Abstract
The curative potential of retroviral vectors for somatic gene therapy has been demonstrated impressively in several clinical trials leading to sustained long-term correction of the underlying genetic defect. Preclinical studies and clinical monitoring of gene modified hematopoietic stem and progenitor cells in patients have shown that biologically relevant vector induced side effects, ranging from in vitro immortalization to clonal dominance and oncogenesis in vivo, accompany therapeutic efficiency of integrating retroviral gene transfer systems. Most importantly, it has been demonstrated that the genotoxic potential is not identical among all retroviral vector systems designed for clinical application. Large scale viral integration site determination has uncovered significant differences in the target site selection of retrovirus subfamilies influencing the propensity for inducing genetic alterations in the host genome. In this review we will summarize recent insights gained on the mechanisms of insertional mutagenesis based on intrinsic target site selection of different retrovirus families. We will also discuss examples of side effects occurring in ongoing human gene therapy trials and future prospectives in the field.
1. Introduction
The defining feature of retroviral replication is the integration of the reverse transcribed viral DNA into the genome of the host cell [,]. Efficient integration of the viral DNA into the host genome is a hallmark of the retroviral life cycle making replication-incompetent retroviral vectors attractive gene transfer vehicles for stable ectopic expression of transgenes in target cells. The understanding of basic principles of the retroviral replication cycle has led to the development of replication incompetent retroviral vectors capable of a single integration event upon infection of target cells in the absence of superinfection [,,,]. Since their first description in the beginning of the 1980s, a variety of vector systems from the two different retroviral subfamilies—Orthoretrovirinae and Spumaretrovirinae—have been developed, which are broadly applied in basic and clinical research as ectopic gene delivery vehicles [,]. The viral integration reaction catalyzed by the viral integrase protein has been extensively analyzed revealing complex interactions with cellular host proteins regulating nuclear import, chromatin tethering and integration into the host genome [,].
Being applied in >357 initiated clinical phase I/II gene therapy trials, retroviral based vectors represent the second most commonly used gene delivery vehicles after adenoviral vectors []. Stable integration into the host genome and subsequent long-term ectopic expression of therapeutic transgenes underlines the potential of retroviral gene transfer systems for correction of inherited diseases [].
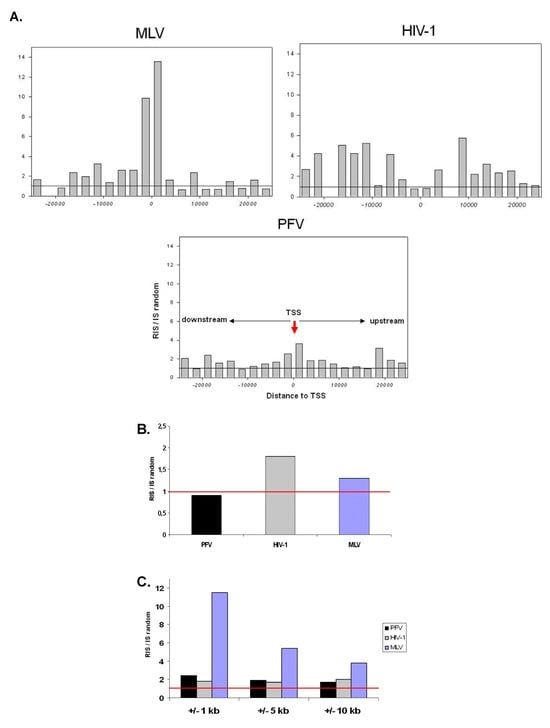
Gene therapy for inherited diseases has demonstrated that modification and retransplantation of gene corrected (stem) cells cures severe disorders. The success of these gene therapy trials has been accomplished by replacing genetically non-functional genes present in the patients’ cells with retroviral vectors constitutively expressing the therapeutical gene after random integration into the host genome. Consequently, the retroviral integration sites (RIS) create unique genetic signatures which can be amplified and sequenced to follow the fate of individual retrovirally ‘molecular marked’ cells and their clonal progeny in the respective target tissue []. Development of technologies for identification and sequencing of retroviral integration sites together with the publication of the human genome sequence have led to precise insights in the global integration pattern of different retrovirus subfamilies [,,,,,,]. These integration site profiling studies uncovered unexpected virus specific integration preferences most likely resulting from different interactions of the viral integration machinery and host factors []. Comprehensive studies of retrovirally transduced cells and their fate in vivo by large scale integration site analyses of preclinical and clinical samples have provided evidence that the propensity for insertional mutagenesis is, at least in part, influenced by the non-random integration site selection of retroviral vectors [].
In this chapter we will focus on recent insights in the target site selection of retroviral vectors and the molecular mechanisms underlying retroviral vector induced mutagenesis. An overview of subsequent biological effects of insertional mutagenesis is given based on preclinical and clinical data. We will introduce recent advances in next generation sequencing technologies and their impact on future high-throughput integration site analyses, both for mutation and vector biosafety research, and highlight their potential for a comprehensive clinical monitoring of current and future stem cell gene therapy trials using retroviral based vectors.
3. Side Effects in Clinical and Preclinical Gene Therapy Studies
First molecular insights in the oncogenic potential of integrated wild type retroviruses were obtained from bursal lymphomas in chicken. The majority of the identified tumor cells contained a provirus integrated in the vicinity of the proto-oncogene c-myc that was overexpressed by the viral promoters/enhancers [,,,]. These genetic alterations caused by retroviruses, termed proviral insertional mutagenesis, have been identified in many types of retrovirus induced tumors [,]. Thereby, a variety of genes could be identified that modulate growth and differentiation and significantly contributed to tumor formation. Despite these insights obtained from wild-type retroviruses and replication-competent retroviral vectors, the risk of insertional mutagenesis (Figure 3) and cellular transformation with replication-incompetent vectors specifically developed for clinical purposes was considered to be rather low [].

Figure 3.
Retroviral vectors may induce mutations in multiple ways by integration of the retrovirus in the host genome. (A,B) Mutagenic proviral insertions in most reported cases induce an activation of neighboring genes by enhancer elements present within the wildtype LTR. Such “enhancer insertions” can induce gene activation from distances up to 100 kb. (C,D) In case of SIN-type retroviral vectors strong internal promoters driving transgene expression may induce deregulation of genes in close proximity similar to so-called “promotor insertions” which result in viral-host gene-fusion transcripts. (E) Genotoxic side effects resulting from retroviral integration sites leading to inactivation of cellular genes may be induced by viral insertion within a host gene leading to truncated non functional transcripts.
However, after first cases of severe adverse events in a minority of patients in the X-SCID gene therapy trial were reported (see next chapter), the first malignant transformation that developed as a result of gene transfer using replication-deficient retroviral vectors designed for clinical use in a murine model was observed in 2002 []. In a murine model system the serial transplantation of bone marrow cells marked with gammaretroviral vectors led to tumor development in secondary and tertiary recipients []. All secondary transplanted animals showed alterations in their hematopoiesis after 22 weeks, and six out of ten animals developed acute myeloid leukemia. Molecular analyses of the malignant clone in respect to the RIS using LM-PCR revealed that in all developed tumors derived from secondary and tertiary recipients an insertion in the Evi1 gene locus triggering malignant transformation was detected []. Although it was shown that the promoter and enhancer elements in the proviral LTR caused an overexpression of Evi1, a synergistic effect of the low-affinity nerve growth factor receptor (LNGFR) remains unclear []. In terms of clinical safety it is important to note that vector dose has an impact on leukemogenesis as shown in a murine study where Leukemia not only occurred due to the growth advantage of single clones but also correlated with high vector doses using MLV based vectors []. Soon after, gene marking studies in a non-human primate model provided evidence for clonal dominance due to an insertional effect of MLV based vectors in vicinity of the Mds1/Evi or Prdm16 gene. This insertional event led to a growth advantage compared to other marked cell clones without causing malignant transformation []. Since the majority of these cell clones contribute to long-term hematopoiesis, a higher engraftment or survival probability as a result of insertional mutagenesis was discussed [,]. Vector induced side effects with possible roles in hematopoietic activity have also been reported in a murine gene marking study in which proviral integration within or nearby particular cellular genes promoted the growth of single transduced cells and contributed to their clonal expansion in vivo []. The first retroviral vector induced acute myeloid leukemia in a non-human primate model was described after retroviral gene transfer in hematopoietic precursor cells. The treated animal died five years after gene therapy due to a myeloid sarcoma caused by two insertions in the Bcl2-A1 and Cdw91 genes. In this study the clone harboring these two insertions was both detectable in the blood where it became dominant one year after transplantation as well as in the tumor, strengthening a cooperative functional role of these insertions in tumor development [].
Human gene therapy using retroviral vectors is a specialized form of therapy that is mainly applied to patients for whom no therapeutic alternatives are available. Therefore, the benefit of gene therapy always has to be opposed to its potential risks. The field has suffered from a lot of initial hype without taking into account that any new kind of specialized therapy may also be accompanied by side effects. The success of human gene therapy for hematopoietic diseases has been enabled by the greater efficiency in performing ex vivo transduction of human CD34+ cells using retroviral vectors and reinfusion of a high number (up to 108−109) of gene-corrected cells. In retrospect, it is now evident that improvement in effectiveness for curing otherwise challenging lethal diseases is accompanied by a higher risk of biologically relevant side effects of this type of gene therapy. Up until now most gene therapy trials in the hematopoietic system involve the use of first generation MLV derived retroviral vectors. Classical MLV based vectors carrying the full LTR are now based on their integration site selection and vector design considered to have a higher potential for genotoxic events as compared to lentiviral based vectors (see below).
Monitoring the in vivo fate of gene corrected CD34+ cells retransplanted into patients by high-throughput integration site analysis in five independent clinical gene therapy studies have shown that distribution of gammeretroviral vectors in vivo is skewed. Apart from subtle effects, clonal dominance and leukemogenesis influencing the growth and differentiation of clones when inserted near to particular genes and loci have been reported in minority of treated patients [64–66,111-114,131]. We have recently described that in addition to overt carcinogenesis, even as a single vector copy, many insertion locations more subtly influence the biological fate of a cell clone in vivo [,,]. With LAM-PCR technology specifically developed for this purpose, it has been identified that up to 40% of circulating cells carry insertions in a rather small set of frequently affected common insertion sites (CIS) [,]. Such CIS are almost always in the direct vicinity of known and novel genes likely to be involved in cellular growth, survival and self-renewal processes of immature progenitor and stem cells.
In the gene therapy trial for X-SCID, two to six years after successful correction of the otherwise lethal disease the development of T-cell leukemia in five of 19 patients has been observed [,]. In four of these patients the leukemic clone harbored a vector integration in the LMO2 proto-oncogene inducing its overexpression. Together with acquired mutations these events have led to expansion of these clones and the development of leukemia [,]. Although under dispute, the combinatorial effect of the IL2RG transgene in the development of the lymphoproliferative disease in the X-SCID trial has been discussed [,] and recent work from Copeland and colleagues provides supportive evidence that IL2RG and LMO2 do cooperate in leukemia induction []. At the same time skewing of RIS was observed in the healthy patients causing subtle insertional effects without any signs of malignant transformation [,].
Evidence for vector induced effects influencing hematopoietic activity have been gained in the gene therapy for the correction of X-CGD [,]. Here, in two adult patients, retroviral gene therapy resulted in the restoration of oxidative antimicrobial activity in phagocytes after gene transfer. Integration site analysis revealed a clonal dominance triggered by insertion sites in the genes MDS1-EVI1, PRDM16 or SETBP1 (Figure 4A), resulting in an expansion of gene corrected myelopoiesis [,]. In the follow up of the clinical trial a substantial gene transfer in neutrophil cells had produced a high number of functional phagocytes, however, after the initial resolution of bacterial and fungal infections, both subjects showed silencing of transgene expression due to methylation of the viral promoter, and myelodysplasia with monosomy 7 as a result of insertional activation of ecotropic viral integration site 1 (EVI1). It has been recently suggested that the overexpression of Evi1 disrupts normal centrosome duplication leading to genomic instability, monosomy 7 and clonal progression toward myelodysplasia [].

Figure 4.
Retroviral integration into common insertion sites in clinical gene therapy trials. (A) Clustering of retroviral integration sites (RIS) within clones sharing integrations in MDS1-EVI1, PRDM16 and SETBP1 in the X-CGD clinical trial identified by linear amplification-mediated PCR (LAM-PCR) (modified from Ott et al. []). (B) Clinical monitoring of retroviral gene corrected hematopoietic stem/progenitor cells (HSC) by LAM-PCR and 454 sequencing in two patients from the WAS gene therapy trial over time reveal multiple clones sharing insertion sites into common integration sites located near genes previously known to induce malignant clonal expansion. For measuring clonal contribution to hematopoiesis over time at every time point analyzed, sequence counts for all RIS contributing to an individual common insertion sites (CIS) derived from PBL and BM were clustered and related to total sequence count at the respective time point (modified from Boztug et al. []).
From these severe adverse events it is tempting to reason that the use of gammaretroviral vectors is per se oncogenic. However, given that severe events have occurred in a minority of treated patients in the X-SCID trial, the clinical follow up of the ADA-SCID [] trial using the same vector backbone as in the X-SCID trial has up to now not been accompanied by any adverse events up to 8 years post therapy. This may indicate that the risk for vector induced side effects may also be dependent on the disease and the clinical protocol. We note that clear evidence for this discussion has not been reported so far.
In the most recent gene therapy trial for Wiskott-Aldrich Syndrome (WAS) an MLV-based vector backbone expressing the WAS protein was used for transduction of autologous CD34+ HSC which were transfused into two patients. WAS is a primary immunodeficiency disorder associated with thrombocytopenia, eczema and autoimmunity and is alternatively treatable with haploidentical BM transplantation []. Here, monitoring the clonal contribution of gene corrected cells to hematopoietic regeneration three years post transfusion by a comprehensive integration site analysis using next generation sequencing technologies has identified over 10,000 unique clones contributing to short- and long-term hematopoieses. This genome-wide insertion site analysis demonstrated that vector integration targeted multiple genes controlling growth development and immunological responses in a persistently polyclonal hematopoiesis. However, many of the previously observed CIS, which occasionally triggered adverse events, were detectable in both subjects to a similar degree suggesting vector induced skewing similar to what was observed in previous gene therapy trials using the MLV backbones (Figure 4B, C). In fact, it was further shown that in sorted cell populations of the lymphoid or myeloid fraction lineage specific CIS could be identified, which in previous gene therapy trials had triggered either lymphoid or myeloid proliferation suggesting that insertional activation of these genes programs cell fate in the hematopoietic system []. In total, 9/10 patients have been treated successfully in this trial. Very recently it has been reported that one patient has developed a T-cell leukemia similar to the patients in the X-SCID trial. Whether vector induced effects have played a role in this side effect is under current investigation but it is most likely that vector induced side effects similar to the side effects observed in the minority of X-SCID patients may play a role here.
First proof that switching the integration pattern of the therapeutical retroviral vector improves genotoxic safety has been provided by the first LV-based gene therapy trial for the treatment of the cerebral form of X-chromosomal linked adrenoleukodystrophy (X-ALD), a demyelinating disease of the central nervous system caused by mutations in the ABCD1 gene []. Progress of X-ALD is treatable by haploidentical BM transplantation, but with patients where no matching donor is found gene therapy is a highly promising therapeutic option []. In 2006, two patients with no sibling BM donors were treated with HIV-1 based vectors, which show distinct differences in target site selection and efficiency in transducing quiescent cells compared to MLV-based vectors. Mobilized hematopoietic precursor cells were harvested from two seven year-old patients, transduced ex vivo with lentiviral SIN-vectors encoding the ABCD1 transgene and reinfused into the patients. This treatment proved efficient to arrest the progression of the disease in both patients through the constitutive expression of the functional ABCD1 therapeutic transgene. More importantly, no signs of vector induced side effects have been reported so far in two treated patients continuously being monitored. Up to now, the clonal dynamics of transduced and reinfused CD34+ HSC show transduction of multipotent HSC and no signs of enrichment near to CIS genes previously being identified as integration hot spots of MLV-based vectors in vivo []. The improved clinical safety of SIN-type LV-based vectors compared to classical MLV-based vectors has previously been proposed based on evidence gained in sensitive mouse models designed to measure genotoxic safety [,]. Deduced with murine genotoxicity assays, although at low rate, vector induced genetic alterations have also been reported with target site selection of LV-based vectors [,,]. Such insertional alterations in the regulation of the cellular genome by LV-vectors have recently been reported in the gene therapy trial for human β-thalassaemia []. Clinical benefit of lentiviral β-globin gene transfer has been achieved in an adult patient 33 months post treatment by clonal dominance initiated by vector induced activation of the HMGA2 gene. This particular clonal dominance has been proposed to accompany clinical efficacy for β-thalassaemia, a challenging hematopoietic disease, and the most common form of severe thalassaemia in southeast Asian countries and their diasporas [].
New Strategies for Vector Biosafety in Gene Therapy
The risk of side effects in future gene therapy trials will be dependent on the choice of the most suitable gene transfer vector dependent on the individual disease and the propensity for genomic alterations driven by vector design and integration pattern. The usage of SIN-type vectors with weak or tissue-specific promoters or non-integrating vector systems—if appropriate—are considered to be safer than classical MLV-vectors. The use of AAV- or Adenovirus-vectors predominantly persisting episomaly in post-mitotic tissue (i.e., retina, brain, muscle or liver) are promising for particular diseases []. However, insertional mutagenesis caused by rare but detectable integration events with AAV-vectors in the liver has also been reported [,] underlining the need for highly sensitive strategies to detect such rare insertions in a clinical setting. In postmitotic tissues, dilution of unintegrated episomal vector forms does not occur thus allowing the use of integrase deficient lentiviral vector systems []. Mutations in the core domain of the viral integrase prevent integration, thereby reducing the risk of insertional mutagenesis. Recently, the long-term functional correction of retinal degeneration in a well-established rodent model for ocular gene therapy was reported under the use of an integrase-deficient HIV-1 vector without apparent side effects [].
Alternative modification of the viral integrase for targeted integration into desired potentially safe chromosomal regions [], together with significant improvements in the efficiency of homologous recombination or gene disruption using novel Zinc-Finger-Nucleases (ZFN) will in future define alternative gene delivery approaches []. Other non-viral integrating vector systems based on sleeping beauty or piggybac transposons have been identified to have a potentially safer integration pattern in vitro []. However, given that any integrating vector system by chance, if combined with genotoxic vector elements, may induce genetic alterations of the cellular genome also account for transposon based vectors which are also used to identify novel cancer genes in murine models [].
With significant improvements in the controlled genetic modification of particular loci in targeted integration approaches and a variety of vectors with alternative integration patterns available it becomes evident that the advance of highly sensitive technologies for a comprehensive genomic screening is required to monitor safety on a genetic level. Whole genome next generation sequencing technologies to evaluate genomic stability and strategies for an unbiased retrieval of integrated sites [] and induced DNA double-strand breaks will be essential for dissecting the genetic specificity and safety of novel targeted gene editing protocols. In this context, recent pioneering advances in the field of cellular reprogramming and the availability of sources of induced pluripotent cells (iPS) [,,] or lineage specific progenitor cells [,,] generated by integrating vector systems expressing particular pluripotency of lineage regulating transcription factors have made prediagnostic genomic safety screening of hundreds of clones feasible for potential future cell based therapies []. New vector systems and the potential use of new induced clinical applicable cell sources will greatly benefit from vector biosafety models established in the gene therapy field [,].
During the last years several in vitro and in vivo systems have been developed assessing the genotoxic potential of different integrating vectors. Transplantation of gammaretroviral transduced hematopoietic cells in mice with knocked-out Cdkn2a tumor suppressor gene leads to accelerated tumor growth in case a cellular proto-oncogene is virally activated [,]. Such conducted insertional mutagenesis screens have resulted in the establishment of the most extensive database of murine genes with oncogenic potential, the so-called mouse “Retrovirally Tagged Cancer Gene Database” (RTCGD) []. Recently also sleeping beauty transposon systems have been applied in a similar manner [,]. Du and colleagues showed that identification of protooncogenes is feasible by retroviral gene transfer in cell culture. Transduction of murine bone marrow cells with replication deficient retroviruses expressing marker genes resulted in immortalized cell lines, many of which contained integrations in the Mds1/Evi1 and Prdm16 genes [,]. Vector integration into the Evi1 locus and overexpression of Evi1 appeared to be sufficient for a cell to be immortalized [,]. Modlich and colleagues adapted and improved this method so that the results could be analyzed in a quantitative manner [].
The tumor prone mouse model in which the tumor suppressor gene Arf as well as the Il2rg gene had been knocked-out originally developed by Lund et al. was suitable to perform comparative tests on the genotoxic potential of different gene transfer vectors [,]. Mice that had received hematopoietic precursor cell transplants transduced with gammaretroviral vectors developed tumors much more rapidly than mice that had received cells harboring lentiviral SIN-vectors with an internal human phosphoglycerate kinase (hPGK) promoter []. Later, it was demonstrated that gammaretroviral vectors with SIN-LTRs show a significant reduction in genotoxicity, but when combined with strong (viral) internal promoters still have the ability to induce leukemia in transplanted mice []. Given that in follow-up of the first LV-vector gene therapy trial for X-ALD no signs of clonal dominance or side effects have been observed up to the present it seems reasonable that such mouse models realistically modulate clinical safety of integrating vector systems. Insertion of insulator elements in the LTRs aims to reduce the influence of the integrated vector on the surrounding host genome and vice versa [,]. However, evidence for increased genotoxic safety with the use of insulators [] needs to be further provided in common in vitro and in vivo models for vector safety assessments, especially after insertional mutagenesis mediated clonal dominance has been reported in the lentiviral β-thalassaemia gene therapy trial in which the cHS4 chromatin insulator was implanted in the LTR of the therapeutic vector [].
Improvement of genotoxic safety using alternative and modified vector systems together with progress in targeted integration approaches and new cell sorting abilities provide a tremendous potential for future gene and cell therapy with increased safety. This will further improve the treatment of patients with otherwise not curable and often lethal inherited diseases.
4. Conclusion and Future Perspective
Large scale genome wide investigations of retroviral target site selection have shown that different retroviral subfamilies have developed different mechanisms to integrate their DNA into the host genome. Such differences result in three main integration patterns with MLV based vectors showing strong preferences for regulatory regions, LV-based vectors integrating preferentially inside RefSeq genes and PFV as well as ASLV having close to random insertional preferences. Towards safe clinical application of retroviral vectors uncovered target site preferences have proven to play substantial roles in the likelihood for insertional mutagenesis and cellular transformation. Knowledge gained from comprehensive integration site analysis of gene therapy patients and preclinical animal models to assess genotoxic safety of retroviral vectors are currently being actively transferred into the development of new and safer clinical protocols for the treatment of hematological and neurodegenerative diseases. The vision of using vectors with targeted synthetic integrases and improving the frequency of homologous recombination with ZFN or Meganucleases in order to correct defective genes or insert therapeutic genes into potentially “safe harbors” is rapidly evolving to become clinically feasible. In the era of whole genome sequencing projects aiming to understand progress of cancer and other diseases on a genetic level, the treatment of many new diseases in which the pathogenesis is dependent on a gene defect may become treatable with novel safe retroviral vectors imposing minimal genomic side effects for the patients.
Acknowledgments
The authors acknowledge all active and former members of the laboratory as well as collaborators for their contributions. This research has been supported in parts by the Deutsche Forschungsgemeinschaft DFG, grant SCHM 2134/1-1 and 2134/1-2 (SPP1230), by a grant of the Tumor Center Heidelberg/Mannheim, by the Bundesministerium für Bildung und Forschung BMBF, grant 01GU0809 (iGene), by the 6th and 7th Framework Programs of the European Commission, Contracts LSHB-CT-2004-005242-CONSERT, LSHB-CT-2006-018933-CLINIGENE and HEALTH-F5-2009-222878-PERSIST and by the Initiative and Networking Fund of the Helmholtz Association within the Helmholtz Alliance on Immunotherapy of Cancer.
References
- Vogt, P.K. Historical introduction to the general properties of retroviruses. In Retroviruses; Coffin, J.M., Hughes, S.H., Varmus, E.H., Eds.; ColdSpring Harbor Laboratory Press: New York, NY, USA, 1997. [Google Scholar][Green Version]
- Brown, P.O. Integration. In Retroviruses; Coffin, J.M., Hughes, S.H., Varmus, E.H., Eds.; ColdSpring Harbor Laboratory Press: New York, NY, USA, 1997. [Google Scholar][Green Version]
- Eglitis, M.A.; Kantoff, P.; Gilboa, E.; Anderson, W.F. Gene expression in mice after high efficiency retroviral-mediated gene transfer. Science 1985, 230, 1395–1398. [Google Scholar] [CrossRef]
- Dick, J.E.; Magli, M.C.; Huszar, D.; Phillips, R.A.; Bernstein, A. Introduction of a selectable gene into primitive stem cells capable of long-term reconstitution of the hemopoietic system of W/Wv mice. Cell 1985, 42, 71–79. [Google Scholar] [CrossRef]
- Williams, D.A.; Lemischka, I.R.; Nathan, D.G.; Mulligan, R.C. Introduction of new genetic material into pluripotent haematopoietic stem cells of the mouse. Nature 1984, 310, 476–80. [Google Scholar] [CrossRef] [PubMed]
- Mann, R.; Mulligan, R.C.; Baltimore, D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell 1983, 33, 153–159. [Google Scholar] [CrossRef]
- Miller, A.D. Development and applications of retroviral vectors. In Retroviruses; Coffin, J.M., Hughes, S.H., Varmus, E.H., Eds.; ColdSpring Harbor Laboratory Press: New York, NY, USA, 1997. [Google Scholar][Green Version]
- Anderson, W.F. Prospects for human gene therapy. Science 1984, 226, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Craigie, R. The road to chromatin - nuclear entry of retroviruses. Nat. Rev. Microbiol. 2007, 5, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Turlure, F.; Devroe, E.; Silver, P.A.; Engelman, A. Human cell proteins and human immunodeficiency virus DNA integration. Front. Biosci. 2004, 9, 3187–3208. [Google Scholar] [CrossRef]
- Edelstein, M.L.; Abedi, M.R.; Wixon, J. Gene therapy clinical trials worldwide to 2007—An update. J. Gene Med. 2007, 9, 833–842. [Google Scholar] [CrossRef]
- Verma, I.M.; Weitzman, M.D. Gene therapy: twenty-first century medicine. Annu. Rev. Biochem. 2005, 74, 711–738. [Google Scholar] [CrossRef]
- von Kalle, C.; Fehse, B.; Layh-Schmitt, G.; Schmidt, M.; Kelly, P.; Baum, C. Stem cell clonality and genotoxicity in hematopoietic cells: Gene activation side effects should be avoidable. Semin. Hematol. 2004, 41, 303–318. [Google Scholar] [CrossRef]
- Trobridge, G.D.; Miller, D.G.; Jacobs, M.A.; Allen, J.M.; Kiem, H.P.; Kaul, R.; Russell, D.W. Foamy virus vector integration sites in normal human cells. Proc. Nat. Acad. Sci. USA 2006, 103, 1498–1503. [Google Scholar] [CrossRef] [PubMed]
- Nowrouzi, A.; Dittrich, M.; Klanke, C.; Heinkelein, M.; Rammling, M.; Dandekar, T.; von Kalle, C.; Rethwilm, A. Genome-wide mapping of foamy virus vector integrations into a human cell line. J. Gen. Virol. 2006, 87, 1339–1347. [Google Scholar] [CrossRef] [PubMed]
- Hacker, C.V.; Vink, C.A.; Wardell, T.W.; Lee, S.; Treasure, P.; Kingsman, S.M.; Mitrophanous, K.A.; Miskin, J.E. The integration profile of EIAV-based vectors. Mol. Ther. 2006, 14, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Narezkina, A.; Taganov, K.D.; Litwin, S.; Stoyanova, R.; Hayashi, J.; Seeger, C.; Skalka, A.M.; Katz, R.A. Genome-wide analyses of avian sarcoma virus integration sites. J. Virol. 2004, 78, 11656–11663. [Google Scholar] [CrossRef]
- Mitchell, R.S.; Beitzel, B.F.; Schroder, A.R.; Shinn, P.; Chen, H.; Berry, C.C.; Ecker, J.R.; Bushman, F.D. Retroviral DNA integration: ASLV, HIV, and MLV show distinct target site preferences. PLoS Biol. 2004, 2, E234. [Google Scholar] [CrossRef]
- Wu, X.; Li, Y.; Crise, B.; Burgess, S.M. Transcription start regions in the human genome are favored targets for MLV integration. Science 2003, 300, 1749–1751. [Google Scholar] [CrossRef]
- Schroder, A.R.; Shinn, P.; Chen, H.; Berry, C.; Ecker, J.R.; Bushman, F. HIV-1 integration in the human genome favors active genes and local hotspots. Cell 2002, 110, 521–529. [Google Scholar] [CrossRef]
- Bushman, F.; Lewinski, M.; Ciuffi, A.; Barr, S.; Leipzig, J.; Hannenhalli, S.; Hoffmann, C. Genome-wide analysis of retroviral DNA integration. Nat. Rev. Microbiol. 2005, 3, 848–858. [Google Scholar] [CrossRef]
- Nienhuis, A.W.; Dunbar, C.E.; Sorrentino, B.P. Genotoxicity of retroviral integration in hematopoietic cells. Mol. Ther. 2006, 13, 1031–1049. [Google Scholar] [CrossRef]
- Temin, H.M.; Mizutani, S. RNA-dependent DNA polymerase in virions of Rous sarcoma virus. Nature 1970, 226, 1211–1213. [Google Scholar] [CrossRef]
- Baltimore, D. RNA-dependent DNA polymerase in virions of RNA tumour viruses. Nature 1970, 226, 1209–1211. [Google Scholar] [CrossRef] [PubMed]
- Temin, H.M. The DNA provirus hypothesis. Science 1976, 192, 1075–1080. [Google Scholar] [CrossRef] [PubMed]
- Temin, H.M. The protovirus hypothesis: speculations on the significance of RNA-directed DNA synthesis for normal development and for carcinogenesis. J. Nat. Canc. Ins. 1971, 46, 3–7. [Google Scholar]
- Temin, H.M. Origin of retroviruses from cellular moveable genetic elements. Cell 1980, 21, 599–600. [Google Scholar] [CrossRef]
- Cohen, J.C.; Shank, P.R.; Morris, V.L.; Cardiff, R.; Varmus, H.E. Integration of the DNA of mouse mammary tumor virus in virus-infected normal and neoplastic tissue of the mouse. Cell 1979, 16, 333–345. [Google Scholar] [CrossRef]
- Steffen, D.; Weinberg, R.A. The integrated genome of murine leukemia virus. Cell 1978, 15, 1003–1010. [Google Scholar] [CrossRef]
- Shank, P.R.; Hughes, S.H.; Kung, H.J.; Majors, J.E.; Quintrell, N.; Guntaka, R.V.; Bishop, J.M.; Varmus, H.E. Mapping unintegrated avian sarcoma virus DNA: termini of linear DNA bear 300 nucleotides present once or twice in two species of circular DNA. Cell 1978, 15, 1383–1395. [Google Scholar] [CrossRef]
- Hughes, S.H.; Shank, P.R.; Spector, D.H.; Kung, H.J.; Bishop, J.M.; Varmus, H.E.; Vogt, P.K.; Breitman, M.L. Proviruses of avian sarcoma virus are terminally redundant, co-extensive with unintegrated linear DNA and integrated at many sites. Cell 1978, 15, 1397–1410. [Google Scholar] [CrossRef]
- Battula, N.; Temin, H.M. Sites of integration of infectious DNA of avian reticuloendotheliosis viruses in different avian cellular DNAs. Cell 1978, 13, 387–398. [Google Scholar] [CrossRef]
- Moebes, A.; Enssle, J.; Bieniasz, P.D.; Heinkelein, M.; Lindemann, D.; Bock, M.; McClure, M.O.; Rethwilm, A. Human foamy virus reverse transcription that occurs late in the viral replication cycle. J. Virol. 1997, 71, 7305–7311. [Google Scholar] [CrossRef]
- Rethwilm, A. The replication strategy of foamy viruses. Curr. Topics Microbiol. Immunol. 2003, 277, 1–26. [Google Scholar]
- Telesnitsky, A.; Goff, S.P. Reverse transkriptase and the generation of retroviral DNA. In Retroviruses; Coffin, J.M., Hughes, S.H., Varmus, E.H., Eds.; ColdSpring Harbor Laboratory Press: New York, NY, USA, 1997. [Google Scholar]
- Bowerman, B.; Brown, P.O.; Bishop, J.M.; Varmus, H.E. A nucleoprotein complex mediates the integration of retroviral DNA. Gene. Develop. 1989, 3, 469–478. [Google Scholar] [CrossRef]
- Fujiwara, T.; Mizuuchi, K. Retroviral DNA integration: structure of an integration intermediate. Cell 1988, 54, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.O.; Bowerman, B.; Varmus, H.E.; Bishop, J.M. Correct integration of retroviral DNA in vitro. Cell 1987, 49, 347–356. [Google Scholar] [CrossRef]
- Petit, C.; Giron, M.L.; Tobaly-Tapiero, J.; Bittoun, P.; Real, E.; Jacob, Y.; Tordo, N.; De The, H.; Saib, A. Targeting of incoming retroviral Gag to the centrosome involves a direct interaction with the dynein light chain 8. J. Cell Sci. 2003, 116, 3433–3442. [Google Scholar] [CrossRef] [PubMed]
- McDonald, D.; Vodicka, M.A.; Lucero, G.; Svitkina, T.M.; Borisy, G.G.; Emerman, M.; Hope, T.J. Visualization of the intracellular behavior of HIV in living cells. J. Cell Biol. 2002, 159, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Saib, A.; Puvion-Dutilleul, F.; Schmid, M.; Peries, J.; de The, H. Nuclear targeting of incoming human foamy virus Gag proteins involves a centriolar step. J. Virol. 1997, 71, 1155–1161. [Google Scholar] [CrossRef]
- Roe, T.; Reynolds, T.C.; Yu, G.; Brown, P.O. Integration of murine leukemia virus DNA depends on mitosis. EMBO J. 1993, 12, 2099–2108. [Google Scholar] [CrossRef]
- Naldini, L.; Blomer, U.; Gallay, P.; Ory, D.; Mulligan, R.; Gage, F.H.; Verma, I.M.; Trono, D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 1996, 272, 263–267. [Google Scholar] [CrossRef]
- Bushman, F.D. Targeting survival: integration site selection by retroviruses and LTR-retrotransposons. Cell 2003, 115, 135–138. [Google Scholar] [CrossRef]
- Craigie, R.; Fujiwara, T.; Bushman, F. The IN protein of Moloney murine leukemia virus processes the viral DNA ends and accomplishes their integration in vitro. Cell 1990, 62, 829–837. [Google Scholar] [CrossRef]
- Hare, S.; Gupta, S.S.; Valkov, E.; Engelman, A.; Cherepanov, P. Retroviral intasome assembly and inhibition of DNA strand transfer. Nature 2010, 464, 232–236. [Google Scholar] [CrossRef]
- Maertens, G.N.; Hare, S.; Cherepanov, P. The mechanism of retroviral integration from X-ray structures of its key intermediates. Nature 2010, 468, 326–329. [Google Scholar] [CrossRef]
- Katzman, M.; Mack, J.P.; Skalka, A.M.; Leis, J. A covalent complex between retroviral integrase and nicked substrate DNA. Proc. Natl. Acad. Sci. USA 1991, 88, 4695–1699. [Google Scholar] [CrossRef]
- Engelman, A.; Mizuuchi, K.; Craigie, R. HIV-1 DNA integration: mechanism of viral DNA cleavage and DNA strand transfer. Cell 1991, 67, 1211–1221. [Google Scholar] [CrossRef] [PubMed]
- Bushman, F.D.; Fujiwara, T.; Craigie, R. Retroviral DNA integration directed by HIV integration protein in vitro. Science 1990, 249, 1555–1558. [Google Scholar] [CrossRef] [PubMed]
- Yoder, K.E.; Bushman, F.D. Repair of gaps in retroviral DNA integration intermediates. J. Virol. 2000, 74, 11191–1200. [Google Scholar] [CrossRef] [PubMed]
- Holman, A.G.; Coffin, J.M. Symmetrical base preferences surrounding HIV-1, avian sarcoma/leukosis virus, and murine leukemia virus integration sites. Proc. Natl. Acad. Sci. USA 2005, 102, 6103–6107. [Google Scholar] [CrossRef]
- Wu, X.; Li, Y.; Crise, B.; Burgess, S.M.; Munroe, D.J. Weak palindromic consensus sequences are a common feature found at the integration target sites of many retroviruses. J. Virol. 2005, 79, 5211–5214. [Google Scholar] [CrossRef]
- Pryciak, P.M.; Sil, A.; Varmus, H.E. Retroviral integration into minichromosomes in vitro. EMBO J. 1992, 11, 291–303. [Google Scholar] [CrossRef]
- Pryciak, P.M.; Varmus, H.E. Nucleosomes, DNA-binding proteins, and DNA sequence modulate retroviral integration target site selection. Cell 1992, 69, 769–780. [Google Scholar] [CrossRef] [PubMed]
- Muller, H.P.; Varmus, H.E. DNA bending creates favored sites for retroviral integration: an explanation for preferred insertion sites in nucleosomes. EMBO J. 1994, 13, 4704–4714. [Google Scholar] [CrossRef]
- Pruss, D.; Reeves, R.; Bushman, F.D.; Wolffe, A.P. The influence of DNA and nucleosome structure on integration events directed by HIV integrase. J. Biol. Chem. 1994, 269, 25031–25041. [Google Scholar] [CrossRef] [PubMed]
- Scherdin, U.; Rhodes, K.; Breindl, M. Transcriptionally active genome regions are preferred targets for retrovirus integration. J. Virol. 1990, 64, 907–912. [Google Scholar] [CrossRef] [PubMed]
- Mooslehner, K.; Karls, U.; Harbers, K. Retroviral integration sites in transgenic Mov mice frequently map in the vicinity of transcribed DNA regions. J. Virol. 1990, 64, 3056–3058. [Google Scholar] [CrossRef] [PubMed]
- Rohdewohld, H.; Weiher, H.; Reik, W.; Jaenisch, R.; Breindl, M. Retrovirus integration and chromatin structure: Moloney murine leukemia proviral integration sites map near DNase I-hypersensitive sites. J. Virol. 1987, 61, 336–343. [Google Scholar] [CrossRef]
- Vijaya, S.; Steffen, D.L.; Robinson, H.L. Acceptor sites for retroviral integrations map near DNase I-hypersensitive sites in chromatin. J. Virol. 1986, 60, 683–692. [Google Scholar] [CrossRef]
- Hacein-Bey-Abina, S.; Le Deist, F.; Carlier, F.; Bouneaud, C.; Hue, C.; De Villartay, J.P.; Thrasher, A.J.; Wulffraat, N.; Sorensen, R.; Dupuis-Girod, S.; et al. Sustained correction of X-linked severe combined immunodeficiency by ex vivo gene therapy. N. Engl. J. Med. 2002, 346, 1185–1193. [Google Scholar] [CrossRef]
- Hacein-Bey-Abina, S.; von Kalle, C.; Schmidt, M.; Le Deist, F.; Wulffraat, N.; McIntyre, E.; Radford, I.; Villeval, J.L.; Fraser, C.C.; Cavazzana-Calvo, M.; et al. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N. Engl. J. Med. 2003, 348, 255–256. [Google Scholar] [CrossRef]
- Hacein-Bey-Abina, S.; Von Kalle, C.; Schmidt, M.; McCormack, M.P.; Wulffraat, N.; Leboulch, P.; Lim, A.; Osborne, C.S.; Pawliuk, R.; Morillon, E.; et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science 2003, 302, 415–419. [Google Scholar] [CrossRef]
- Hacein-Bey-Abina, S.; Garrigue, A.; Wang, G.P.; Soulier, J.; Lim, A.; Morillon, E.; Clappier, E.; Caccavelli, L.; Delabesse, E.; Beldjord, K.; et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J. Clin. Invest. 2008, 118, 3132–3142. [Google Scholar] [CrossRef] [PubMed]
- Howe, S.J.; Mansour, M.R.; Schwarzwaelder, K.; Bartholomae, C.; Hubank, M.; Kempski, H.; Brugman, M.H.; Pike-Overzet, K.; Chatters, S.J.; de Ridder, D.; et al. Insertional mutagenesis combined with acquired somatic mutations causes leukemogenesis following gene therapy of SCID-X1 patients. J. Clin. Invest. 2008, 118, 3143–3150. [Google Scholar] [CrossRef] [PubMed]
- Hematti, P.; Hong, B.K.; Ferguson, C.; Adler, R.; Hanawa, H.; Sellers, S.; Holt, I.E.; Eckfeldt, C.E.; Sharma, Y.; Schmidt, M.; et al. Distinct genomic integration of MLV and SIV vectors in primate hematopoietic stem and progenitor cells. PLoS Biol. 2004, 2, e423. [Google Scholar] [CrossRef] [PubMed]
- Themis, M.; Waddington, S.N.; Schmidt, M.; von Kalle, C.; Wang, Y.; Al-Allaf, F.; Gregory, L.G.; Nivsarkar, M.; Themis, M.; Holder, M.V.; et al. Oncogenesis following delivery of a nonprimate lentiviral gene therapy vector to fetal and neonatal mice. Mol. Ther. 2005, 12, 763–771. [Google Scholar] [CrossRef]
- Lewinski, M.K.; Yamashita, M.; Emerman, M.; Ciuffi, A.; Marshall, H.; Crawford, G.; Collins, F.; Shinn, P.; Leipzig, J.; Hannenhalli, S. Retroviral DNA integration: Viral and cellular determinants of target-site selection. PLoS Pathog. 2006, 2, e60. [Google Scholar] [CrossRef] [PubMed]
- Engelman, A.; Cherepanov, P. The lentiviral integrase binding protein LEDGF/p75 and HIV-1 replication. PLoS Pathog. 2008, 4, e1000046. [Google Scholar] [CrossRef] [PubMed]
- Vandekerckhove, L.; Christ, F.; Van Maele, B.; De Rijck, J.; Gijsbers, R.; Van den Haute, C.; Witvrouw, M.; Debyser, Z. Transient and stable knockdown of the integrase cofactor LEDGF/p75 reveals its role in the replication cycle of human immunodeficiency virus. J. Virol. 2006, 80, 1886–1896. [Google Scholar] [CrossRef]
- Llano, M.; Delgado, S.; Vanegas, M.; Poeschla, E.M. Lens epithelium-derived growth factor/p75 prevents proteasomal degradation of HIV-1 integrase. J. Biol. Chem. 2004, 279, 55570–55577. [Google Scholar] [CrossRef]
- Busschots, K.; Vercammen, J.; Emiliani, S.; Benarous, R.; Engelborghs, Y.; Christ, F.; Debyser, Z. The interaction of LEDGF/p75 with integrase is lentivirus-specific and promotes DNA binding. J. Biol. Chem. 2005, 280, 17841–17847. [Google Scholar] [CrossRef]
- Emiliani, S.; Mousnier, A.; Busschots, K.; Maroun, M.; Van Maele, B.; Tempe, D.; Vandekerckhove, L.; Moisant, F.; Ben-Slama, L.; Witvrouw, M. Integrase mutants defective for interaction with LEDGF/p75 are impaired in chromosome tethering and HIV-1 replication. J. Biol. Chem. 2005, 280, 25517–25523. [Google Scholar] [CrossRef]
- Ciuffi, A.; Llano, M.; Poeschla, E.; Hoffmann, C.; Leipzig, J.; Shinn, P.; Ecker, J.R.; Bushman, F. A role for LEDGF/p75 in targeting HIV DNA integration. Nat. Med. 2005, 11, 1287–1289. [Google Scholar] [CrossRef] [PubMed]
- Gijsbers, R.; Ronen, K.; Vets, S.; Malani, N.; De Rijck, J.; McNeely, M.; Bushman, F.D.; Debyser, Z. LEDGF hybrids efficiently retarget lentiviral integration into heterochromatin. Mol. Ther. 2010, 18, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Marshall, H.M.; Ronen, K.; Berry, C.; Llano, M.; Sutherland, H.; Saenz, D.; Bickmore, W.; Poeschla, E.; Bushman, F.D. Role of PSIP1/LEDGF/p75 in lentiviral infectivity and integration targeting. PLoS ONE 2007, 2, e1340. [Google Scholar] [CrossRef] [PubMed]
- Shun, M.C.; Raghavendra, N.K.; Vandegraaff, N.; Daigle, J.E.; Hughes, S.; Kellam, P.; Cherepanov, P.; Engelman, A. LEDGF/p75 functions downstream from preintegration complex formation to effect gene-specific HIV-1 integration. Gene. Develop. 2007, 21, 1767–1778. [Google Scholar] [CrossRef]
- Bartholomae, C.C.; Arens, A.; Balaggan, K.S.; Yanez-Munoz, R.J.; Montini, E.; Howe, S.J.; Paruzynski, A.; Korn, B.; Appelt, J.U.; Macneil, A. Lentiviral Vector Integration Profiles Differ in Rodent Postmitotic Tissues. Mol. Ther. 2011, 19, 703–710. [Google Scholar] [CrossRef]
- Hendrix, J.; Gijsbers, R.; De Rijck, J.; Voet, A.; Hotta, J.; McNeely, M.; Hofkens, J.; Debyser, Z.; Engelborghs, Y. The transcriptional co-activator LEDGF/p75 displays a dynamic scan-and-lock mechanism for chromatin tethering. Nucl. Acid. Res. 2011, 39, 1310–1325. [Google Scholar] [CrossRef]
- Silvers, R.M.; Smith, J.A.; Schowalter, M.; Litwin, S.; Liang, Z.; Geary, K.; Daniel, R. Modification of integration site preferences of an HIV-1-based vector by expression of a novel synthetic protein. Hum. Gene. Ther. 2010, 21, 337–349. [Google Scholar] [CrossRef]
- Meehan, A.M.; Poeschla, E.M. Chromatin tethering and retroviral integration: recent discoveries and parallels with DNA viruses. Biochim. Biophys. Acta 2010, 1799, 182–191. [Google Scholar] [CrossRef]
- Ferris, A.L.; Wu, X.; Hughes, C.M.; Stewart, C.; Smith, S.J.; Milne, T.A.; Wang, G.G.; Shun, M.C.; Allis, C.D.; Engelman, A.; Hughes, S.H. Lens epithelium-derived growth factor fusion proteins redirect HIV-1 DNA integration. Proc. Natl. Acad. Sci. USA 2010, 107, 3135–3140. [Google Scholar] [CrossRef]
- Meekings, K.N.; Leipzig, J.; Bushman, F.D.; Taylor, G.P.; Bangham, C.R. HTLV-1 integration into transcriptionally active genomic regions is associated with proviral expression and with HAM/TSP. PLoS Pathog. 2008, 4, e1000027. [Google Scholar] [CrossRef]
- Derse, D.; Crise, B.; Li, Y.; Princler, G.; Lum, N.; Stewart, C.; McGrath, C.F.; Hughes, S.H.; Munroe, D.J.; Wu, X. Human T-cell leukemia virus type 1 integration target sites in the human genome: comparison with those of other retroviruses. J. Virol. 2007, 81, 6731–6741. [Google Scholar] [CrossRef] [PubMed]
- Maxfield, L.F.; Fraize, C.D.; Coffin, J.M. Relationship between retroviral DNA-integration-site selection and host cell transcription. Proc. Nat. Acad. Sci. USA 2005, 102, 1436–1441. [Google Scholar] [CrossRef]
- Lewinski, M.K.; Bisgrove, D.; Shinn, P.; Chen, H.; Hoffmann, C.; Hannenhalli, S.; Verdin, E.; Berry, C.C.; Ecker, J.R.; Bushman, F.D. Genome-wide analysis of chromosomal features repressing human immunodeficiency virus transcription. J. Virol. 2005, 79, 6610–6619. [Google Scholar] [CrossRef] [PubMed]
- Taganov, K.D.; Cuesta, I.; Daniel, R.; Cirillo, L.A.; Katz, R.A.; Zaret, K.S.; Skalka, A.M. Integrase-specific enhancement and suppression of retroviral DNA integration by compacted chromatin structure in vitro. J. Virol. 2004, 78, 5848–5855. [Google Scholar] [CrossRef] [PubMed]
- Felice, B.; Cattoglio, C.; Cittaro, D.; Testa, A.; Miccio, A.; Ferrari, G.; Luzi, L.; Recchia, A.; Mavilio, F. Transcription factor binding sites are genetic determinants of retroviral integration in the human genome. PLoS ONE 2009, 4, e4571. [Google Scholar] [CrossRef] [PubMed]
- Biasco, L.; Ambrosi, A.; Pellin, D.; Bartholomae, C.; Brigida, I.; Roncarolo, M.G.; Di Serio, C.; von Kalle, C.; Schmidt, M.; Aiuti, A. Integration profile of retroviral vector in gene therapy treated patients is cell-specific according to gene expression and chromatin conformation of target cell. EMBO Mol. Med. 2011, 3, 89–101. [Google Scholar] [CrossRef]
- Baum, C. Parachuting in the epigenome: the biology of gene vector insertion profiles in the context of clinical trials. EMBO Mol. Med. 2011, 3, 75–77. [Google Scholar] [CrossRef]
- Baum, C.; Dullmann, J.; Li, Z.; Fehse, B.; Meyer, J.; Williams, D.A.; von Kalle, C. Side effects of retroviral gene transfer into hematopoietic stem cells. Blood 2003, 101, 2099–2114. [Google Scholar] [CrossRef]
- Montini, E.; Cesana, D.; Schmidt, M.; Sanvito, F.; Bartholomae, C.C.; Ranzani, M.; Benedicenti, F.; Sergi, L.S.; Ambrosi, A.; Ponzoni, M.; et al. The genotoxic potential of retroviral vectors is strongly modulated by vector design and integration site selection in a mouse model of HSC gene therapy. J. Clin. Invest. 2009, 119, 964–975. [Google Scholar] [CrossRef]
- Maruggi, G.; Porcellini, S.; Facchini, G.; Perna, S.K.; Cattoglio, C.; Sartori, D.; Ambrosi, A.; Schambach, A.; Baum, C.; Bonini, C.; et al. Transcriptional enhancers induce insertional gene deregulation independently from the vector type and design. Mol. Ther. 2009, 17, 851–856. [Google Scholar] [CrossRef]
- Modlich, U.; Bohne, J.; Schmidt, M.; von Kalle, C.; Knoss, S.; Schambach, A.; Baum, C. Cell-culture assays reveal the importance of retroviral vector design for insertional genotoxicity. Blood 2006, 108, 2545–2553. [Google Scholar] [CrossRef] [PubMed]
- Lund, A.H.; Turner, G.; Trubetskoy, A.; Verhoeven, E.; Wientjens, E.; Hulsman, D.; Russell, R.; DePinho, R.A.; Lenz, J.; van Lohuizen, M. Genome-wide retroviral insertional tagging of genes involved in cancer in Cdkn2a-deficient mice. Nat. Genet. 2002, 32, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Montini, E.; Cesana, D.; Schmidt, M.; Sanvito, F.; Ponzoni, M.; Bartholomae, C.; Sergi, L.S.; Benedicenti, F.; Ambrosi, A.; Di Serio, C. Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration. Nat. Biotechnol. 2006, 24, 687–696. [Google Scholar] [CrossRef]
- Bauer, T.R. Jr.; Allen, J.M.; Hai, M.; Tuschong, L.M.; Khan, I.F.; Olson, E.M.; Adler, R.L.; Burkholder, T.H.; Gu, Y.C.; Russell, D.W. Successful treatment of canine leukocyte adhesion deficiency by foamy virus vectors. Nat. Med. 2008, 14, 93–97. [Google Scholar] [CrossRef]
- Li, J.; Shen, H.; Himmel, K.L.; Dupuy, A.J.; Largaespada, D.A.; Nakamura, T.; Shaughnessy, J.D.Jr.; Jenkins, N.A.; Copeland, N.G. Leukaemia disease genes: large-scale cloning and pathway predictions. Nat. Genet. 1999, 23, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, G.P.; Steigerwald, S.D.; Mueller, P.R.; Wold, B.; Riggs, A.D. Genomic sequencing and methylation analysis by ligation mediated PCR. Science 1989, 246, 810–813. [Google Scholar] [CrossRef]
- Mueller, P.R.; Wold, B. In vivo footprinting of a muscle specific enhancer by ligation mediated PCR. Science 1989, 246, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Silver, J.; Keerikatte, V. Novel use of polymerase chain reaction to amplify cellular DNA adjacent to an integrated provirus. J. Virol. 1989, 63, 1924–1928. [Google Scholar] [CrossRef]
- Schmidt, M.; Schwarzwaelder, K.; Bartholomae, C.; Zaoui, K.; Ball, C.; Pilz, I.; Braun, S.; Glimm, H.; von Kalle, C. High-resolution insertion-site analysis by linear amplification-mediated PCR (LAM-PCR). Nat. Methods 2007, 4, 1051–1057. [Google Scholar] [CrossRef]
- Schmidt, M.; Hoffmann, G.; Wissler, M.; Lemke, N.; Mussig, A.; Glimm, H.; Williams, D.A.; Ragg, S.; Hesemann, C.U.; von Kalle, C. Detection and direct genomic sequencing of multiple rare unknown flanking DNA in highly complex samples. Hum. Gene. Ther. 2001, 12, 743–749. [Google Scholar] [CrossRef]
- Schmidt, M.; Carbonaro, D.A.; Speckmann, C.; Wissler, M.; Bohnsack, J.; Elder, M.; Aronow, B.J.; Nolta, J.A.; Kohn, D.B.; von Kalle, C. Clonality analysis after retroviral-mediated gene transfer to CD34+ cells from the cord blood of ADA-deficient SCID neonates. Nat. Med. 2003, 9, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, R.; Eckenberg, R.; Paruzynski, A.; Bartholomae, C.C.; Nowrouzi, A.; Arens, A.; Howe, S.J.; Recchia, A.; Cattoglio, C.; Wang, W.; et al. Comprehensive genomic access to vector integration in clinical gene therapy. Nat. Med. 2009, 15, 1431–1436. [Google Scholar] [CrossRef] [PubMed]
- Paruzynski, A.; Arens, A.; Gabriel, R.; Bartholomae, C.C.; Scholz, S.; Wang, W.; Wolf, S.; Glimm, H.; Schmidt, M.; von Kalle, C. Genome-wide high-throughput integrome analyses by nrLAM-PCR and next-generation sequencing. Nat. Protocol. 2010, 5, 1379–1395. [Google Scholar] [CrossRef] [PubMed]
- Warlich, E.; Kuehle, J.; Cantz, T.; Brugman, M.H.; Maetzig, T.; Galla, M.; Filipczyk, A.A.; Halle, S.; Klump, H.; Scholer, H.R.; et al. Lentiviral Vector Design and Imaging Approaches to Visualize the Early Stages of Cellular Reprogramming. Mol. Ther. 2011, 19, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Schwarzwaelder, K.; Bartholomae, C.C.; Glimm, H.; von Kalle, C. Detection of retroviral integration sites by linear amplification-mediated PCR and tracking of individual integration clones in different samples. Meth. Mol. Biol. 2009, 506, 363–372. [Google Scholar]
- Cartier, N.; Hacein-Bey-Abina, S.; Bartholomae, C.C.; Veres, G.; Schmidt, M.; Kutschera, I.; Vidaud, M.; Abel, U.; Dal-Cortivo, L.; Caccavelli, L.; et al. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science 2009, 326, 818–823. [Google Scholar] [CrossRef]
- Schwarzwaelder, K.; Howe, S.J.; Schmidt, M.; Brugman, M.H.; Deichmann, A.; Glimm, H.; Schmidt, S.; Prinz, C.; Wissler, M.; King, D.J.; et al. Gammaretrovirus-mediated correction of SCID-X1 is associated with skewed vector integration site distribution in vivo. J. Clin. Invest. 2007, 117, 2241–2249. [Google Scholar] [CrossRef]
- Deichmann, A.; Hacein-Bey-Abina, S.; Schmidt, M.; Garrigue, A.; Brugman, M.H.; Hu, J.; Glimm, H.; Gyapay, G.; Prum, B.; Fraser, C.C.; et al. Vector integration is nonrandom and clustered and influences the fate of lymphopoiesis in SCID-X1 gene therapy. J. Clin. Invest. 2007, 117, 2225–2232. [Google Scholar] [CrossRef]
- Ott, M.G.; Schmidt, M.; Schwarzwaelder, K.; Stein, S.; Siler, U.; Koehl, U.; Glimm, H.; Kuhlcke, K.; Schilz, A.; Kunkel, H.; et al. Correction of X-linked chronic granulomatous disease by gene therapy, augmented by insertional activation of MDS1-EVI1, PRDM16 or SETBP1. Nat. Med. 2006, 12, 401–409. [Google Scholar] [CrossRef]
- Boztug, K.; Schmidt, M.; Schwarzer, A.; Banerjee, P.P.; Diez, I.A.; Dewey, R.A.; Bohm, M.; Nowrouzi, A.; Ball, C.R.; Glimm, H.; et al. Stem-cell gene therapy for the Wiskott-Aldrich syndrome. N. Engl. J. Med. 2010, 363, 1918–1927. [Google Scholar] [CrossRef]
- Wang, G.P.; Garrigue, A.; Ciuffi, A.; Ronen, K.; Leipzig, J.; Berry, C.; Lagresle-Peyrou, C.; Benjelloun, F.; Hacein-Bey-Abina, S.; Fischer, A.; et al. DNA bar coding and pyrosequencing to analyze adverse events in therapeutic gene transfer. Nucl. Acid. Res. 2008, 36, e49. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.P.; Berry, C.C.; Malani, N.; Leboulch, P.; Fischer, A.; Hacein-Bey-Abina, S.; Cavazzana-Calvo, M.; Bushman, F.D. Dynamics of gene-modified progenitor cells analyzed by tracking retroviral integration sites in a human SCID-X1 gene therapy trial. Blood 2010, 115, 4356–4366. [Google Scholar] [CrossRef] [PubMed]
- Stein, S.; Ott, M.G.; Schultze-Strasser, S.; Jauch, A.; Burwinkel, B.; Kinner, A.; Schmidt, M.; Kramer, A.; Schwable, J.; Glimm, H.; et al. Genomic instability and myelodysplasia with monosomy 7 consequent to EVI1 activation after gene therapy for chronic granulomatous disease. Nat. Med. 2010, 16, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Cavazzana-Calvo, M.; Payen, E.; Negre, O.; Wang, G.; Hehir, K.; Fusil, F.; Down, J.; Denaro, M.; Brady, T.; Westerman, K.; et al. Transfusion independence and HMGA2 activation after gene therapy of human beta-thalassaemia. Nature 2010, 467, 318–322. [Google Scholar] [CrossRef]
- Payne, G.S.; Bishop, J.M.; Varmus, H.E. Multiple arrangements of viral DNA and an activated host oncogene in bursal lymphomas. Nature 1982, 295, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Payne, G.S.; Courtneidge, S.A.; Crittenden, L.B.; Fadly, A.M.; Bishop, J.M.; Varmus, H.E. Analysis of avian leukosis virus DNA and RNA in bursal tumours: viral gene expression is not required for maintenance of the tumor state. Cell 1981, 23, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Neel, B.G.; Hayward, W.S.; Robinson, H.L.; Fang, J.; Astrin, S.M. Avian leukosis virus-induced tumors have common proviral integration sites and synthesize discrete new RNAs: oncogenesis by promoter insertion. Cell 1981, 23, 323–334. [Google Scholar] [CrossRef]
- Hayward, W.S.; Neel, B.G.; Astrin, S.M. Activation of a cellular onc gene by promoter insertion in ALV-induced lymphoid leukosis. Nature 1981, 290, 475–480. [Google Scholar] [CrossRef]
- Kool, J.; Berns, A. High-throughput insertional mutagenesis screens in mice to identify oncogenic networks. Nat. Rev. Canc. 2009, 9, 389–399. [Google Scholar] [CrossRef]
- Rosenberg, N.; Jolicoeur, P. Retroviral pathogenesis. In Retroviruses; Coffin, J.M., Hughes, S.H., Varmus, E.H., Eds.; ColdSpring Harbor Laboratory Press: New York, NY, USA, 1997; pp. S475–S586. [Google Scholar]
- Moolten, F.L.; Cupples, L.A. A model for predicting the risk of cancer consequent to retroviral gene therapy. Hum. Gene. Ther. 1992, 3, 479–486. [Google Scholar] [CrossRef]
- Li, Z.; Dullmann, J.; Schiedlmeier, B.; Schmidt, M.; von Kalle, C.; Meyer, J.; Forster, M.; Stocking, C.; Wahlers, A.; Frank, O.; Ostertag, W.; et al. Murine leukemia induced by retroviral gene marking. Science 2002, 296, 497. [Google Scholar] [CrossRef] [PubMed]
- Modlich, U.; Kustikova, O.S.; Schmidt, M.; Rudolph, C.; Meyer, J.; Li, Z.; Kamino, K.; von Neuhoff, N.; Schlegelberger, B.; Kuehlcke, K.; et al. Leukemias following retroviral transfer of multidrug resistance 1 (MDR1) are driven by combinatorial insertional mutagenesis. Blood 2005, 105, 4235–4246. [Google Scholar] [CrossRef]
- Calmels, B.; Ferguson, C.; Laukkanen, M.O.; Adler, R.; Faulhaber, M.; Kim, H.J.; Sellers, S.; Hematti, P.; Schmidt, M.; von Kalle, C.; et al. Recurrent retroviral vector integration at the Mds1/Evi1 locus in nonhuman primate hematopoietic cells. Blood 2005, 106, 2530–2533. [Google Scholar] [CrossRef] [PubMed]
- Kustikova, O.; Fehse, B.; Modlich, U.; Yang, M.; Dullmann, J.; Kamino, K.; von Neuhoff, N.; Schlegelberger, B.; Li, Z.; Baum, C. Clonal dominance of hematopoietic stem cells triggered by retroviral gene marking. Science 2005, 308, 1171–1174. [Google Scholar] [CrossRef] [PubMed]
- Seggewiss, R.; Pittaluga, S.; Adler, R.L.; Guenaga, F.J.; Ferguson, C.; Pilz, I.H.; Ryu, B.; Sorrentino, B.P.; Young, W.S.; Donahue, R.E.; et al. Acute myeloid leukemia is associated with retroviral gene transfer to hematopoietic progenitor cells in a rhesus macaque. Blood 2006, 107, 3865–3867. [Google Scholar] [CrossRef]
- Aiuti, A.; Cattaneo, F.; Galimberti, S.; Benninghoff, U.; Cassani, B.; Callegaro, L.; Scaramuzza, S.; Andolfi, G.; Mirolo, M.; Brigida, I.; et al. Gene therapy for immunodeficiency due to adenosine deaminase deficiency. N. Engl. J. Med. 2009, 360, 447–458. [Google Scholar] [CrossRef]
- Woods, N.B.; Bottero, V.; Schmidt, M.; von Kalle, C.; Verma, I.M. Gene therapy: Therapeutic gene causing lymphoma. Nature 2006, 440, 1123. [Google Scholar] [CrossRef]
- Thrasher, A.J.; Gaspar, H.B.; Baum, C.; Modlich, U.; Schambach, A.; Candotti, F.; Otsu, M.; Sorrentino, B.; Scobie, L.; Cameron, E.; et al. Gene therapy: X-SCID transgene leukaemogenicity. Nature 2006, 443, E5–E6; discussion E6–E7. [Google Scholar] [CrossRef]
- Dave, U.P.; Akagi, K.; Tripathi, R.; Cleveland, S.M.; Thompson, M.A.; Yi, M.; Stephens, R.; Downing, J.R.; Jenkins, N.A.; Copeland, N.G. Murine leukemias with retroviral insertions at Lmo2 are predictive of the leukemias induced in SCID-X1 patients following retroviral gene therapy. PLoS Genet. 2009, 5, e1000491. [Google Scholar] [CrossRef]
- Cartier, N.; Aubourg, P. Hematopoietic stem cell transplantation and hematopoietic stem cell gene therapy in X-linked adrenoleukodystrophy. Brain Pathol. 2010, 20, 857–862. [Google Scholar] [CrossRef]
- Montini, E.; Cesana, D.; Schmidt, M.; Sanvito, F.; Ponzoni, M.; Bartholomae, C.; Sergi Sergi, L.; Benedicenti, F.; Ambrosi, A.; Di Serio, C.; et al. Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration. Nat. Biotechnol. 2006, 24, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Modlich, U.; Navarro, S.; Zychlinski, D.; Maetzig, T.; Knoess, S.; Brugman, M.H.; Schambach, A.; Charrier, S.; Galy, A.; Thrasher, A.J.; et al. Insertional transformation of hematopoietic cells by self-inactivating lentiviral and gammaretroviral vectors. Mol. Ther. 2009, 17, 1919–1928. [Google Scholar] [CrossRef] [PubMed]
- Daya, S.; Berns, K.I. Gene therapy using adeno-associated virus vectors. Clin. Microbiol. Rev. 2008, 21, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Russell, D.W. AAV vectors, insertional mutagenesis, and cancer. Mol. Ther. 2007, 15, 1740–1743. [Google Scholar] [CrossRef]
- Donsante, A.; Miller, D.G.; Li, Y.; Vogler, C.; Brunt, E.M.; Russell, D.W.; Sands, M.S. AAV vector integration sites in mouse hepatocellular carcinoma. Science 2007, 317, 477. [Google Scholar] [CrossRef] [PubMed]
- Wanisch, K.; Yanez-Munoz, R.J. Integration-deficient lentiviral vectors: A slow coming of age. Mol. Ther. 2009, 17, 1316–1332. [Google Scholar] [CrossRef]
- Yanez-Munoz, R.J.; Balaggan, K.S.; MacNeil, A.; Howe, S.J.; Schmidt, M.; Smith, A.J.; Buch, P.; MacLaren, R.E.; Anderson, P.N.; Barker, S.E.; et al. Effective gene therapy with nonintegrating lentiviral vectors. Nat. Med. 2006, 12, 348–353. [Google Scholar] [CrossRef]
- Bushman, F. Targeting retroviral integration? Mol. Ther. 2002, 6, 570–571. [Google Scholar] [CrossRef]
- Urnov, F.D.; Rebar, E.J.; Holmes, M.C.; Zhang, H.S.; Gregory, P.D. Genome editing with engineered zinc finger nucleases. Nat. Rev. Genet. 2010, 11, 636–646. [Google Scholar] [CrossRef]
- Ivics, Z.; Izsvak, Z. The expanding universe of transposon technologies for gene and cell engineering. Mob. DNA 2010, 1, 25. [Google Scholar] [CrossRef]
- Copeland, N.G.; Jenkins, N.A. Harnessing transposons for cancer gene discovery. Nat. Rev. Canc. 2010, 10, 696–706. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, S.; Blau, H.M. Nuclear reprogramming to a pluripotent state by three approaches. Nature 2010, 465, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Vierbuchen, T.; Ostermeier, A.; Pang, Z.P.; Kokubu, Y.; Sudhof, T.C.; Wernig, M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature 2010, 463, 1035–1041. [Google Scholar] [CrossRef]
- Szabo, E.; Rampalli, S.; Risueno, R.M.; Schnerch, A.; Mitchell, R.; Fiebig-Comyn, A.; Levadoux-Martin, M.; Bhatia, M. Direct conversion of human fibroblasts to multilineage blood progenitors. Nature 2010, 468, 521–526. [Google Scholar] [CrossRef]
- Ieda, M.; Fu, J.D.; Delgado-Olguin, P.; Vedantham, V.; Hayashi, Y.; Bruneau, B.G.; Srivastava, D. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell 2010, 142, 375–386. [Google Scholar] [CrossRef]
- Papapetrou, E.P.; Lee, G.; Malani, N.; Setty, M.; Riviere, I.; Tirunagari, L.M.; Kadota, K.; Roth, S.L.; Giardina, P.; Viale, A.; et al. Genomic safe harbors permit high beta-globin transgene expression in thalassemia induced pluripotent stem cells. Nat. Biotechnol. 2011, 29, 73–78. [Google Scholar] [CrossRef]
- Yamanaka, S. Strategies and new developments in the generation of patient-specific pluripotent stem cells. Cell Stem. Cell 2007, 1, 39–49. [Google Scholar] [CrossRef]
- Ellis, J.; Baum, C.; Benvenisty, N.; Mostoslavsky, G.; Okano, H.; Stanford, W.L.; Porteus, M.; Sadelain, M. Benefits of utilizing gene-modified iPSCs for clinical applications. Cell Stem. Cell 2010, 7, 429–430. [Google Scholar] [CrossRef]
- Suzuki, T.; Shen, H.; Akagi, K.; Morse, H.C.; Malley, J.D.; Naiman, D.Q.; Jenkins, N.A.; Copeland, N.G. New genes involved in cancer identified by retroviral tagging. Nat. Genet. 2002, 32, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Akagi, K.; Suzuki, T.; Stephens, R.M.; Jenkins, N.A.; Copeland, N.G. RTCGD: Retroviral tagged cancer gene database. Nucl. Acid. Res. 2004, 32, D523–D527. [Google Scholar] [CrossRef] [PubMed]
- Starr, T.K.; Allaei, R.; Silverstein, K.A.; Staggs, R.A.; Sarver, A.L.; Bergemann, T.L.; Gupta, M.; O’Sullivan, M.G.; Matise, I.; Dupuy, A.J.; et al. A transposon-based genetic screen in mice identifies genes altered in colorectal cancer. Science 2009, 323, 1747–1750. [Google Scholar] [CrossRef] [PubMed]
- Dupuy, A.J.; Rogers, L.M.; Kim, J.; Nannapaneni, K.; Starr, T.K.; Liu, P.; Largaespada, D.A.; Scheetz, T.E.; Jenkins, N.A.; Copeland, N.G. A modified sleeping beauty transposon system that can be used to model a wide variety of human cancers in mice. Canc. Res. 2009, 69, 8150–8156. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Spence, S.E.; Jenkins, N.A.; Copeland, N.G. Cooperating cancer-gene identification through oncogenic-retrovirus-induced insertional mutagenesis. Blood 2005, 106, 2498–2505. [Google Scholar] [CrossRef]
- Du, Y.; Jenkins, N.A.; Copeland, N.G. Insertional mutagenesis identifies genes that promote the immortalization of primary bone marrow progenitor cells. Blood 2005, 106, 3932–3939. [Google Scholar] [CrossRef]
- Copeland, N.G.; Jenkins, N.A. Retroviral integration in murine myeloid tumors to identify Evi-1, a novel locus encoding a zinc-finger protein. Adv. Canc. Res. 1990, 54, 141–157. [Google Scholar]
- Neff, T.; Shotkoski, F.; Stamatoyannopoulos, G. Stem cell gene therapy, position effects and chromatin insulators. Stem Cells 1997, 15, 265–271. [Google Scholar]
- Valenzuela, L.; Kamakaka, R.T. Chromatin insulators. Annu. Rev. Genet. 2006, 40, 107–138. [Google Scholar] [CrossRef]
- Arumugam, P.I.; Higashimoto, T.; Urbinati, F.; Modlich, U.; Nestheide, S.; Xia, P.; Fox, C.; Corsinotti, A.; Baum, C.; Malik, P. Genotoxic potential of lineage-specific lentivirus vectors carrying the beta-globin locus control region. Mol. Ther. 2009, 17, 1929–1937. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2011 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).