Epidemiol. Immunobiol. 1974, 00, 129. [Google Scholar]
- Buletsa, B.A.; Turak Iu, A.; Korol, M.; Ignatovich, II; Vitvitskii, A.A. [Neurologic manifestations of West Nile fever in the Transcarpathian region]. Zh. Nevropatol. Psikhiatr. Im. S. S. Korsakova 1989, 89, 29–30. [Google Scholar]
- Vinograd, I.A.; Beletskaia, G.V.; Chumachenko, S.S.; Ardamatskaia, T.B.; Rogochii, E.G.; Lutsik, B.D.; Palchevski, N.V.; Vigovksy, A.I.; Olmelchenko, A.A. [Isolation of West Nile virus in the Southern Ukraine]. Vopr. Virusol. 1982, 27, 567–569. [Google Scholar]
- Lozyns'kyi, I.M.; Vynohrad, I.A. [Arboviruses and arbovirus infections in the forest steppe zone of Ukraine]. Mikrobiol. Z. 1998, 60, 49–60. [Google Scholar]
- ECDC, European Centers for Disease Prevention and Control: West Nile Fever Map – Table on cases 2011. Available online: http://ecdc.europa.eu/en/healthtopics/west_nile_fever/West–Nile–fever–maps/Pages/2010–table.aspx (accessed on 13 August 2013).
- ECDC, E.C.f.D.P.a.C.: West Nile Fever Map – Table on cases 2012. Available online: http://ecdc.europa.eu/en/healthtopics/west_nile_fever/West–Nile–fever–maps/Pages/2012–table.aspx (accessed on 13 August 2013).
- Giese, C.; Ait el Belghiti, F.; Barboza, P.; all National EpiSouth Focal Points. West Nile Virus Circulation in the EpiSouth countries and neighbouring areas, Seasons 2010 and 2011. Update 1st July 2012. Available online: http://www.episouthnetwork.org/sites/default/files/outputs/note_west_nile_episouth_2010_2011_july2012.pdf (accessed on 13 August 2013).
- Ziegler, U.; Seidowski, D.; Angenvoort, J.; Eiden, M.; Müller, K.; Nowotny, N.; Groschup, M.H. Monitoring of West Nile virus infections in Germany. Zoon. Public Health 2012, 59 Suppl 2, 95–101. [Google Scholar]
- Rushton, J.O.; Lecollinet, S.; Hubalek, Z.; Svobodova, P.; Lussy, H.; Nowotny, N. Tick–borne Encephalitis Virus in Horses, Austria, 2011. Emerg. Infect. Dis. 2013, 19, 635–637. [Google Scholar] [CrossRef]
- Ziegler, U.; Angenvoort, J.; Klaus, C.; Nagel–Kohl, U.; Sauerwald, C.; Thalheim, S.; Horner, S.; Braun, B.; Kenklies, S.; Tyczka, J.; et al. Use of competition ELISA for monitoring of west nile virus infections in horses in Germany. Int. J. Environ. Res. Public Health 2013, 10, 3112–3120. [Google Scholar] [CrossRef]
- Hubalek, Z.; Halouzka, J. West Nile fever – a reemerging mosquito–borne viral disease in Europe. Emerg. Infect. Dis. 1999, 5, 643–650. [Google Scholar] [CrossRef]
- Vinograd, I.A.; Beletskaia, G.V.; Chumachenko, S.S.; Omelchenko, G.A.; Lozinski, I.N.; Yartys, O.S.; et al. [Ecological aspects of arbovirus studies in the Ukrainian SSR]. In [Ecology of viruses and diagnostics of arbovirus infections.]; Lvov, D.K., Gaidamovich, S.Y., Eds.; Acad Med Sci USSR: Moscow, 1989; pp. 21–27. [Google Scholar]
- Atkinson, P.W.; Clark, J.A.; Delany, S.; Diagana, C.H.; du Feu, C.; Fiedler, W.; Fransson, T.; Gaulthier–Clerc, M.; Grantham, M.; Gschweng, M.; et al. Urgent preliminary assessment of ornithological data relevant to the spread of Avian Influenza in Europe. Report to the European Commission. May 2006. Available online: http://ec.europa.eu/environment/nature/conservation/wildbirds/birdflue/index_en.htm (accessed on 18 August 2013).
- Reiter, P. West Nile virus in Europe: understanding the present to gauge the future. Euro surveillance : bulletin Européen sur les maladies transmissibles = European communicable disease bulletin. 2010, 15, 19508. [Google Scholar]
- Seidowski, D.; Ziegler, U.; von Rönn, J.A.; Müller, K.; Hüppop, K.; Müller, T.; Freuling, C.; Mühle, R.U.; Nowotny, N.; Ulrich, R.G.; et al. West Nile virus monitoring of migratory and resident birds in Germany. Vector Borne Zoonotic Dis. 2010, 10, 639–647. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ziegler, U.; Skrypnyk, A.; Keller, M.; Staubach, C.; Bezymennyi, M.; Damiani, A.M.; Osterrieder, N.; Groschup, M.H. West Nile Virus Antibody Prevalence in Horses of Ukraine. Viruses 2013, 5, 2469-2482. https://doi.org/10.3390/v5102469
Ziegler U, Skrypnyk A, Keller M, Staubach C, Bezymennyi M, Damiani AM, Osterrieder N, Groschup MH. West Nile Virus Antibody Prevalence in Horses of Ukraine. Viruses. 2013; 5(10):2469-2482. https://doi.org/10.3390/v5102469
Chicago/Turabian StyleZiegler, Ute, Artem Skrypnyk, Markus Keller, Christoph Staubach, Maksym Bezymennyi, Armando M. Damiani, Nikolaus Osterrieder, and Martin H. Groschup. 2013. "West Nile Virus Antibody Prevalence in Horses of Ukraine" Viruses 5, no. 10: 2469-2482. https://doi.org/10.3390/v5102469
APA StyleZiegler, U., Skrypnyk, A., Keller, M., Staubach, C., Bezymennyi, M., Damiani, A. M., Osterrieder, N., & Groschup, M. H. (2013). West Nile Virus Antibody Prevalence in Horses of Ukraine. Viruses, 5(10), 2469-2482. https://doi.org/10.3390/v5102469




