Vaccines in Development against West Nile Virus
Abstract
:1. Introduction: West Nile Virus Dissemination
2. West Nile Virus Vaccine Requirements
3. West Nile Virus Veterinary Vaccines
| Vaccine | Antigen |
|---|---|
| West Nile Innovator–Pfizer | Whole inactivated WNV [37] |
| Vetera vaccine–Boehringer Ingelheim | Whole inactivated WNV |
| West Nile-Innovator DNA Pfizer (discontinued) | Plasmid DNA PrM/E |
| Recombiteck–Merial | Canarypox expressing PrM/E [38,39] |
| Prevenile–Intervet (recalled) | YF17D backbone expressing WNV PrM/E |
4. West Nile Virus Human Vaccines
4.1. Vaccines under Clinical Trials
| Vaccine name | Antigen | Clinical trial |
|---|---|---|
| ChimeriVax-WN02 | Chimeric YF17D backbone expressing WNV PrM/E | Phase II [40,41,42,43]. |
| Chimeric WN/DEN4-3’delta30 | Chimeric DV4 backbone expressing WNV PrM/E | Phase I [45]. |
| Clinical trial VRC303 | Plasmid DNA expressing PrM/E | Phase I [47,48]. |
| WN-80E | Soluble E lacking the trans membrane domain | Phase I [49,50,51,52]. |
4.2. Vaccines in Preclinical Development (Table 3)
4.2.1. Subunit Vaccines
4.2.2. DNA Vaccines
4.2.3. Non-Replicating Single-Cycle Vaccines
4.2.4. Inactivated Virus Vaccines
4.2.5. Recombinant Viral Vector Vaccines
4.2.6. Chimeric Vaccines
4.2.7. Live-Attenuated Vaccines Derived from Infectious Clones
| Vaccine | Strategy | Animal model |
|---|---|---|
| Truncated E (Drosophila) | - | Mice and horses [55,56] |
| Truncated E (Baculovirus) | - | Mice and Hamsters [52,53] |
| Truncated E -Nanoparticles LPS covered | Inflammasome-activating nanoparticle + LPS | Mice [60] |
| Truncated E –Nanoparticles | Inflammasome-activating nanoparticle + TLR9 Ligand | Mice [60] |
| CpG | ||
| EDIII - bacterial flagellin | TLR5 Ligand | Mice [20] |
| Continuous B-cell epitope from EDIII + HSP60 p458 peptide as carrier | - | Mice [62] |
| VLP (bacteriophage AP205)-EDIII | - | Mice [63] |
| WNV VLP (insect cells) | - | Mice [64] |
| Retroviral VLP (gag)-EDIII | VLPs expressing EDIII | Mice [65] |
| Plasmid DNA-PrM/E | - | Mice and Horses [61] |
| Plasmid DNA PrM/E+ Inactivated vaccine | Synergetic effect | Mice [67] |
| Plasmid DNA EDIII + IL15 adjuvant plasmid | Enhance T-cell memory | Mice [68] |
| Plasmid DNA PrM/E + LAMP | Target MHC-II compartment | Mice [69] |
| WNV DNA lacking the capsid protein | Disable the formation of infectious particles | Mice [65] |
| Kunjin DNA vaccine infectious clone (RepliVax) | Non-replicating single-cycle vaccine | Mice, Hamsters and Monkeys [77,78] |
| Capsid-deleted Kunjin DNA vaccine + co-expression of C protein (SRIP) | In vivo expression of single round infectious particles | Mice and Horses [72] |
| Adenovirus vector expressing WNV C, PrM, E and NS1 | Replication-incompetent vaccine | Mice [79] |
| Formalin-inactivated WNV vaccine (WN-VAX) | - | Mice [80] |
| Formaldehyde-inactivated vaccine Israel 98 (ISR98) strain | - | Geese [81] |
| H2O2-inactivated WN-KUN strain | Kunjin is naturally attenuated | Mice [80] |
| 6 contiguous cDNA fragments encoding NY99 genome | Bipartite infectious clone | Mice [86] |
| VSV vector expressing WNV E | Live attenuated vaccine | Mice [88] |
| Lentiviral HIV1-vector sE ISR98 WNV | - | Mice [89] |
| Non-integrative lentiviral HIV1 vector sE ISR98 WNV | Increased safety compared to HIV1-TRIP | Mice [85] |
| Chimeric DEN2 backbone expressing WNV PrM/E | LAV, highly attenuated | Mice [90,91] |
| Infectious clone (WN1415) | LAV, highly attenuated | Mice [92] |
| Live attenuated virus (LAV) mutations in the E and NS1 glycosylation sites | LAV, highly attenuated | Mice [93] |
| Recombinant measles vaccine expressing sE ISR98 WNV | LAV, highly attenuated | Mice[100], Monkeys [102] |
5. A WNV Vaccine Candidate Based on Recombinant Measles Vaccine
5.1. MVSchw-sEWNV Vaccine
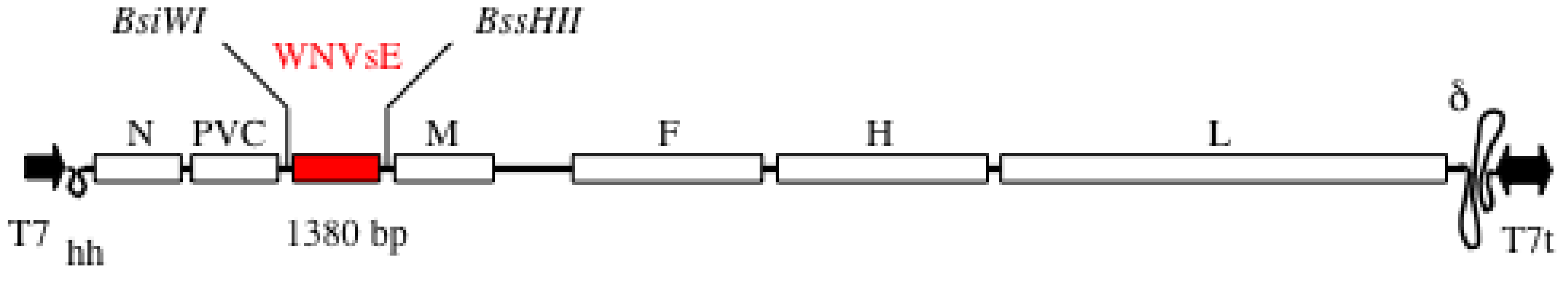
5.2. Immunogenicity in Mice
5.3. Immunogenicity in Non-Human Primates
5.3.1. Squirrel Monkey: A New Primate Model for West Nile Virus Infection

5.3.2. MV-WNV Induces Protective Immunity in Squirrel Monkeys
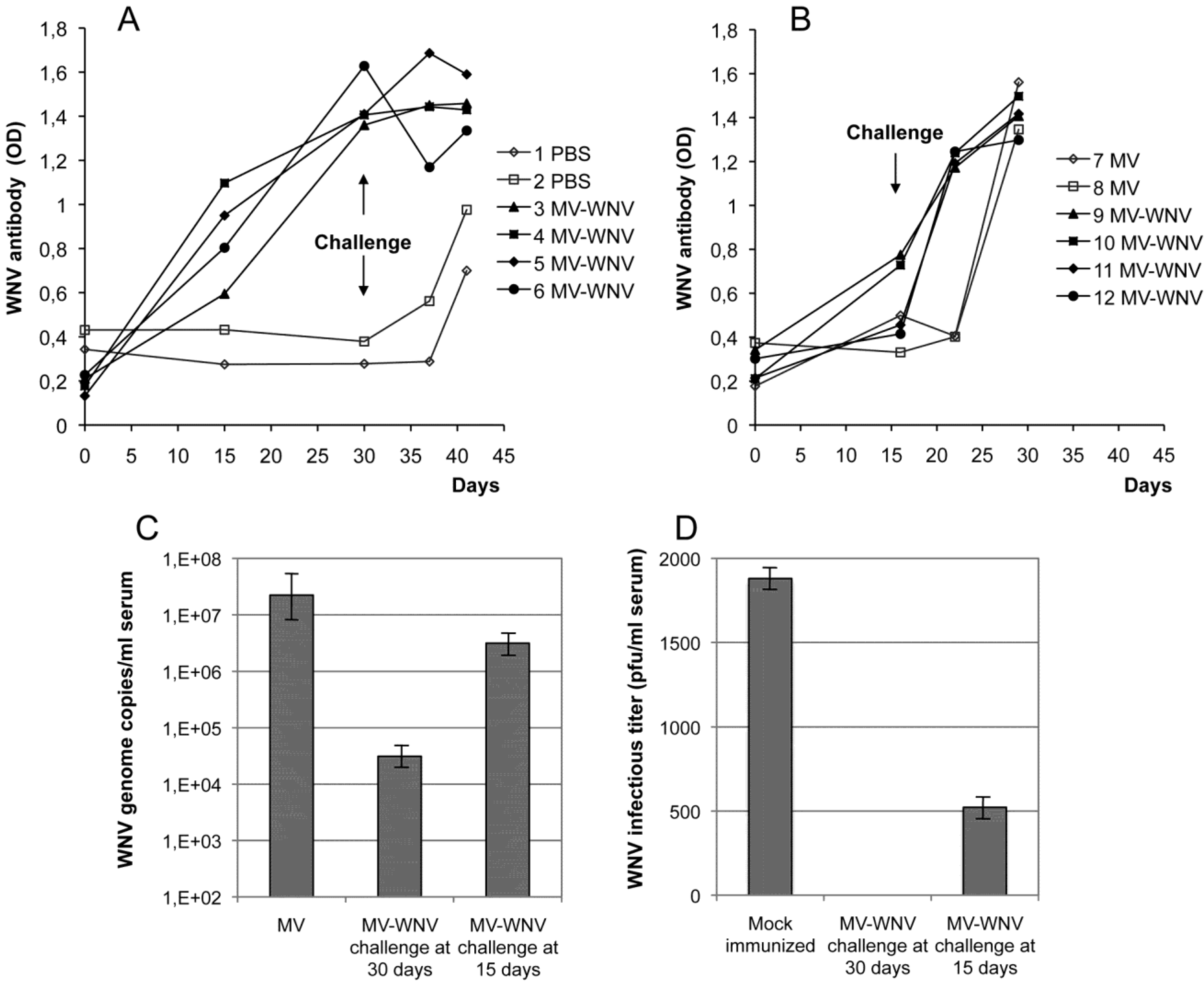
5.4. Other Recombinant Measles-Arbovirus Vaccines
6. Concluding Remarks
Acknowledgments
Conflicts of Interest
References
- Smithburn, K.C.H.; Burke, A.W.; Paul, J.H. A neurotropic virus isolated from the blood of a native of Uganda. Am. J. Trop. Med. Hyg. 1940, 20, 471–472. [Google Scholar]
- Zeller, H.G.; Schuffenecker, I. West Nile virus: an overview of its spread in Europe and the Mediterranean basin in contrast to its spread in the Americas. European journal of clinical microbiology & infectious diseases : official publication of the European Society of Clinical Microbiology 2004, 23, 147–156. [Google Scholar] [CrossRef]
- Malkinson, M.; Banet, C.; Weisman, Y.; Pokamunski, S.; King, R.; Drouet, M.T.; Deubel, V. Introduction of West Nile virus in the Middle East by migrating white storks. Emerg. Infect. Dis. 2002, 8, 392–397. [Google Scholar] [CrossRef]
- Murray, K.O.; Mertens, E.; Despres, P. West Nile virus and its emergence in the United States of America. Veterinary research 2010, 41, 67. [Google Scholar] [CrossRef]
- Prevention, Centers for Disease Control and Prevention (CDC). West Nile Virus (WNV) Human Infections Reported to ArboNET, by State, United States, 2012 (as of December 11, 2012). 2012 [cited 2012 21–02–2013]. Available online: http://www.cdc.gov/ncidod/dvbid/westnile/surv&controlCaseCount12_detailed.htm (accessed on 6 October 2013).
- Prevention, European Center for Disease Control and Prevention (ECDC). Available online: http://ecdc.europa.eu/en/healthtopics/west_nile_fever/West-Nile-fever-maps/Pages/index.aspx (accessed on 6 October 2013).
- Allain, J.P.; Stramer, S.L.; Carneiro-Proietti, A.B.; Martins, M.L.; Lopes da Silva, S.N.; Ribeiro, M.; Proietti, F.A.; Reesink, H.W. Transfusion-transmitted infectious diseases. Biologicals: J. Int. Ass. of Biol. Stand. 2009, 37, 71–77. [Google Scholar]
- Hinckley, A.F.; O'Leary, D.R.; Hayes, E.B. Transmission of West Nile virus through human breast milk seems to be rare. Pediatrics 2007, 119, e666–671. [Google Scholar] [CrossRef]
- Interim guidelines for the evaluation of infants born to mothers infected with West Nile virus during pregnancy. MMWR 2004, 53, 154–157.
- Mackenzie, J.S.; Williams, D.T. The zoonotic flaviviruses of southern, south-eastern and eastern Asia, and Australasia: the potential for emergent viruses. Zoon. Publ. Health 2009, 56, 338–356. [Google Scholar] [CrossRef]
- Lanciotti, R.S.; Roehrig, J.T.; Deubel, V.; Smith, J.; Parker, M.; Steele, K.; Crise, B.; Volpe, K.E.; Crabtree, M.B.; Scherret, J.H.; Hall, R.A.; MacKenzie, J.S.; Cropp, C.B.; Panigrahy, B.; Ostlund, E.; Schmitt, B.; Malkinson, M.; Banet, C.; Weissman, J.; Komar, N.; Savage, H.M.; Stone, W.; McNamara, T.; Gubler, D.J. Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science 1999, 286, 2333–2337. [Google Scholar] [CrossRef]
- Lanciotti, R.S.; Ebel, G.D.; Deubel, V.; Kerst, A.J.; Murri, S.; Meyer, R.; Bowen, M.; McKinney, N.; Morrill, W.E.; Crabtree, M.B.; Kramer, L.D.; Roehrig, J.T. Complete genome sequences and phylogenetic analysis of West Nile virus strains isolated from the United States, Europe, and the Middle East. Virology 2002, 298, 96–105. [Google Scholar] [CrossRef]
- Papa, A. West Nile virus infections in Greece: an update. Expert Rev. of Anti-Infect. Ther. 2012, 10, 743–750. [Google Scholar] [CrossRef]
- Papa, A.; Politis, C.; Tsoukala, A.; Eglezou, A.; Bakaloudi, V.; Hatzitaki, M.; Tsergouli, K. West Nile virus lineage 2 from blood donor, Greece. Emerg. Infect. Dis. 2012, 18, 688–689. [Google Scholar] [CrossRef]
- Bakonyi, T.; Hubalek, Z.; Rudolf, I.; Nowotny, N. Novel flavivirus or new lineage of West Nile virus, central Europe. Emerg. Infect. Dis. 2005, 11, 225–231. [Google Scholar] [CrossRef]
- Bondre, V.P.; Jadi, R.S.; Mishra, A.C.; Yergolkar, P.N.; Arankalle, V.A. West Nile virus isolates from India: evidence for a distinct genetic lineage. J. Gen. Virol. 2007, 88, 875–884. [Google Scholar] [CrossRef]
- Arnold, C. West Nile virus bites back. Lancet Neurol. 2012, 11, 1023–1024. [Google Scholar] [CrossRef]
- Beasley, D.W. Vaccines and immunotherapeutics for the prevention and treatment of infections with West Nile virus. Immunotherapy 2011, 3, 269–285. [Google Scholar] [CrossRef]
- Martina, B.E.; Koraka, P.; van den Doel, P.; van Amerongen, G.; Rimmelzwaan, G.F.; Osterhaus, A.D. Immunization with West Nile virus envelope domain III protects mice against lethal infection with homologous and heterologous virus. Vaccine 2008, 26, 153–157. [Google Scholar] [CrossRef]
- McDonald, W.F.; Huleatt, J.W.; Foellmer, H.G.; Hewitt, D.; Tang, J.; Desai, P.; Price, A.; Jacobs, A.; Takahashi, V.N.; Huang, Y.; Nakaar, V.; Alexopoulou, L.; Fikrig, E.; Powell, T.J. A West Nile virus recombinant protein vaccine that coactivates innate and adaptive immunity. J. Infect. Dis. 2007, 195, 1607–1617. [Google Scholar] [CrossRef]
- Heinz, F.X.; Stiasny, K. Flaviviruses and flavivirus vaccines. Vaccine 2012, 30, 4301–4306. [Google Scholar] [CrossRef]
- Nybakken, G.E.; Nelson, C.A.; Chen, B.R.; Diamond, M.S.; Fremont, D.H. Crystal structure of the West Nile virus envelope glycoprotein. J. Virol. 2006, 80, 11467–11474. [Google Scholar] [CrossRef]
- Kanai, R.; Kar, K.; Anthony, K.; Gould, L.H.; Ledizet, M.; Fikrig, E.; Marasco, W.A.; Koski, R.A.; Modis, Y. Crystal structure of west nile virus envelope glycoprotein reveals viral surface epitopes. J. Virol. 2006, 80, 11000–11008. [Google Scholar] [CrossRef]
- Modis, Y.; Ogata, S.; Clements, D.; Harrison, S.C. A ligand-binding pocket in the dengue virus envelope glycoprotein. Proc. Natl. Acad. Sci. U.S.A. 2003, 100, 6986–6991. [Google Scholar] [CrossRef]
- Modis, Y.; Ogata, S.; Clements, D.; Harrison, S.C. Structure of the dengue virus envelope protein after membrane fusion. Nature 2004, 427, 313–319. [Google Scholar] [CrossRef]
- Nayak, V.; Dessau, M.; Kucera, K.; Anthony, K.; Ledizet, M.; Modis, Y. Crystal structure of dengue virus type 1 envelope protein in the postfusion conformation and its implications for membrane fusion. J. Virol. 2009, 83, 4338–4344. [Google Scholar] [CrossRef]
- Rey, F.A.; Heinz, F.X.; Mandl, C.; Kunz, C.; Harrison, SC. The envelope glycoprotein from tick-borne encephalitis virus at 2 A resolution. Nature 1995, 375, 291–298. [Google Scholar] [CrossRef]
- Throsby, M.; Geuijen, C.; Goudsmit, J.; Bakker, A.Q.; Korimbocus, J.; Kramer, R.A.; Clijsters-van der Horst, M.; de Jong, M.; Jongeneelen, M.; Thijsse, S.; Smit, R.; Visser, T.J.; Bijl, N.; Marissen, W.E.; Loeb, M.; Kelvin, D.J.; Preiser, W.; ter Meulen, J.; de Kruif, J. Isolation and characterization of human monoclonal antibodies from individuals infected with West Nile Virus. J. Virol. 2006, 80, 6982–6992. [Google Scholar] [CrossRef]
- Thomas, S.; Redfern, J.B.; Lidbury, B.A.; Mahalingam, S. Antibody-dependent enhancement and vaccine development. Expert. Rev. Vaccines 2006, 5, 409–412. [Google Scholar] [CrossRef]
- Diamond, M.S.; Pierson, T.C.; Fremont, D.H. The structural immunology of antibody protection against West Nile virus. Immunol. Rev. 2008, 225, 212–225. [Google Scholar] [CrossRef]
- Pierson, T.C.; Fremont, D.H.; Kuhn, R.J.; Diamond, M.S. Structural insights into the mechanisms of antibody-mediated neutralization of flavivirus infection: implications for vaccine development. Cell host & microbe 2008, 4, 229–238. [Google Scholar]
- White, L.J.; Sariol, C.A.; Mattocks, M.D.; Wahala, M.P.B.W.; Yingsiwaphat, V.; Collier, M.L.; Whitley, J.; Mikkelsen, R.; Rodriguez, I.V.; Martinez, M.I.; de Silva, A.; Johnston, R.E. An alphavirus vector-based tetravalent dengue vaccine induces a rapid and protective immune response in macaques that differs qualitatively from immunity induced by live virus infection. J. Virol. 2013, 87, 3409–3424. [Google Scholar] [CrossRef]
- de Alwis, R.; Smith, S.A.; Olivarez, N.P.; Messer, W.B.; Huynh, J.P.; Wahala, W.M.; White, L.J.; Diamond, M.S.; Baric, R.S.; Crowe, J.E., Jr.; de Silva, A.M. Identification of human neutralizing antibodies that bind to complex epitopes on dengue virions. Proc. Natl. Acad. Sci. U.S.A. 2012, 109, 7439–7444. [Google Scholar] [CrossRef]
- Kaufmann, B.; Vogt, M.R.; Goudsmit, J.; Holdaway, H.A.; Aksyuk, A.A.; Chipman, P.R.; Kuhn, R.J.; Diamond, M.S.; Rossmann, M.G. Neutralization of West Nile virus by cross-linking of its surface proteins with Fab fragments of the human monoclonal antibody CR4354. Proc. Natl. Acad. Sci. U.S.A. 2010, 107, 18950–18955. [Google Scholar] [CrossRef]
- Vogt, M.R.; Moesker, B.; Goudsmit, J.; Jongeneelen, M.; Austin, S.K.; Oliphant, T.; Nelson, S.; Pierson, T.C.; Wilschut, J.; Throsby, M.; Diamond, M.S. Human monoclonal antibodies against West Nile virus induced by natural infection neutralize at a postattachment step. J. Virol. 2009, 83, 6494–6507. [Google Scholar] [CrossRef]
- Lok, S.M.; Kostyuchenko, V.; Nybakken, G.E.; Holdaway, H.A.; Battisti, A.J.; Sukupolvi-Petty, S.; Sedlak, D.; Fremont, D.H.; Chipman, P.R.; Roehrig, J.T.; Diamond, M.S.; Kuhn, R.J.; Rossmann, M.G. Binding of a neutralizing antibody to dengue virus alters the arrangement of surface glycoproteins. Nat. Struct. Mol. Biol. 2008, 15, 312–317. [Google Scholar] [CrossRef]
- Ng, T.; Hathaway, D.; Jennings, N.; Champ, D.; Chiang, Y.W.; Chu, H.J. Equine vaccine for West Nile virus. Dev. Biol. 2003, 114, 221–227. [Google Scholar]
- El Garch, H.; Minke, J.M.; Rehder, J.; Richard, S.; Edlund Toulemonde, C.; Dinic, S.; Andreoni, C.; Audonnet, J.C.; Nordgren, R.; Juillard, V. A West Nile virus (WNV) recombinant canarypox virus vaccine elicits WNV-specific neutralizing antibodies and cell-mediated immune responses in the horse. Vet. Immunol. Immunopathol. 2008, 123, 230–239. [Google Scholar] [CrossRef]
- Karaca, K.; Bowen, R.; Austgen, L.E.; Teehee, M.; Siger, L.; Grosenbaugh, D.; Loosemore, L.; Audonnet, J.C.; Nordgren, R.; Minke, J.M. Recombinant canarypox vectored West Nile virus (WNV) vaccine protects dogs and cats against a mosquito WNV challenge. Vaccine 2005, 23, 3808–2813. [Google Scholar] [CrossRef]
- Monath, T.P.; Liu, J.; Kanesa-Thasan, N.; Myers, G.A.; Nichols, R.; Deary, A.; McCarthy, K.; Johnson, C.; Ermak, T.; Shin, S.; Arroyo, J.; Guirakhoo, F.; Kennedy, J.S.; Ennis, F.A.; Green, S.; Bedford, P. A live, attenuated recombinant West Nile virus vaccine. Proc. Natl. Acad. Sci. U.S.A. 2006, 103, 6694–6699. [Google Scholar] [CrossRef]
- Smith, H. L.; Monath, T.P.; Pazoles, P.; Rothman, A.L.; Casey, D.M.; Terajima, M.; Ennis, F.A.; Guirakhoo, F.; Green, S. Development of antigen-specific memory CD8+ T cells following live-attenuated chimeric West Nile virus vaccination. J. Infect. Dis. 2011, 203, 513–522. [Google Scholar] [CrossRef]
- Guy, B.; Guirakhoo, F.; Barban, V.; Higgs, S.; Monath, T.P.; Lang, J. Preclinical and clinical development of YFV 17D-based chimeric vaccines against dengue, West Nile and Japanese encephalitis viruses. Vaccine 2010, 28, 632–649. [Google Scholar] [CrossRef]
- Dayan, G.H.; Bevilacqua, J.; Coleman, D.; Buldo, A.; Risi, G. Phase II, dose ranging study of the safety and immunogenicity of single dose West Nile vaccine in healthy adults >/= 50 years of age. Vaccine 2012, 30, 6656–6664. [Google Scholar] [CrossRef]
- Pletnev, A.G.; Swayne, D.E.; Speicher, J.; Rumyantsev, A.A.; Murphy, B.R. Chimeric West Nile/dengue virus vaccine candidate: preclinical evaluation in mice, geese and monkeys for safety and immunogenicity. Vaccine 2006, 24, 6392–6404. [Google Scholar] [CrossRef]
- De Filette, M.; Ulbert, S.; Diamond, M.; Sanders, N.N. Recent progress in West Nile virus diagnosis and vaccination. Vet. Res. 2012, 43, 16. [Google Scholar] [CrossRef]
- Hanley, K.A.; Goddard, L.B.; Gilmore, L.E.; Scott, T.W.; Speicher, J.; Murphy, B.R.; Pletnev, A.G. Infectivity of West Nile/dengue chimeric viruses for West Nile and dengue mosquito vectors. Vector Borne Zoonotic. Dis. 2005, 5, 1–10. [Google Scholar] [CrossRef]
- Ledgerwood, J.E.; Pierson, T.C.; Hubka, S.A.; Desai, N.; Rucker, S.; Gordon, I.J.; Enama, M.E.; Nelson, S.; Nason, M.; Gu, W.; Bundrant, N.; Koup, R.A.; Bailer, R.T.; Mascola, J.R.; Nabel, G.J.; Graham, B.S. A West Nile virus DNA vaccine utilizing a modified promoter induces neutralizing antibody in younger and older healthy adults in a phase I clinical trial. J. Infect. Dis. 2011, 203, 1396–1404. [Google Scholar] [CrossRef]
- Martin, J.E.; Pierson, T.C.; Hubka, S.; Rucker, S.; Gordon, I.J.; Enama, M.E.; Andrews, C.A.; Xu, Q.; Davis, B.S.; Nason, M.; Fay, M.; Koup, R.A.; Roederer, M.; Bailer, R.T.; Gomez, P. L.; Mascola, J.R.; Chang, G.J.; Nabel, G.J.; Graham, B.S. A West Nile virus DNA vaccine induces neutralizing antibody in healthy adults during a phase 1 clinical trial. J. Infect. Dis. 2007, 196, 1732–1740. [Google Scholar] [CrossRef]
- Lieberman, M.M.; Clements, D.E.; Ogata, S.; Wang, G.; Corpuz, G.; Wong, T.; Martyak, T.; Gilson, L.; Coller, B.A.; Leung, J.; Watts, D.M.; Tesh, R.B.; Siirin, M.; Travassos da Rosa, A.; Humphreys, T.; Weeks-Levy, C. Preparation and immunogenic properties of a recombinant West Nile subunit vaccine. Vaccine 2007, 25, 414–423. [Google Scholar] [CrossRef]
- Lieberman, M.M.; Nerurkar, V.R.; Luo, H.; Cropp, B.; Carrion, R., Jr.; de la Garza, M.; Coller, B.A.; Clements, D.; Ogata, S.; Wong, T.; Martyak, T.; Weeks-Levy, C. Immunogenicity and protective efficacy of a recombinant subunit West Nile virus vaccine in rhesus monkeys. Clin. Vaccine Immunol. 2009, 16, 1332–1337. [Google Scholar] [CrossRef]
- Watts, D.M.; Tesh, R.B.; Siirin, M.; Rosa, A.T.; Newman, P.C.; Clements, D.E.; Ogata, S.; Coller, B.A.; Weeks-Levy, C.; Lieberman, M.M. Efficacy and durability of a recombinant subunit West Nile vaccine candidate in protecting hamsters from West Nile encephalitis. Vaccine 2007, 25, 2913–2918. [Google Scholar] [CrossRef]
- Jarvi, S.I.; Hu, D.; Misajon, K.; Coller, B.A.; Wong, T.; Lieberman, M.M. Vaccination of captive nene (Branta sandvicensis) against West Nile virus using a protein-based vaccine (WN-80E). J. Wildlife Dis. 2013, 49, 152–156. [Google Scholar] [CrossRef]
- database, N. c. t. clinicaltrials.gov
- Coller, B.-A.; Weeks-Levy, C.; Ogata, S. RECOMBINANT SUBUNIT WEST NILE VIRUS VACCINE FOR PROTECTION OF HUMAN SUBJECTS. Patent application number: 20120141520 2012. [Google Scholar]
- Wang, T.; Anderson, J.F.; Magnarelli, L.A.; Wong, S.J.; Koski, R.A.; Fikrig, E. Immunization of mice against West Nile virus with recombinant envelope protein. J. Immunol. 2001, 167, 5273–5277. [Google Scholar]
- Ledizet, M.; Kar, K.; Foellmer, H.G.; Wang, T.; Bushmich, S.L.; Anderson, J.F.; Fikrig, E.; Koski, R.A. A recombinant envelope protein vaccine against West Nile virus. Vaccine 2005, 23, 3915–3924. [Google Scholar] [CrossRef]
- Bonafe, N.; Rininger, J.A.; Chubet, R.G.; Foellmer, H.G.; Fader, S.; Anderson, J.F.; Bushmich, S.L.; Anthony, K.; Ledizet, M.; Fikrig, E.; Koski, R.A.; Kaplan, P. A recombinant West Nile virus envelope protein vaccine candidate produced in Spodoptera frugiperda expresSF+ cells. Vaccine 2009, 27, 213–222. [Google Scholar] [CrossRef]
- Zhu, B.; Ye, J.; Lu, P.; Jiang, R.; Yang, X.; Fu, Z.F.; Chen, H.; Cao, S. Induction of antigen-specific immune responses in mice by recombinant baculovirus expressing premembrane and envelope proteins of West Nile virus. Virol. J. 2012, 9, 132. [Google Scholar] [CrossRef]
- Demento, S.L.; Eisenbarth, S.C.; Foellmer, H.G.; Platt, C.; Caplan, M.J.; Mark Saltzman, W.; Mellman, I.; Ledizet, M.; Fikrig, E.; Flavell, R.A.; Fahmy, T.M. Inflammasome-activating nanoparticles as modular systems for optimizing vaccine efficacy. Vaccine 2009, 27, 3013–3021. [Google Scholar] [CrossRef]
- Demento, S.L.; Bonafe, N.; Cui, W.; Kaech, S.M.; Caplan, M.J.; Fikrig, E.; Ledizet, M.; Fahmy, T.M. TLR9-targeted biodegradable nanoparticles as immunization vectors protect against West Nile encephalitis. J. Immunol. 2010, 185, 2989–2997. [Google Scholar] [CrossRef]
- Chu, J.H.; Chiang, C.C.; Ng, M.L. Immunization of flavivirus West Nile recombinant envelope domain III protein induced specific immune response and protection against West Nile virus infection. J. Immunol. 2007, 178, 2699–2705. [Google Scholar]
- Gershoni-Yahalom, O.; Landes, S.; Kleiman-Shoval, S.; Ben-Nathan, D.; Kam, M.; Lachmi, B.E.; Khinich, Y.; Simanov, M.; Samina, I.; Eitan, A.; Cohen, I.R.; Rager-Zisman, B.; Porgador, A. Chimeric vaccine composed of viral peptide and mammalian heat-shock protein 60 peptide protects against West Nile virus challenge. Immunology 2010, 130, 527–535. [Google Scholar] [CrossRef]
- Spohn, G.; Jennings, G.T.; Martina, B.E.; Keller, I.; Beck, M.; Pumpens, P.; Osterhaus, A.D.; Bachmann, M.F. A VLP-based vaccine targeting domain III of the West Nile virus E protein protects from lethal infection in mice. Virol. J. 2010, 7, 146. [Google Scholar] [CrossRef]
- Qiao, M.; Ashok, M.; Bernard, K.A.; Palacios, G.; Zhou, Z.H.; Lipkin, W.I.; Liang, T.J. Induction of sterilizing immunity against West Nile Virus (WNV), by immunization with WNV-like particles produced in insect cells. J. Infect. Dis. 2004, 190, 2104–2108. [Google Scholar] [CrossRef]
- Chua, A.J.; Vituret, C.; Tan, M.L.; Gonzalez, G.; Boulanger, P.; Ng, M.L.; Hong, S.S. A novel platform for virus-like particle-display of flaviviral envelope domain III: induction of Dengue and West Nile virus neutralizing antibodies. Virol. J. 2013, 10, 129. [Google Scholar]
- Davis, B.S.; Chang, G.J.; Cropp, B.; Roehrig, J.T.; Martin, D.A.; Mitchell, C.J.; Bowen, R.; Bunning, M.L. West Nile virus recombinant DNA vaccine protects mouse and horse from virus challenge and expresses in vitro a noninfectious recombinant antigen that can be used in enzyme-linked immunosorbent assays. J. Virol. 2001, 75, 4040–4047. [Google Scholar] [CrossRef]
- Ishikawa, T.; Takasaki, T.; Kurane, I.; Nukuzuma, S.; Kondo, T.; Konishi, E. Co-immunization with West Nile DNA and inactivated vaccines provides synergistic increases in their immunogenicities in mice. Microbes. Infect. 2007, 9, 1089–1095. [Google Scholar] [CrossRef]
- Ramanathan, M.P.; Kutzler, M.A.; Kuo, Y.C.; Yan, J.; Liu, H.; Shah, V.; Bawa, A.; Selling, B.; Sardesai, N.Y.; Kim, J.J.; Weiner, D.B. Coimmunization with an optimized IL15 plasmid adjuvant enhances humoral immunity via stimulating B cells induced by genetically engineered DNA vaccines expressing consensus JEV and WNV E DIII. Vaccine 2009, 27, 4370–4380. [Google Scholar] [CrossRef]
- Anwar, A.; Chandrasekaran, A.; Ng, M.L.; Marques, E.; August, J.T. West Nile premembrane-envelope genetic vaccine encoded as a chimera containing the transmembrane and cytoplasmic domains of a lysosome-associated membrane protein: increased cellular concentration of the transgene product, targeting to the MHC II compartment, and enhanced neutralizing antibody response. Virology 2005, 332, 66–77. [Google Scholar] [CrossRef]
- Seregin, A.; Nistler, R.; Borisevich, V.; Yamshchikov, G.; Chaporgina, E.; Kwok, C.W.; Yamshchikov, V. Immunogenicity of West Nile virus infectious DNA and its noninfectious derivatives. Virology 2006, 356, 115–125. [Google Scholar] [CrossRef]
- Hall, R.A.; Nisbet, D.J.; Pham, K.B.; Pyke, A.T.; Smith, G.A.; Khromykh, A.A. DNA vaccine coding for the full-length infectious Kunjin virus RNA protects mice against the New York strain of West Nile virus. Proc. Natl. Acad. Sci. U.S.A. 2003, 100, 10460–10464. [Google Scholar]
- Chang, D.C.; Liu, W.J.; Anraku, I.; Clark, D.C.; Pollitt, C.C.; Suhrbier, A.; Hall, R.A.; Khromykh, A.A. Single-round infectious particles enhance immunogenicity of a DNA vaccine against West Nile virus. Nat. Biotechnol. 2008, 26, 571–577. [Google Scholar] [CrossRef]
- Widman, D.G.; Ishikawa, T.; Winkelmann, E.R.; Infante, E.; Bourne, N.; Mason, P.W. RepliVAX WN, a single-cycle flavivirus vaccine to prevent West Nile disease, elicits durable protective immunity in hamsters. Vaccine 2009, 27, 5550–5553. [Google Scholar] [CrossRef]
- Widman, D.G.; Ishikawa, T.; Giavedoni, L.D.; Hodara, V.L.; Garza Mde, L.; Montalbo, J.A.; Travassos Da Rosa, A.P.; Tesh, R.B.; Patterson, J.L.; Carrion, R., Jr.; Bourne, N.; Mason, P. W. Evaluation of RepliVAX WN, a single-cycle flavivirus vaccine, in a non-human primate model of West Nile virus infection. Am. J. Trop. Med. Hyg. 2010, 82, 1160–1167. [Google Scholar] [CrossRef]
- Suzuki, R.; Fayzulin, R.; Frolov, I.; Mason, P.W. Identification of mutated cyclization sequences that permit efficient replication of West Nile virus genomes: use in safer propagation of a novel vaccine candidate. J. Virol. 2008, 82, 6942–6951. [Google Scholar] [CrossRef]
- Widman, D.G.; Frolov, I.; Mason, P.W. Third-generation flavivirus vaccines based on single-cycle, encapsidation-defective viruses. Adv. Virus Res. 2008, 72, 77–126. [Google Scholar] [CrossRef]
- Ishikawa, T.; Widman, D.G.; Bourne, N.; Konishi, E.; Mason, P.W. Construction and evaluation of a chimeric pseudoinfectious virus vaccine to prevent Japanese encephalitis. Vaccine 2008, 26, 2772–2781. [Google Scholar] [CrossRef]
- Widman, D.G.; Ishikawa, T.; Fayzulin, R.; Bourne, N.; Mason, P.W. Construction and characterization of a second-generation pseudoinfectious West Nile virus vaccine propagated using a new cultivation system. Vaccine 2008, 26, 2762–2771. [Google Scholar] [CrossRef]
- Schepp-Berglind, J.; Luo, M.; Wang, D.; Wicker, J.A.; Raja, N.U.; Hoel, B.D.; Holman, D.H.; Barrett, A.D.; Dong, J.Y. Complex adenovirus-mediated expression of West Nile virus C, PreM, E, and NS1 proteins induces both humoral and cellular immune responses. Clin. Vaccine Immunol. 2007, 14, 1117–1126. [Google Scholar] [CrossRef]
- Lim, C.K.; Takasaki, T.; Kotaki, A.; Kurane, I. Vero cell-derived inactivated West Nile (WN) vaccine induces protective immunity against lethal WN virus infection in mice and shows a facilitated neutralizing antibody response in mice previously immunized with Japanese encephalitis vaccine. Virology 2008, 374, 60–70. [Google Scholar] [CrossRef]
- Samina, I.; Havenga, M.; Koudstaal, W.; Khinich, Y.; Koldijk, M.; Malkinson, M.; Simanov, M.; Perl, S.; Gijsbers, L.; Weverling, G.J.; Uytdehaag, F.; Goudsmit, J. Safety and efficacy in geese of a PER.C6-based inactivated West Nile virus vaccine. Vaccine 2007, 25, 8338–8345. [Google Scholar] [CrossRef]
- Scherret, J.H.; Mackenzie, J.S.; Hall, R.A.; Deubel, V.; Gould, E.A. Phylogeny and molecular epidemiology of West Nile and Kunjin viruses. Curr. Top. Microbiol. Immunol. 2002, 267, 373–390. [Google Scholar] [CrossRef]
- Scherret, J.H.; Poidinger, M.; Mackenzie, J.S.; Broom, A.K.; Deubel, V.; Lipkin, W.I.; Briese, T.; Gould, E.A.; Hall, R.A. The relationships between West Nile and Kunjin viruses. Emerg. Infect. Dis. 2001, 7, 697–705. [Google Scholar]
- Gray, T.J.; Burrow, J.N.; Markey, P.G.; Whelan, P.I.; Jackson, J.; Smith, D.W.; Currie, B.J. West nile virus (Kunjin subtype) disease in the northern territory of Australia--a case of encephalitis and review of all reported cases. Am. J. Trop. Med. Hyg. 2011, 85, 952–956. [Google Scholar] [CrossRef]
- Pinto, A.K.; Richner, J.M.; Poore, E.A.; Patil, P.P.; Amanna, I.J.; Slifka, M.K.; Diamond, M.S. A Hydrogen Peroxide-Inactivated Virus Vaccine Elicits Humoral and Cellular Immunity and Protects against Lethal West Nile Virus Infection in Aged Mice. J. Virol. 2013, 87, 1926–1936. [Google Scholar] [CrossRef]
- Orlinger, K.K.; Holzer, G.W.; Schwaiger, J.; Mayrhofer, J.; Schmid, K.; Kistner, O.; Noel Barrett, P.; Falkner, F.G. An inactivated West Nile Virus vaccine derived from a chemically synthesized cDNA system. Vaccine 2010, 28, 3318–3324. [Google Scholar] [CrossRef]
- Rosas, C.T.; Tischer, B.K.; Perkins, G.A.; Wagner, B.; Goodman, L.B.; Osterrieder, N. Live-attenuated recombinant equine herpesvirus type 1 (EHV-1) induces a neutralizing antibody response against West Nile virus (WNV). Virus Res. 2007, 125, 69–78. [Google Scholar] [CrossRef]
- Iyer, A.V.; Pahar, B.; Boudreaux, M.J.; Wakamatsu, N.; Roy, A.F.; Chouljenko, V.N.; Baghian, A.; Apetrei, C.; Marx, P.A.; Kousoulas, K.G. Recombinant vesicular stomatitis virus-based west Nile vaccine elicits strong humoral and cellular immune responses and protects mice against lethal challenge with the virulent west Nile virus strain LSU-AR01. Vaccine 2009, 27, 893–903. [Google Scholar] [CrossRef]
- Iglesias, M.C.; Frenkiel, M.P.; Mollier, K.; Souque, P.; Despres, P.; Charneau, P. A single immunization with a minute dose of a lentiviral vector-based vaccine is highly effective at eliciting protective humoral immunity against West Nile virus. J. Gene Med. 2006, 8, 265–274. [Google Scholar] [CrossRef]
- Coutant, F.; Frenkiel, M.P.; Despres, P.; Charneau, P. Protective antiviral immunity conferred by a nonintegrative lentiviral vector-based vaccine. PLoS ONE 2008, 3, e3973. [Google Scholar]
- Huang, C.Y.; Silengo, S.J.; Whiteman, M.C.; Kinney, R.M. Chimeric dengue 2 PDK-53/West Nile NY99 viruses retain the phenotypic attenuation markers of the candidate PDK-53 vaccine virus and protect mice against lethal challenge with West Nile virus. J. Virol. 2005, 79, 7300–7310. [Google Scholar] [CrossRef]
- Yamshchikov, G.; Borisevich, V.; Seregin, A.; Chaporgina, E.; Mishina, M.; Mishin, V.; Kwok, C.W.; Yamshchikov, V. An attenuated West Nile prototype virus is highly immunogenic and protects against the deadly NY99 strain: a candidate for live WN vaccine development. Virology 2004, 330, 304–312. [Google Scholar] [CrossRef]
- Whiteman, M.C.; Li, L.; Wicker, J.A.; Kinney, R.M.; Huang, C.; Beasley, D.W.; Chung, K.M.; Diamond, M.S.; Solomon, T.; Barrett, A.D. Development and characterization of non-glycosylated E and NS1 mutant viruses as a potential candidate vaccine for West Nile virus. Vaccine 2010, 28, 1075–1083. [Google Scholar] [CrossRef]
- Yu, L.; Robert Putnak, J.; Pletnev, A.G.; Markoff, L. Attenuated West Nile viruses bearing 3'SL and envelope gene substitution mutations. Vaccine 2008, 26, 5981–5988. [Google Scholar] [CrossRef]
- Wicker, J.A.; Whiteman, M.C.; Beasley, D.W.; Davis, C.T.; Zhang, S.; Schneider, B.S.; Higgs, S.; Kinney, R.M.; Barrett, A.D. A single amino acid substitution in the central portion of the West Nile virus NS4B protein confers a highly attenuated phenotype in mice. Virology 2006, 349, 245–253. [Google Scholar] [CrossRef]
- Liu, W.J.; Wang, X.J.; Clark, D.C.; Lobigs, M.; Hall, R.A.; Khromykh, A.A. A single amino acid substitution in the West Nile virus nonstructural protein NS2A disables its ability to inhibit alpha/beta interferon induction and attenuates virus virulence in mice. J. Virol. 2006, 80, 2396–2404. [Google Scholar] [CrossRef]
- Combredet, C.; Labrousse, V.; Mollet, L.; Lorin, C.; Delebecque, F.; Hurtrel, B.; McClure, H.; Feinberg, M.B.; Brahic, M.; Tangy, F. A molecularly cloned Schwarz strain of measles virus vaccine induces strong immune responses in macaques and transgenic mice. J. Virol. 2003, 77, 11546–11554. [Google Scholar] [CrossRef]
- Tangy, F.; Lucas, M.; Navarro-Sanchez, E.; Frenkiel, M.; Combredet, C.; Despres, P. Chimeric poly peptides and their therapeutic use against a flaviviridae infection. CA2549086; Filed 20-06-2006 and issued 20-12-2006,
- Lorin, C.; Mollet, L.; Delebecque, F.; Combredet, C.; Charneau, P.; Hurtrel, B.; Brahic, M.; Tangy, F. A Single Injection of Recombinant Measles Vaccines Expressing HIV-1 Clade B Envelope Glycoproteins Induces Neutralizing Antibodies and Cellular Immune Responses to HIV. J. Virol. 2004, 78, 146–157. [Google Scholar] [CrossRef]
- Desprès, P.; Combredet, C.; Frenkiel, M.; Lorin, C.; Brahic, M.; Tangy, F. Live measles vaccine expressing the secreted form of the West Nile virus envelope glycoprotein protects against West Nile virus encephalitis. J. Infect. Dis. 2005, 191, 207–214. [Google Scholar] [CrossRef]
- Brandler, S.; Lucas-Hourani, M.; Moris, A.; Frenkiel, M.P.; Combredet, C.; Fevrier, M.; Bedouelle, H.; Schwartz, O.; Despres, P.; Tangy, F. Pediatric Measles Vaccine Expressing a Dengue Antigen Induces Durable Serotype-specific Neutralizing Antibodies to Dengue Virus. PLoS neglected tropical diseases 2007, 1, e96. [Google Scholar] [CrossRef]
- Brandler, S.; Marianneau, P.; Loth, P.; Lacote, S.; Combredet, C.; Frenkiel, M.P.; Despres, P.; Contamin, H.; Tangy, F. Measles vaccine expressing the secreted form of west nile virus envelope glycoprotein induces protective immunity in squirrel monkeys, a new model of west nile virus infection. J. Infect. Dis. 2012, 206, 212–219. [Google Scholar] [CrossRef]
- Brandler, S.; Ruffie, C.; Combredet, C.; Brault, J.B.; Najburg, V.; Prevost, M.C.; Habel, A.; Tauber, E.; Despres, P.; Tangy, F. A recombinant measles vaccine expressing chikungunya virus-like particles is strongly immunogenic and protects mice from lethal challenge with chikungunya virus. Vaccine 2013.
- Brandler, S.; Ruffie, C.; Najburg, V.; Frenkiel, M.P.; Bedouelle, H.; Despres, P.; Tangy, F. Pediatric measles vaccine expressing a dengue tetravalent antigen elicits neutralizing antibodies against all four dengue viruses. Vaccine 2010, 28, 6730–6739. [Google Scholar] [CrossRef]
- Stebbings, R.; Fevrier, M.; Li, B.; Lorin, C.; Koutsoukos, M.; Mee, E.; Rose, N.; Hall, J.; Page, M.; Almond, N.; Voss, G.; Tangy, F. Immunogenicity of a recombinant measles-HIV-1 clade B candidate vaccine. PLoS ONE 2012, 7, e50397. [Google Scholar] [CrossRef]
- Lorin, C.; Segal, L.; Mols, J.; Morelle, D.; Bourguignon, P.; Rovira, O.; Mettens, P.; Silvano, J.; Dumey, N.; Le Goff, F.; Koutsoukos, M.; Voss, G.; Tangy, F. Toxicology, biodistribution and shedding profile of a recombinant measles vaccine vector expressing HIV-1 antigens, in cynomolgus macaques. Naunyn-Schmiedeberg's Arch. Pharmacol. 2012, 385, 1211–1225. [Google Scholar] [CrossRef]
- Lucas, M.; Mashimo, T.; Frenkiel, M.-P.; Simon-Chazottes, D.; Montagutelli, X.; Ceccaldi, P.-E.; Guénet, J.-L.; Desprès, P. Infection of mouse neurons by West Nile virus is modulated by the interferon-inducible 2’-5’ oligoadenylate synthetase 1b protein. Immun. Cell Biol. 2003, 81, 230–236. [Google Scholar] [CrossRef]
- Ratterree, M.S.; da Rosa, A.P.; Bohm, R.P., Jr.; Cogswell, F.B.; Phillippi, K.M.; Caillouet, K.; Schwanberger, S.; Shope, R.E.; Tesh, R.B. West Nile virus infection in nonhuman primate breeding colony, concurrent with human epidemic, southern Louisiana. Emerg. Infect. Dis. 2003, 9, 1388–1394. [Google Scholar] [CrossRef]
- Arroyo, J.; Miller, C.; Catalan, J.; Myers, G.A.; Ratterree, M.S.; Trent, D.W.; Monath, T.P. ChimeriVax-West Nile virus live-attenuated vaccine: preclinical evaluation of safety, immunogenicity, and efficacy. J. Virol. 2004, 78, 12497–12507. [Google Scholar] [CrossRef]
- Ratterree, M.; Gutierrez, R.; Travassos da Rosa, A.; Dille, B.; Beasley, D.; Bohm, R.; Desai, S.; Didier, P.; Bikenmeyer, L.; Dawson, G.; Leary, T.; Schochetman, G.; Phillippi-Falkenstein, K.; Arroyo, J.; Barrett, A.; Tesh, R. Experimental infection of rhesus macaques with West Nile virus: level and duration of viremia and kinetics of the antibody response after infection. J. Infect. Dis. 2004, 189, 669–676. [Google Scholar] [CrossRef]
- Wertheimer, A.M.; Uhrlaub, J.L.; Hirsch, A.; Medigeshi, G.; Sprague, J.; Legasse, A.; Wilk, J.; Wiley, C.A.; Didier, P.; Tesh, R.B.; Murray, K.O.; Axthelm, M.K.; Wong, S.W.; Nikolich-Zugich, J. Immune response to the West Nile virus in aged non-human primates. PLoS ONE 5, e15514.
- Pogodina, V.; Frolova, M.; Malenko, G.; Fokina, G.; Koreshkova, G.; Kiseleva, L.; Bochkova, N.; Ralph, N.; Related Articles, L. A. Study on West Nile virus persistence in monkeys. Arch. Virol. 1983, 75, 71–86. [Google Scholar] [CrossRef]
- Wolf, R.F.; Papin, J.F.; Hines-Boykin, R.; Chavez-Suarez, M.; White, G.L.; Sakalian, M.; Dittmer, D.P. Baboon model for West Nile virus infection and vaccine evaluation. Virology 2006, 355, 44–51. [Google Scholar] [CrossRef]
- Nathanson, N.; Davis, M.; Thind, I.S.; Price, W.H. Histological studies of the monkey neurovirulence of group B arboviruses. II. Selection of indicator centers. Am. J. Epidemiol. 1966, 84, 524–540. [Google Scholar]
- Manuelidis, E.E. Neuropathology of experimental West Nile virus infection in monkeys. J. Neuropath. Exp. Neur. 1956, 15, 448–460. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Brandler, S.; Tangy, F. Vaccines in Development against West Nile Virus. Viruses 2013, 5, 2384-2409. https://doi.org/10.3390/v5102384
Brandler S, Tangy F. Vaccines in Development against West Nile Virus. Viruses. 2013; 5(10):2384-2409. https://doi.org/10.3390/v5102384
Chicago/Turabian StyleBrandler, Samantha, and Frederic Tangy. 2013. "Vaccines in Development against West Nile Virus" Viruses 5, no. 10: 2384-2409. https://doi.org/10.3390/v5102384
APA StyleBrandler, S., & Tangy, F. (2013). Vaccines in Development against West Nile Virus. Viruses, 5(10), 2384-2409. https://doi.org/10.3390/v5102384




