The Curious Case of Arenavirus Entry, and Its Inhibition
Abstract
:1. Introduction

2. The Unusual GPC Envelope Glycoprotein
4. GPC Is a Class I Virus Fusion Protein
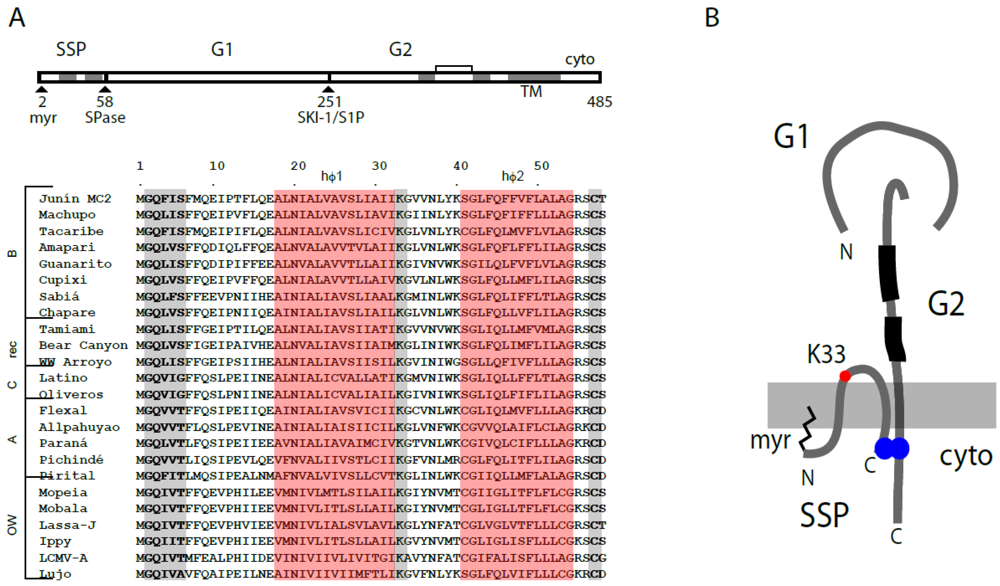
5. The Unusual SSP Signal Peptide
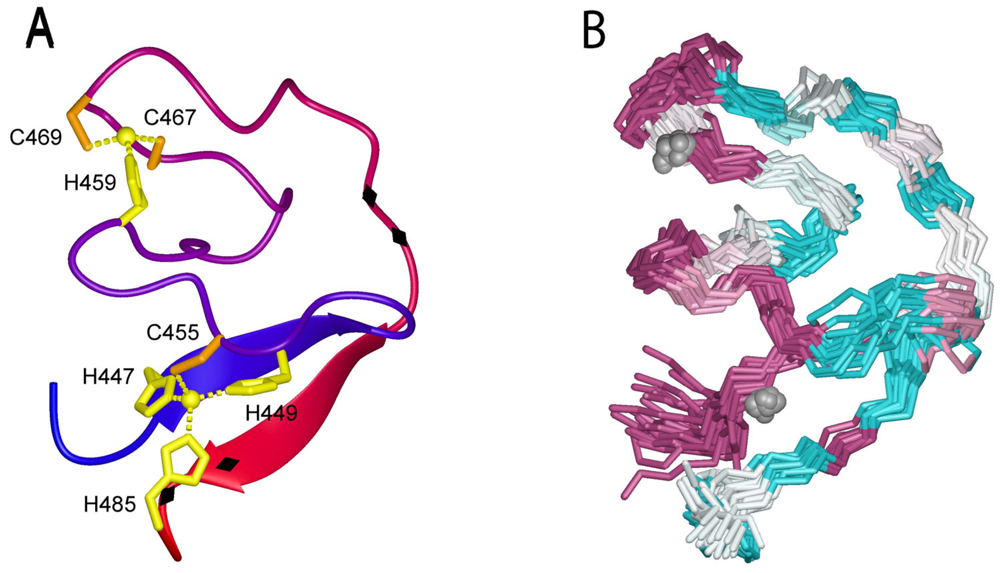
6. The Role of SSP in GPC-Mediated Membrane Fusion
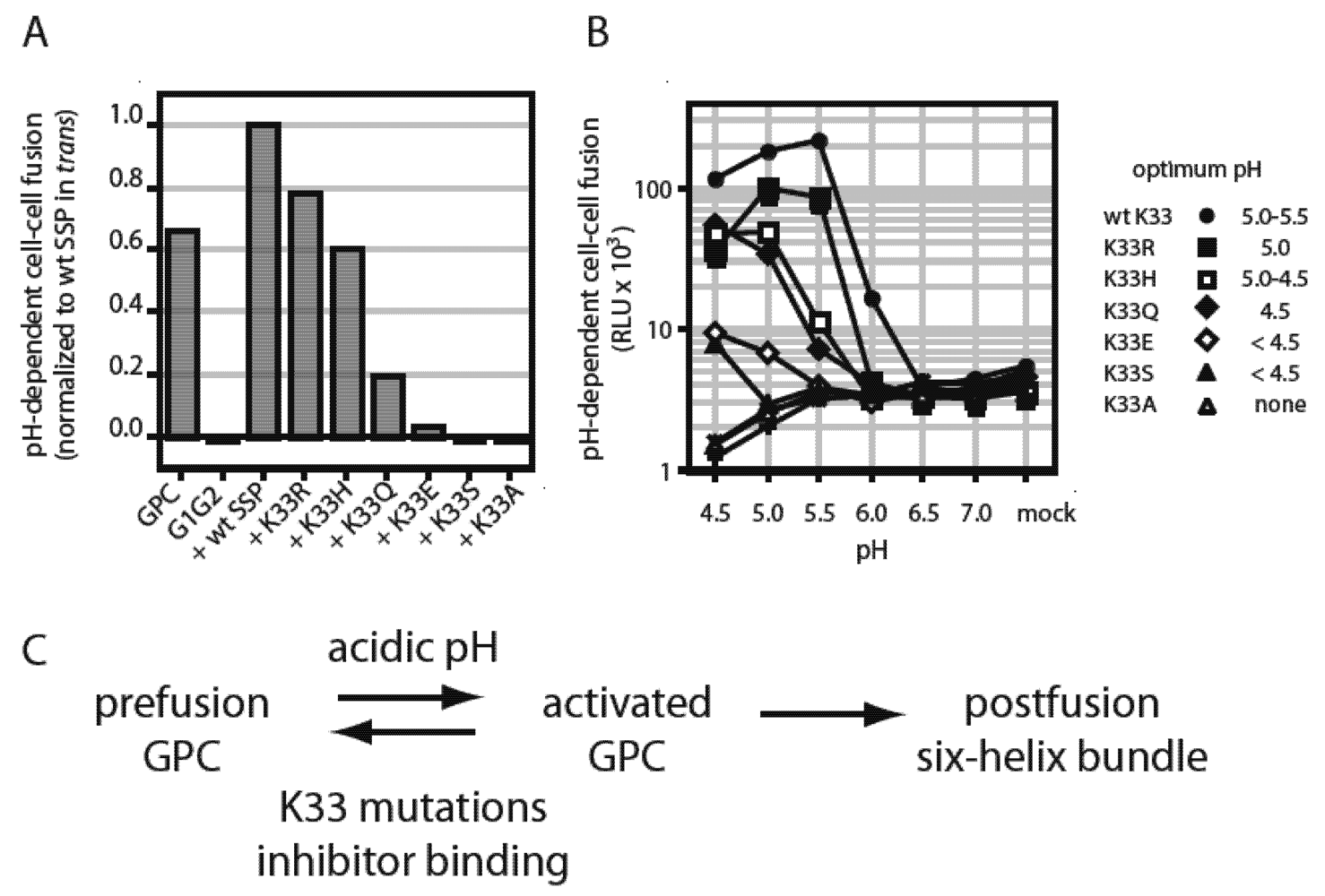
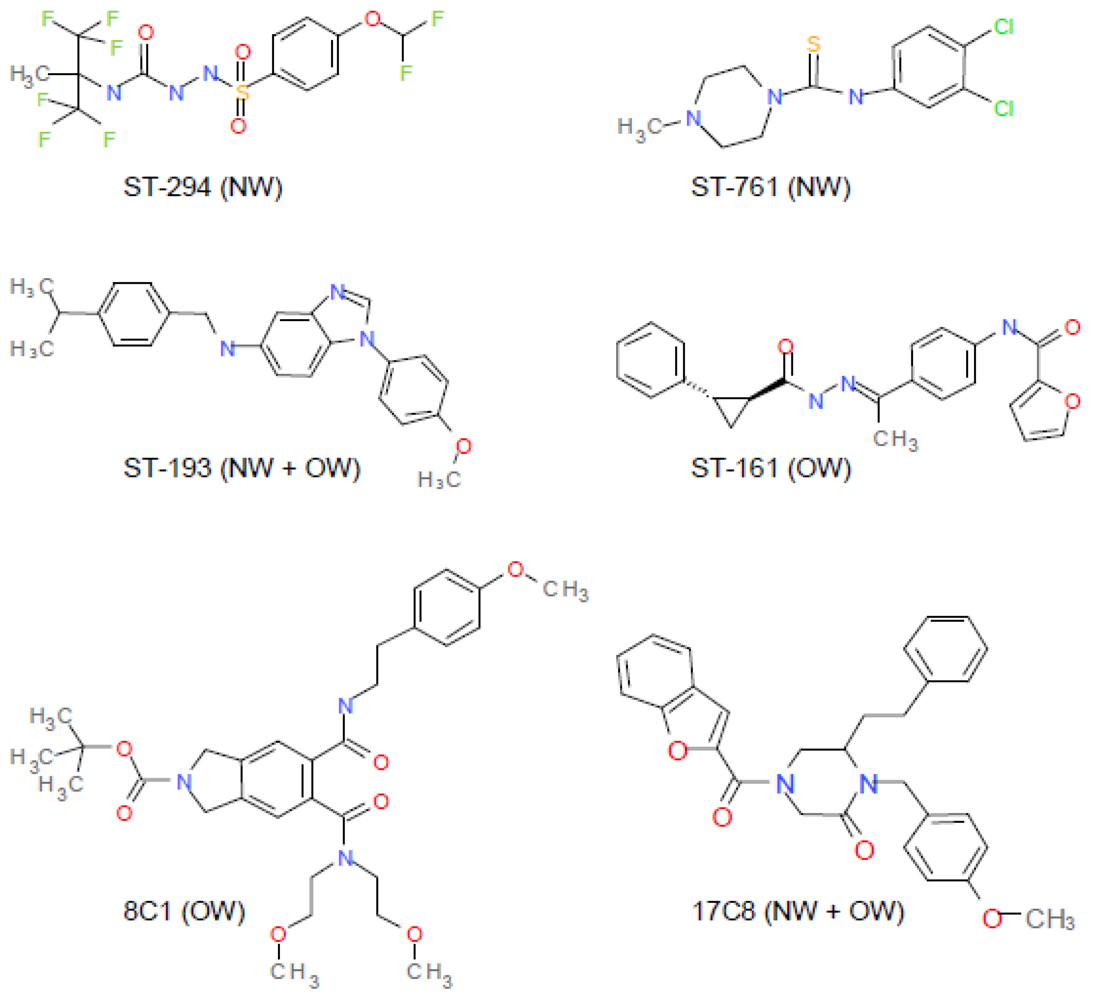
7. Small-Molecule Inhibitors of pH-Induced Activation
8. Future Directions
Acknowledgments
References and Notes
- Charrel, R.N.; de Lamballerie, X. Zoonotic aspects of arenavirus infections. Vet. Microbiol. 2010, 140, 213–220. [Google Scholar]
- Palacios, G.; Savji, N.; Hui, J.; Travassos da Rosa, A.; Popov, V.; Briese, T.; Tesh, R.; Lipkin, W.I. Genomic and phylogenetic characterization of Merino Walk virus, a novel arenavirus isolated in South Africa. J. Gen. Virol. 2010, 91, 1315–1324. [Google Scholar]
- Coulibaly-N'Golo, D.; Allali, B.; Kouassi, S.K.; Fichet-Calvet, E.; Becker-Ziaja, B.; Rieger, T.; Olschläger, S.; Dosso, H.; Denys, C.; Ter Meulen, J.; et al. Novel arenavirus sequences in Hylomyscus sp. and Mus (Nannomys) setulosus from Côte d'Ivoire: Implications for evolution of arenaviruses in Africa. PLoS One 2011, 6, e20893. [Google Scholar] [PubMed]
- Clegg, J.C.S. Molecular phylogeny of the arenaviruses. Curr. Top. Microbiol. Immunol. 2002, 262, 1–24. [Google Scholar]
- Salazar-Bravo, J.; Ruedas, L.A.; Yates, T.L. Mammalian reservoirs of arenaviruses. Curr. Top. Microbiol. Immunol. 2002, 262, 25–63. [Google Scholar]
- Gonzalez, J.P.; Emonet, S.; de Lamballerie, X.; Charrel, R. Arenaviruses. Curr. Top. Microbiol. Immunol. 2007, 315, 253–288. [Google Scholar]
- de Bellocq, J.G.; Borremans, B.; Katakweba, A.; Makundi, R.; Baird, S.J.; Becker-Ziaja, B.; Günther, S.; Leirs, H. Sympatric occurrence of 3 arenaviruses, Tanzania. Emerg. Infect. Dis. 2010, 16, 692–695. [Google Scholar]
- Haas, W.H.; Breuer, T.; Pfaff, G.; Schmitz, H.; Köhler, P.; Asper, M.; Emmerich, P.; Drosten, C.; Gölnitz, U.; Fleischer, K.; et al. Imported Lassa fever in Germany: Surveillance and management of contact persons. Clin. Infect. Dis. 2003, 36, 1254–1258. [Google Scholar] [PubMed]
- Amorosa, V.; MacNeil, A.; McConnell, R.; Patel, A.; Dillon, K.E.; Hamilton, K.; Erickson, B.R.; Campbell, S.; Knust, B.; Cannon, D.; et al. Imported Lassa fever, Pennsylvania, USA, 2010. Emerg. Infect. Dis. 2010, 16, 1598–1600. [Google Scholar] [PubMed]
- Fischer, S.A.; Graham, M.B.; Kuehnert, M.J.; Kotton, C.N.; Srinivasan, A.; Marty, F.M.; Comer, J.A.; Guarner, J.; Paddock, C.D.; DeMeo, D.L.; et al. Transmission of lymphocytic choriomeningitis virus by organ transplantation. N. Engl. J. Med. 2006, 354, 2235–2249. [Google Scholar]
- Palacios, G.; Druce, J.; Du, L.; Tran, T.; Birch, C.; Briese, T.; Conlan, S.; Quan, P.L.; Hui, J.; Marshall, J.; et al. A new arenavirus in a cluster of fatal transplant-associated diseases. N. Engl. J. Med. 2008, 358, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Peters, C.J. Human infection with arenaviruses in the Americas. Curr. Top. Microbiol. Immunol. 2002, 262, 65–74. [Google Scholar]
- McCormick, J.B.; Fisher-Hoch, S.P. Lassa fever. Curr. Top. Microbiol. Immunol. 2002, 262, 75–109. [Google Scholar]
- Gunther, S.; Lenz, O. Lassa virus. Crit. Rev. Clin. Lab. Sci. 2004, 41, 339–390. [Google Scholar]
- Bolken, T.C.; Laquerre, S.; Zhang, Y.; Bailey, T.R.; Pevear, D.C.; Kickner, S.S.; Sperzel, L.E.; Jones, K.F.; Warren, T.K.; Amanda Lund, S.; et al. Identification and characterization of potent small molecule inhibitor of hemorrhagic fever New World arenaviruses. Antivir. Res. 2006, 69, 86–89. [Google Scholar]
- Larson, R.A.; Dai, D.; Hosack, V.T.; Tan, Y.; Bolken, T.C.; Hruby, D.E.; Amberg, S.M. Identification of a broad-spectrum arenavirus entry inhibitor. J. Virol. 2008, 82, 10768–10775. [Google Scholar]
- Lee, A.M.; Rojek, J.M.; Spiropoulou, C.F.; Gundersen, A.T.; Jin, W.; Shaginian, A.; York, J.; Nunberg, J.H.; Boger, D.L.; Oldstone, M.B.A.; et al. Unique small molecule entry inhibitors of hemorrhagic fever arenaviruses. J. Biol. Chem. 2008, 283, 18734–18742. [Google Scholar] [PubMed]
- Whitby, L.R.; Lee, A.M.; Kunz, S.; Oldstone, M.B.; Boger, D.L. Characterization of lassa virus cell entry inhibitors: Determination of the active enantiomer by asymmetric synthesis. Bioorg. Med. Chem. Lett. 2009, 19, 3771–3774. [Google Scholar]
- Cashman, K.A.; Smith, M.A.; Twenhafel, N.A.; Larson, R.A.; Jones, K.F.; Allen, R.D., 3rd; Dai, D.; Chinsangaram, J.; Bolken, T.C.; Hruby, D.E.; et al. Evaluation of Lassa antiviral compound ST-193 in a guinea pig model. Antivir. Res. 2011, 90, 70–79. [Google Scholar]
- Enria, D.A.; Briggiler, A.M.; Sánchez, Z. Treatment of Argentine hemorrhagic fever. Antivir. Res. 2008, 78, 132–139. [Google Scholar]
- Maiztegui, J.I.; McKee, K.T., Jr.; Barrera Oro, J.G.; Harrison, L.H.; Gibbs, P.H.; Feuillade, M.R.; Enria, D.A.; Briggiler, A.M.; Levis, S.C.; Ambrosio, A.M.; et al. Protective efficacy of a live attenuated vaccine against Argentine hemorrhagic fever. AHF Study Group. J. Infect. Dis. 1998, 177, 277–283. [Google Scholar] [PubMed]
- Enria, D.A.; Barrera Oro, J.G. Junin virus vaccines. Curr. Top. Microbiol. Immunol. 2002, 263, 239–261. [Google Scholar]
- Albariño, C.G.; Bird, B.H.; Chakrabarti, A.K.; Dodd, K.A.; Flint, M.; Bergeron, E.; White, D.M.; Nichol, S.T. The major determinant of attenuation in mice of the candid1 vaccine for argentine hemorrhagic Fever is located in the g2 glycoprotein transmembrane domain. J. Virol. 2011, 85, 10404–10408. [Google Scholar]
- Droniou-Bonzom, M.E.; Reignier, T.; Oldenburg, J.E.; Cox, A.U.; Exline, C.M.; Rathbun, J.Y.; Cannon, P.M. Substitutions in the glycoprotein (GP) of the Candid#1 vaccine strain of Junin virus increase dependence on human transferrin receptor 1 for entry and destabilize the metastable conformation of GP. J. Virol. 2011. [Google Scholar]
- Geisbert, T.W.; Jones, S.; Fritz, E.A.; Shurtleff, A.C.; Geisbert, J.B.; Liebscher, R.; Grolla, A.; Stroher, U.; Fernando, L.; Daddario, K.M.; et al. Development of a new vaccine for the prevention of Lassa fever. PLoS Med. 2005, 2, e183. [Google Scholar] [PubMed]
- Carrion, R., Jr.; Patterson, J.L.; Johnson, C.; Gonzales, M.; Moreira, C.R.; Ticer, A.; Brasky, K.; Hubbard, G.B.; Moshkoff, D.; Zapata, J.; et al. A ML29 reassortant virus protects guinea pigs against a distantly related Nigerian strain of Lassa virus and can provide sterilizing immunity. Vaccine 2007, 25, 4093–4102. [Google Scholar]
- Cosset, F.L.; Marianneau, P.; Verney, G.; Gallais, F.; Tordo, N.; Pécheur, E.I.; ter Meulen, J.; Deubel, V.; Bartosch, B. Characterization of Lassa virus cell entry and neutralization with Lassa virus pseudoparticles. J. Virol. 2009, 83, 3228–3237. [Google Scholar]
- Emonet, S.E.; Urata, S.; de la Torre, J.C. Arenavirus reverse genetics: new approaches for the investigation of arenavirus biology and development of antiviral strategies. Virology 2011, 411, 416–425. [Google Scholar]
- NIAID. NIAID Category A, B, and C Priority Pathogens. Available online: http://www.niaid.nih.gov/topics/BiodefenseRelated/Biodefense/research/Pages/CatA.aspx (accessed on 31 October 2011).
- Health and Human Services. Implementation plan for chemical, biological, radiological and nuclear threats. Available online: http://www.hhs.gov/aspr/barda/phemce/enterprise/strategy/index.html (accessed on 31 October 2011).
- Buchmeier, M.J.; de la Torre, J.-C.; Peters, C.J. Arenaviridae: The viruses and their replication. In Fields Virology, 5th; Knipe, D.M., Howley, P.M., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007; Volume 2, pp. 1791–1828. [Google Scholar]
- Briknarova, K.; Thomas, C.J.; York, J.; Nunberg, J.H. Structure of a zinc-binding domain in the Junin virus envelope glycoprotein. J. Biol. Chem. 2011, 286, 1528–1536. [Google Scholar]
- Radoshitzky, S.R.; Abraham, J.; Spiropoulou, C.F.; Kuhn, J.H.; Nguyen, D.; Li, W.; Nagel, J.; Schmidt, P.J.; Nunberg, J.H.; Andrews, N.C.; et al. Transferrin receptor 1 is a cellular receptor for New World haemorrhagic fever arenaviruses. Nature 2007, 446, 92–96. [Google Scholar] [PubMed]
- Flanagan, M.L.; Oldenburg, J.; Reignier, T.; Holt, N.; Hamilton, G.A.; Martin, V.K.; Cannon, P.M. New world clade B arenaviruses can use transferrin receptor 1 (TfR1)-dependent and -independent entry pathways, and glycoproteins from human pathogenic strains are associated with the use of TfR1. J. Virol. 2008, 82, 938–948. [Google Scholar]
- Radoshitzky, S.R.; Kuhn, J.H.; Spiropoulou, C.F.; Albariño, C.G.; Nguyen, D.P.; Salazar-Bravo, J.; Dorfman, T.; Lee, A.S.; Wang, E.; Ross, S.R.; et al. Receptor determinants of zoonotic transmission of New World hemorrhagic fever arenaviruses. Proc. Natl. Acad. Sci. U. S. A. 2008, 105, 2664–2669. [Google Scholar] [PubMed]
- Abraham, J.; Kwong, J.A.; Albariño, C.G.; Lu, J.G.; Radoshitzky, S.R.; Salazar-Bravo, J.; Farzan, M.; Spiropoulou, C.F.; Choe, H. Host-species transferrin receptor 1 orthologs are cellular receptors for nonpathogenic new world clade B arenaviruses. PLoS Pathog. 2009, 5, e1000358. [Google Scholar]
- Martin, V.K.; Droniou-Bonzom, M.E.; Reignier, T.; Oldenburg, J.E.; Cox, A.U.; Cannon, P.M. Investigation of clade B New World arenavirus tropism by using chimeric GP1 proteins. J. Virol. 2010, 84, 1176–1182. [Google Scholar]
- Radoshitzky, S.R.; Longobardi, L.E.; Kuhn, J.H.; Retterer, C.; Dong, L.; Clester, J.C.; Kota, K.; Carra, J.; Bavari, S. Machupo virus glycoprotein determinants for human transferrin receptor 1 binding and cell entry. PLoS One 2011, 6, e21398. [Google Scholar]
- Abraham, J.; Corbett, K.D.; Farzan, M.; Choe, H.; Harrison, S.C. Structural basis for receptor recognition by New World hemorrhagic fever arenaviruses. Nat. Struct. Mol. Biol. 2010, 17, 438–444. [Google Scholar]
- Cheng, Y.; Zak, O.; Aisen, P.; Harrison, S.C.; Walz, T. Structure of the human transferrin receptor-transferrin complex. Cell 2004, 116, 565–576. [Google Scholar]
- Bowden, T.A.; Crispin, M.; Graham, S.C.; Harvey, D.J.; Grimes, J.M.; Jones, E.Y.; Stuart, D.I. Unusual molecular architecture of the machupo virus attachment glycoprotein. J. Virol. 2009, 83, 8259–8265. [Google Scholar]
- Charrel, R.N.; de Lamballerie, X.; Emonet, S. Phylogeny of the genus Arenavirus. Curr. Opin. Microbiol. 2008, 11, 362–368. [Google Scholar]
- Di Simone, C.; Zandonatti, M.A.; Buchmeier, M.J. Acidic pH triggers LCMV membrane fusion activity and conformational change in the glycoprotein spike. Virology 1994, 198, 455–465. [Google Scholar]
- Borrow, P.; Oldstone, M.B.A. Mechanism of lymphocytic choriomeningitis virus entry into cells. Virology 1994, 198, 1–9. [Google Scholar]
- Di Simone, C.; Buchmeier, M.J. Kinetics and pH dependence of acid-induced structural changes in the lymphocytic choriomeningitis virus glycoprotein complex. Virology 1995, 209, 3–9. [Google Scholar]
- Choe, H.; Jemielity, S.; Abraham, J.; Radoshitzky, S.R.; Farzan, M. Transferrin receptor 1 in the zoonosis and pathogenesis of New World hemorrhagic fever arenaviruses. Curr. Opin. Microbiol. 2011, 14, 476–482. [Google Scholar]
- Cao, W.; Henry, M.D.; Borrow, P.; Yamada, H.; Elder, J.H.; Ravkov, E.V.; Nichol, S.T.; Compans, R.W.; Campbell, K.P.; Oldstone, M.B.A. Identification of alpha-dystroglycan as a receptor for lymphocytic choriomeningitis virus and Lassa fever virus. Science 1998, 282, 2079–2081. [Google Scholar]
- Spiropoulou, C.F.; Kunz, S.; Rollin, P.E.; Campbell, K.P.; Oldstone, M.B.A. New World arenavirus clade C, but not clade A and B viruses, utilizes alpha-dystroglycan as its major receptor. J. Virol. 2002, 76, 5140–5146. [Google Scholar] [PubMed]
- Reignier, T.; Oldenburg, J.; Noble, B.; Lamb, E.; Romanowski, V.; Buchmeier, M.J.; Cannon, P.M. Receptor use by pathogenic arenaviruses. Virology 2006, 353, 111–120. [Google Scholar]
- Quirin, K.; Eschli, B.; Scheu, I.; Poort, L.; Kartenbeck, J.; Helenius, A. Lymphocytic choriomeningitis virus uses a novel endocytic pathway for infectious entry via late endosomes. Virology 2008, 378, 21–33. [Google Scholar]
- Pasqual, G.; Rojek, J.M.; Masin, M.; Chatton, J.Y.; Kunz, S. Old World Arenaviruses Enter the Host Cell via the Multivesicular Body and Depend on the Endosomal Sorting Complex Required for Transport. PLoS Pathog. 2011, 7, e1002232. [Google Scholar]
- Klewitz, C.; Klenk, H.D.; ter Meulen, J. Amino acids from both N-terminal hydrophobic regions of the Lassa virus envelope glycoprotein GP-2 are critical for pH-dependent membrane fusion and infectivity. J. Gen. Virol. 2007, 88, 2320–2328. [Google Scholar]
- White, J.M.; Delos, S.E.; Brecher, M.; Schornberg, K. Structures and mechanisms of viral membrane fusion proteins: Multiple variations on a common theme. Crit. Rev. Biochem. Mol. Biol. 2008, 43, 189–219. [Google Scholar]
- Harrison, S.C. Mechanism of membrane fusion by viral envelope proteins. Adv. Virus Res. 2005, 64, 231–261. [Google Scholar]
- Gallaher, W.R.; DiSimone, C.; Buchmeier, M.J. The viral transmembrane superfamily: Possible divergence of Arenavirus and Filovirus glycoproteins from a common RNA virus ancestor. BMC Microbiol. 2001, 1, 1. [Google Scholar]
- Eschli, B.; Quirin, K.; Wepf, A.; Weber, J.; Zinkernagel, R.; Hengartner, H. Identification of an N-terminal trimeric coiled-coil core within arenavirus glycoprotein 2 permits assignment to class I viral fusion proteins. J. Virol. 2006, 80, 5897–5907. [Google Scholar]
- York, J.; Berry, J.D.; Ströher, U.; Li, Q.; Feldmann, H.; Lu, M.; Trahey, M.; Nunberg, J.H. An antibody directed against the fusion peptide of Junin virus envelope glycoprotein GPC inhibits pH-induced membrane fusion. J. Virol. 2010, 84, 6119–6129. [Google Scholar]
- Igonet, S.; Vaney, M.C.; Vonhrein, C.; Bricogne, G.; Stura, E.A.; Hengartner, H.; Eschli, B.; Rey, F.A. X-ray structure of the arenavirus glycoprotein GP2 in its postfusion hairpin conformation. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 19967–19972. [Google Scholar]
- York, J.; Agnihothram, S.S.; Romanowski, V.; Nunberg, J.H. Genetic analysis of heptad-repeat regions in the G2 fusion subunit of the Junin arenavirus envelope glycoprotein. Virology 2005, 343, 267–279. [Google Scholar]
- Lumb, K.; Kim, P. A buried polar interaction imparts structural uniqueness in a designed heterodimeric coiled coil. Biochemistry 1995, 34, 8642–8648. [Google Scholar]
- Delos, S.E.; White, J.M. Critical role for the cysteines flanking the internal fusion peptide of avian sarcoma/leukosis virus envelope glycoprotein. J. Virol. 2000, 74, 9738–9741. [Google Scholar]
- Eichler, R.; Lenz, O.; Garten, W.; Strecker, T. The role of single N-glycans in proteolytic processing and cell surface transport of the Lassa virus glycoprotein GP-C. Virol. J. 2006, 3, 41. [Google Scholar]
- von Heijne, G. Signal sequences. The limits of variation. J. Mol. Biol. 1985, 184, 99–105. [Google Scholar] [CrossRef] [PubMed]
- York, J.; Nunberg, J.H. Distinct requirements for signal peptidase processing and function of the stable signal peptide (SSP) subunit in the Junin virus envelope glycoprotein. Virology 2007, 359, 72–81. [Google Scholar]
- Froeschke, M.; Basler, M.; Groettrup, M.; Dobberstein, B. Long-lived signal peptide of lymphocytic choriomeningitis virus glycoprotein pGP-C. J. Biol. Chem. 2003, 278, 41914–41920. [Google Scholar]
- Sanchez, A.; Pifat, D.Y.; Kenyon, R.H.; J, P.C.; McCormick, J.B.; Kiley, M.P. Junin virus monoclonal antibodies: Characterization and cross-reactivity with other arenaviruses. J. Gen. Virol. 1989, 70, 1125–1132. [Google Scholar]
- York, J.; Romanowski, V.; Lu, M.; Nunberg, J.H. The signal peptide of the Junín arenavirus envelope glycoprotein is myristoylated and forms an essential subunit of the mature G1-G2 complex. J. Virol. 2004, 78, 10783–10792. [Google Scholar]
- Agnihothram, S.S.; York, J.; Nunberg, J.H. Role of the stable signal peptide and cytoplasmic domain of G2 in regulating intracellular transport of the Junin virus envelope glycoprotein complex. J. Virol. 2006, 80, 5189–5198. [Google Scholar]
- Albariño, C.G.; Bird, B.H.; Chakrabarti, A.K.; Dodd, K.A.; White, D.M.; Bergeron, E.; Shrivastava-Ranjan, P.; Nichol, S.T. Reverse genetics generation of chimeric infectious Junin/Lassa virus is dependent on interaction of homologous glycoprotein stable signal peptide and G2 cytoplasmic domains. J. Virol. 2011, 85, 112–122. [Google Scholar]
- Saunders, A.A.; Ting, J.P.; Meisner, J.; Neuman, B.W.; Perez, M.; de la Torre, J.C.; Buchmeier, M.J. Mapping the landscape of the lymphocytic choriomeningitis virus stable signal peptide reveals novel functional domains. J. Virol. 2007, 81, 5649–5657. [Google Scholar]
- Agnihothram, S.S.; Dancho, B.; Grant, K.W.; Grimes, M.L.; Lyles, D.S.; Nunberg, J.H. Arenavirus envelope glycoprotein GPC assembles into detergent-soluble membrane microdomains. J. Virol. 2009, 83, 9890–9900. [Google Scholar]
- Eichler, R.; Lenz, O.; Strecker, T.; Eickmann, M.; Klenk, H.D.; Garten, W. Identification of Lassa virus glycoprotein signal peptide as a trans-acting maturation factor. EMBO Rep. 2003, 4, 1084–1088. [Google Scholar]
- Schlie, K.; Strecker, T.; Garten, W. Maturation cleavage within the ectodomain of Lassa virus glycoprotein relies on stabilization by the cytoplasmic tail. FEBS Lett. 2010, 584, 4379–4382. [Google Scholar]
- Elagoz, A.; Benjannet, S.; Mammarbassi, A.; Wickham, L.; Seidah, N.G. Biosynthesis and cellular trafficking of the convertase SKI-1/S1P: Ectodomain shedding requires SKI-1 activity. J. Biol. Chem. 2002, 277, 11265–11275. [Google Scholar]
- Eichler, R.; Lenz, O.; Strecker, T.; Eickmann, M.; Klenk, H.D.; Garten, W. Lassa virus glycoprotein signal peptide displays a novel topology with an extended ER-luminal region. J. Biol. Chem. 2004, 279, 12293–12299. [Google Scholar]
- Schrempf, S.; Froeschke, M.; Giroglou, T.; von Laer, D.; Dobberstein, B. Signal peptide requirements for lymphocytic choriomeningitis virus glycoprotein C maturation and virus infectivity. J. Virol. 2007, 81, 12515–12524. [Google Scholar]
- Agnihothram, S.S.; York, J.; Trahey, M.; Nunberg, J.H. Bitopic membrane topology of the stable signal peptide in the tripartite Junín virus GP-C envelope glycoprotein complex. J. Virol. 2007, 81, 4331–4337. [Google Scholar]
- York, J.; Nunberg, J.H. A novel zinc-binding domain is essential for formation of the functional Junín virus envelope glycoprotein complex. J. Virol. 2007, 81, 13385–13391. [Google Scholar]
- York, J.; Nunberg, J.H. Role of the stable signal peptide of the Junín arenavirus envelope glycoprotein in pH-dependent membrane fusion. J. Virol. 2006, 80, 7775–7780. [Google Scholar]
- York, J.; Nunberg, J.H. Intersubunit interactions modulate pH-induced activation of membrane fusion by the Junin virus envelope glycoprotein GPC. J. Virol. 2009, 83, 4121–4126. [Google Scholar]
- York, J.; Nunberg, J.H. The University of Montana: Missoula, MT, USA, Unpublished work.
- Kampmann, T.; Mueller, D.S.; Mark, A.E.; Young, P.R.; Kobe, B. The role of histidine residues in low-pH-mediated viral membrane fusion. Structure 2006, 14, 1481–1487. [Google Scholar]
- Thoennes, S.; Li, Z.N.; Lee, B.J.; Langley, W.A.; Skehel, J.J.; Russell, R.J.; Steinhauer, D.A. Analysis of residues near the fusion peptide in the influenza hemagglutinin structure for roles in triggering membrane fusion. Virology 2008, 370, 403–414. [Google Scholar]
- Jasti, J.; Furukawa, H.; Gonzales, E.B.; Gouaux, E. Structure of acid-sensing ion channel 1 at 1.9 A resolution and low pH. Nature 2007, 449, 316–323. [Google Scholar] [PubMed]
- York, J.; Dai, D.; Amberg, S.A.; Nunberg, J.H. pH-induced activation of arenavirus membrane fusion is antagonized by small-molecule inhibitors. J. Virol. 2008, 82, 10932–10939. [Google Scholar]
- Thomas, C.J.; Casquilho-Gray, H.E.; York, J.; DeCamp, D.L.; Dai, D.; Petrilli, E.B.; Boger, D.L.; Slayden, R.A.; Amberg, S.M.; Sprang, S.R.; Nunberg, J.H. A specific interaction of small-molecule entry inhibitors with the envelope glycoprotein complex of the Junín hemorrhagic fever arenavirus. J. Biol. Chem. 2011, 286, 6192–6200. [Google Scholar]
- Sanchez, A.B.; de la Torre, J.C. Rescue of the prototypic Arenavirus LCMV entirely from plasmid. Virology 2006, 350, 370–380. [Google Scholar]
- Lan, S.; McLay Schelde, L.; Wang, J.; Kumar, N.; Ly, H.; Liang, Y. Development of infectious clones for virulent and avirulent pichinde viruses: A model virus to study arenavirus-induced hemorrhagic fevers. J. Virol. 2009, 83, 6357–6362. [Google Scholar]
- Albariño, C.G.; Bergeron, E.; Erickson, B.R.; Khristova, M.L.; Rollin, P.E.; Nichol, S.T. Efficient reverse genetics generation of infectious Junin viruses differing in glycoprotein processing. J. Virol. 2009, 83, 5606–5614. [Google Scholar]
- Emonet, S.F.; Seregin, A.V.; Yun, N.E.; Poussard, A.L.; Walker, A.G.; de la Torre, J.C.; Paessler, S. Rescue from cloned cDNAs and in vivo characterization of recombinant pathogenic Romero and live-attenuated Candid #1 strains of Junin virus, the causative agent of Argentine hemorrhagic fever disease. J. Virol. 2011, 85, 1473–1483. [Google Scholar] [PubMed]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Nunberg, J.H.; York, J. The Curious Case of Arenavirus Entry, and Its Inhibition. Viruses 2012, 4, 83-101. https://doi.org/10.3390/v4010083
Nunberg JH, York J. The Curious Case of Arenavirus Entry, and Its Inhibition. Viruses. 2012; 4(1):83-101. https://doi.org/10.3390/v4010083
Chicago/Turabian StyleNunberg, Jack H., and Joanne York. 2012. "The Curious Case of Arenavirus Entry, and Its Inhibition" Viruses 4, no. 1: 83-101. https://doi.org/10.3390/v4010083
APA StyleNunberg, J. H., & York, J. (2012). The Curious Case of Arenavirus Entry, and Its Inhibition. Viruses, 4(1), 83-101. https://doi.org/10.3390/v4010083