Walleye Dermal Sarcoma Virus: Molecular Biology and Oncogenesis
Abstract
:1. Introduction
2. Epsilonretroviruses and associated diseases
2.1. Disease, pathology and transmission
2.2. Molecular characterization of Epsilonretroviruses.
2.3. WDSV accessory protein function
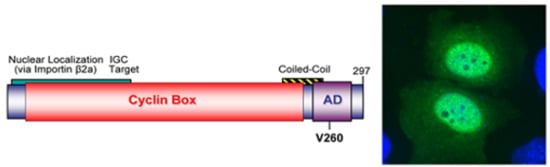
2.3.1. WDSV rv-cyclin
2.3.2. WDSV Orf B.
2.3.3. WDSV Orf C.
3. Conclusions
Acknowledgements
References and Notes
- Quackenbush, S.L.; Casey, J.W. Cornell University: Ithaca, NY, USA, Unpublished work. 1998.
- Poulet, F.M.; Bowser, P.R.; Casey, J.W. Retroviruses of fish, reptiles, and molluscs. In The Retroviridae; Levy, J.A., Ed.; Plenum Press: New York, USA, 1994; Volume 3, pp. 1–38. [Google Scholar]
- Rovnak, J.; Casey, R.N.; Brewster, C.D.; Casey, J.W.; Quackenbush, S.L. Establishment of productively infected walleye dermal sarcoma explant cells. J. Gen. Virol. 2007, 88, 2583–2589. [Google Scholar] [CrossRef] [PubMed]
- Hart, D.; Frerichs, G.N.; Rambaut, A.; Onions, D.E. Complete nucleotide sequence and transcriptional analysis of the snakehead fish retrovirus. J. Virol. 1996, 70, 3606–3616. [Google Scholar] [CrossRef]
- Holzschu, D.L.; Martineau, D.; Fodor, S.K.; Vogt, V.M.; Bowser, P.R.; Casey, J.W. Nucleotide sequence and protein analysis of a complex piscine retrovirus, walleye dermal sarcoma virus. J. Virol. 1995, 69, 5320–5331. [Google Scholar] [CrossRef]
- LaPierre, L.A.; Holzschu, D.L.; Bowser, P.R.; Casey, J.W. Sequence and transcriptional analyses of the fish retroviruses walleye epidermal hyperplasia virus types 1 and 2: Evidence for a gene duplication. J. Virol. 1999, 73, 9393–9403. [Google Scholar] [CrossRef] [PubMed]
- Paul, T.A.; Quackenbush, S.L.; Sutton, C.; Casey, R.N.; Bowser, P.R.; Casey, J.W. Identification and characterization of an exogenous retrovirus from atlantic salmon swim bladder sarcomas. J. Virol. 2006, 80, 2941–2948. [Google Scholar] [CrossRef] [PubMed]
- Duncan, I.B. Evidence for an oncovirus in the swim bladder fibrosarcoma of Atlantic salmon Salmo salar L. J. Fish Dis. 1978, 1, 127–131. [Google Scholar] [CrossRef]
- McKnight, I.J. Sarcoma of the swim bladder of Atlantic salmon (Salmo salar L.). Aquaculture 1978, 13, 55–60. [Google Scholar] [CrossRef]
- Frerichs, G.N.; Morgan, D.; Hart, D.; Skerrow, C.; Roberts, R.J.; Onions, D.E. Spontaneously productive C-type retrovirus infection of fish cell lines. J. Gen. Virol. 1991, 72, 2537–2539. [Google Scholar] [CrossRef]
- LaPierre, L.A.; Holzschu, D.L.; Wooster, G.A.; Bowser, P.R.; Casey, J.W. Two closely related but distinct retroviruses are associated with walleye discrete epidermal hyperplasia. J. Virol. 1998, 72, 3484–3490. [Google Scholar] [CrossRef]
- Martineau, D.; Bowser, P.R.; Renshaw, R.R.; Casey, J.W. Molecular characterization of a unique retrovirus associated with a fish tumor. J. Virol. 1992, 66, 596–599. [Google Scholar] [CrossRef]
- Martineau, D.; Renshaw, R.; Williams, J.R.; Casey, J.W.; Bowser, P.R. A large unintegrated retrovirus DNA species present in a dermal tumor of walleye Stizostedion vitreum. Dis. Aquat. Org. 1991, 10, 153–158. [Google Scholar] [CrossRef]
- Walker, R. Virus associated with epidermal hyperplasia in fish. Natl Cancer Inst Monogr. 1969, 31, 195–207. [Google Scholar]
- Yamamoto, T.; Kelly, R.K.; Nielsen, O. Epidermal hyperplasia of walleye, Stizostedion vitreum vitreum (Mitchill), associated with retrovirus-like type-C particles: prevalence, histologic, and electron microscopic observations. J. Fish Dis. 1985, 19, 425–436. [Google Scholar] [CrossRef]
- Yamamoto, T.; Kelly, R.K.; Nielsen, O. Morphological differentiation of virus-associated skin tumors of walleye (Stizostedion vitreum vitreum). Fish Pathol. 1985, 20, 361–372. [Google Scholar] [CrossRef]
- Bowser, P.R.; Earnest-Koons, K.A.; Wooster, G.A.; LaPierre, L.A.; Holzschu, D.L.; Casey, J.W. Experimental transmission of discrete epidermal hyperplasia in walleyes. J. Aquat. Anim. Health 1998, 10, 282–286. [Google Scholar] [CrossRef]
- Yamamoto, T.; MacDonald, R.D.; Gillespie, D.C.; Kelly, R.K. Viruses associated with lymphocystis and dermal sarcoma of walleye (Stizostedion vitreum vitreum). J. Fish Res. Board Can. 1976, 33, 2408–2419. [Google Scholar] [CrossRef]
- Bowser, P.R.; Wooster, G.A. Regression of dermal sarcoma in adult walleyes (Stizostedion vitreum). J. Aquat. Anim. Health 1991, 3, 147–150. [Google Scholar] [CrossRef]
- Bowser, P.R.; Wolfe, M.J.; Forney, J.L.; Wooster, G.A. Seasonal prevalence of skin tumors from walleye (Stizostedion vitreum) from Oneida Lake, New York. J. Wildl. Dis. 1988, 24, 292–298. [Google Scholar] [CrossRef]
- Martineau, D.; Bowser, P.R.; Wooster, G.A.; Forney, J.L. Histologic and ultrastructural studies of dermal sarcoma of walleye (Pisces: Stizostedion vitreum). Vet. Pathol. 1990, 27, 340–346. [Google Scholar] [CrossRef]
- Poulet, F.M.; Vogt, V.M.; Bowser, P.R.; Casey, J.W. In situ hybridization and immunohistochemical study of walleye dermal sarcoma virus (WDSV) nucleic acids and proteins in spontaneous sarcomas of adult walleyes (Stizostedion vitreum). Vet. Pathol. 1995, 32, 162–172. [Google Scholar] [CrossRef]
- Poulet, F.M.; Bowser, P.R.; Casey, J.W. PCR and RT-PCR analysis of infection and transcriptional activity of walleye dermal sarcoma virus (WDSV) in organs of adult walleyes (Stizotedion vitreum). Vet. Pathol. 1996, 33, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Bowser, P.; Wooster, G.; Getchell, R.; Chen, C.-Y.; Sutton, C.; Casey, J. Naturally occurring invasive walleye dermal sarcoma and attempted experimental transmission of the tumor. J. Aquat. Anim. Health 2002, 14, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Martineau, D.; Bowser, P.R.; Wooster, G.A.; Armstrong, G.A. Experimental transmission of a dermal sarcoma in fingerling walleyes (Stizostedion vitreum vitreum). Vet. Pathol. 1990, 27, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Bowser, P.R.; Wooster, G.A.; Earnest-Koons, K. Effects of fish age and challenge route in experimental transmission of walleye dermal sarcoma in walleyes by cell-free tumor filtrates. J. Aquat. Anim. Health 1997, 9, 274–278. [Google Scholar] [CrossRef]
- Bowser, P.R.; Wooster, G.A.; Quackenbush, S.L.; Casey, R.N.; Casey, J.W. Comparison of fall and spring tumors as inocula for experimental transmission of walleye dermal sarcoma. J. Aquat. Anim. Health 1996, 8, 78–81. [Google Scholar] [CrossRef]
- Earnest-Koons, K.; Wooster, G.A.; Bowser, P.R. Invasive walleye dermal sarcoma in laboratory-maintained walleyes, Stizostedion vitreum. Dis. Aquat. Org. 1996, 24, 227–232. [Google Scholar]
- Bowser, P.R.; Wooster, G.A.; Getchell, R.G.; Paul, T.A.; Casey, R.N.; Casey, J.W. Experimental transmission of walleye dermal sarcoma to yellow perch. J. Aquat. Anim. Health 2001, 13, 214–219. [Google Scholar] [CrossRef]
- Holzschu, D.L.; Wooster, G.A.; Bowser, P.R. Experimental transmission of dermal sarcoma to the sauger Stizostedion canadense. Dis. Aquat. Org. 1998, 32, 9–14. [Google Scholar] [CrossRef]
- Getchell, R.G.; Wooster, G.A.; Bowser, P.R. Temperature-associated regression of walleye dermal sarcoma tumors. J. Aquat. Anim. Health 2000, 12, 189–195. [Google Scholar] [CrossRef]
- Bowser, P.R.; Wooster, G.A.; Getchell, R.G. Transmission of walleye dermal sarcoma and lymphocystis via waterborne exposure. J. Aquat. Anim. Health 1999, 11, 158–161. [Google Scholar] [CrossRef]
- Getchell, R.G.; Wooster, G.A.; Rudstam, L.G.; Van De Valk, A.J.; Brooking, T.E.; Bowser, P.R. Prevalence of walleye dermal sarcoma by age-class in walleyes from Oneida Lake, New York. J. Aquat. Anim. Health 2000, 12, 220–223. [Google Scholar] [CrossRef]
- Getchell, R.G.; Wooster, G.A.; Bowser, P.R. Resistance to walleye drmal sarcoma tumor redevelopment. J. Aquat. Anim. Health 2001, 13, 228–233. [Google Scholar] [CrossRef]
- Claros, M.G.; von Heijne, G. TopPred II: an improved software for membrane protein structure predictions. Comput Appl Biosci 1994, 10, 685–686. [Google Scholar] [CrossRef]
- von Heijne, G. Membrane protein structure prediction. Hydrophobicity analysis and the positive-inside rule. J. Mol. Biol. 1992, 225, 487–494. [Google Scholar] [CrossRef]
- Martineau, D.; Renshaw, R.; Bowser, P.R.; Casey, J.W. In Initial characterization of a retrovirus found in walleyes (Stizostedion vitreum), Second International Symposium of Virus of Lower Vertebrates, Corvallis, Oregon, 1991; Oregon State University Printing Department: Corvallis, Oregon, USA, 1991; pp. 157–163. [Google Scholar]
- Quackenbush, S.L.; Holzschu, D.L.; Bowser, P.R.; Casey, J.W. Transcriptional analysis of walleye dermal sarcoma virus (WDSV). Virology 1997, 237, 107–112. [Google Scholar] [CrossRef]
- Donehower, L.A.; Bohannon, R.C.; Ford, R.J.; Gibbs, R.A. The use of primers from highly conserved pol regions to identify uncharacterized retroviruses by the polymerase chain reaction. J. Virol. Methods 1990, 28, 33–46. [Google Scholar] [CrossRef]
- Hoeppner, S.; Baumli, S.; Cramer, P. Structure of the mediator subunit cyclin C and its implications for CDK8 function. J. Mol. Biol. 2005, 350, 833–842. [Google Scholar] [CrossRef]
- Daniels, C.C.; Rovnak, J.; Quackenbush, S.L. Walleye dermal sarcoma virus Orf B functions through receptor for activated C kinase (RACK1) and protein kinase C. Virology 2008, 375, 550–560. [Google Scholar] [CrossRef]
- Nudson, W.A.; Rovnak, J.; Buechner, M.; Quackenbush, S.L. Walleye dermal sarcoma virus Orf C is targeted to the mitochondria. J. Gen. Virol. 2003, 84, 375–381. [Google Scholar] [CrossRef]
- Rovnak, J.; Casey, J.W.; Quackenbush, S.L. Intracellular targeting of walleye dermal sarcoma virus Orf A (rv-cyclin). Virology 2001, 280, 31–40. [Google Scholar] [CrossRef]
- LaPierre, L.A.; Casey, J.W.; Holzschu, D.L. Walleye retroviruses associated with skin tumors and hyperplasias encode cyclin D homologs. J. Virol. 1998, 72, 8765–8771. [Google Scholar] [CrossRef] [PubMed]
- Rovnak, J.; Hronek, B.W.; Ryan, S.O.; Cai, S.; Quackenbush, S.L. An activation domain within the walleye dermal sarcoma virus retroviral cyclin protein is essential for inhibition of the viral promoter. Virology 2005, 342, 240–251. [Google Scholar] [CrossRef] [PubMed]
- Rovnak, J.; Quackenbush, S.L. Walleye dermal sarcoma virus retroviral cyclin directly contacts TAF9. J. Virol. 2006, 80, 12041–12048. [Google Scholar] [CrossRef] [PubMed]
- Bex, F.; McDowall, A.; Burny, A.; Gaynor, R. The Human T-Cell Leukemia Virus type 1 transactivator protein Tax colocalizes in unique nuclear structures with NF-kB proteins. J. Virol. 1997, 71, 3484–3497. [Google Scholar] [CrossRef] [PubMed]
- Bregman, D.B.; Du, L.; van der Zee, S.; Warren, S.L. Transcription-dependent redistribution of the large subunit of RNA polymerase II to discrete nuclear domains. J. Cell Biol. 1995, 129, 287–298. [Google Scholar] [CrossRef]
- Kim, E.; Du, L.; Bregman, D.B.; Warren, S.L. Splicing factors associate with hyperphosphorylated RNA polymerase II in the absence of pre-mRNA. J. Cell Biol. 1997, 136, 19–28. [Google Scholar] [CrossRef]
- Liao, S.M.; Zhang, J.; Jeffery, D.A.; Koleske, A.J.; Thompson, C.M.; Chao, D.M.; Viljoen, M.; van Vuuren, H.J.; Young, R.A. A kinase-cyclin pair in the RNA polymerase II holoenzyme. Nature 1995, 9, 121–122. [Google Scholar] [CrossRef]
- Mortillaro, M.J.; Blencowe, B.J.; Wei, X.; Nakayasu, H.; Du, L.; Warren, S.L.; Sharp, P.A.; Berezney, R. A hyperphosphorylated form of the large subunit of RNA polymerase II is associated with splicing complexes and the nuclear matrix. Proc. Natl. Acad. Sci. U. S. A. 1996, 93, 8253–8257. [Google Scholar] [CrossRef]
- Tassan, J.-P.; Jaquenoud, M.; Leopold, P.; Schultz, S.J.; Nigg, E.A. Identification of human cyclin-dependent kinase 8, a putative kinase partner for cyclin C. Proc. Natl. Acad. Sci. U. S. A. 1995, 92, 8871–8875. [Google Scholar] [CrossRef]
- Rovnak, J.; Quackenbush, S.L. Walleye dermal sarcoma virus cyclin interacts with components of the Mediator complex and the RNA polymerase II holoenzyme. J. Virol. 2002, 76, 8031–8039. [Google Scholar] [CrossRef]
- Zhang, Z.; Kim, E.; Martineau, D. Functional characterization of a piscine retroviral promoter. J. Gen. Virol. 1999, 80, 3065–3072. [Google Scholar] [CrossRef]
- Quackenbush, S.L.; Linton, A.; Brewster, C.D.; Rovnak, J. Walleye dermal sarcoma virus rv-cyclin inhibits NF-κB-dependent transcription. Virology 2009, 386, 55–60. [Google Scholar] [CrossRef]
- Noble, M.E.M.; Endicott, J.A.; Brown, N.R.; Johnson, L.N. The cyclin box fold: protein recognition in cell-cycle and transcription control. Trends Biol. Science 1997, 22, 482–487. [Google Scholar] [CrossRef]
- Zhang, Z.; Martineau, D. Walleye dermal sarcoma virus: OrfA N-terminal end inhibits the activity of a reporter gene directed by eukaryotic promoters and has a negative effect on the growth of fish and mammalian cells. J. Virol. 1999, 73, 8884–8889. [Google Scholar] [CrossRef]
- Rovnak, J.; Quackenbush, S.L. Colorado State University: Fort Collins, CO, USA, Unpublished work. 2010.
- Donner, A.J.; Ebmeier, C.C.; Taatjes, D.J.; Espinosa, J.M. CDK8 is a positive regulator of transcriptional elongtion within the serum response network. Nat. Struct. Mol. Biol. 2010, 17, 194–201. [Google Scholar] [CrossRef]
- Meyer, K.D.; Donner, A.J.; Knuesel, M.T.; York, A.G.; Espinosa, J.M.; Taatjes, D.J. Cooperative activity of cdk8 and GCN5L within Mediator directs tandem phosphoacetylation of histone H3. Embo J. 2008, 27, 1447–1457. [Google Scholar] [CrossRef]
- Morris, E.J.; Ji, J.Y.; Yang, F.; Di Stefano, L.; Herr, A.; Moon, N.S.; Kwon, E.J.; Haigis, K.M.; Naar, A.M.; Dyson, N.J. E2F1 represses beta-catenin transcription and is antagonized by both pRB and CDK8. Nature 2008, 455, 552–556. [Google Scholar] [CrossRef]
- Ren, S.; Rollins, B.J. CyclinC/Cdk3 Promotes Rb-Dependent G0 Exit. Cell 2004, 117, 239–251. [Google Scholar] [CrossRef]
- Lahue, E.E.; Smith, A.V.; Orr-Weaver, T.L. A novel cyclin gene from Drosophila complements CLN function in yeast. Genes Dev. 1991, 5, 2166–2175. [Google Scholar] [CrossRef]
- Leopold, P.; O’Farrell, P.H. An evolutionarily conserved cyclin homolog from Drosophila rescues yeast deficient in G1 cyclins. Cell 1991, 66, 1207–1216. [Google Scholar] [CrossRef]
- Lew, D.J.; Dulic, V.; Reed, S.I. Isolation of three novel human cyclins by rescue of G1 cyclin (Cln) function in yeast. Cell 1991, 66, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Lairmore, M.D.; Stanley, J.R.; Weber, S.A.; Holzschu, D.L. Squamous epithelial proliferation induced by walleye dermal sarcoma retrovirus cyclin in transgenic mice. Proc. Natl. Acad. Sci. U. S. A. 2000, 97, 6114–6119. [Google Scholar] [CrossRef] [PubMed]
- Paul, T.A.; Rovnak, J.; Quackenbush, S.L.; Whitlock, K.; Zhan, H.; Gong, Z.; Spitsbergen, J.; Bowser, P.R.; Casey, J.W. Transgenic expression of walleye dermal sarcom virus rv-cyclin (orf A) in Zebrafish does not result in tissue proliferation. Mar. Biotechnol 2010. [Google Scholar]
- Zhan, H.; Spitsbergern, J.M.; Qing, W.; Wu, Y.L.; Paul, T.A.; Casey, J.W.; Her, G.M.; Gong, Z. Trasngenic expression of walleye dermal sarcoma virus rv-cyclin gene in zebrafish and its suppressive effect on liver tumor developemtn after carcinogen treatment. Mar. Biotechnol. 2010. [Google Scholar] [CrossRef]
- Dorn, G.N.; Mochly-Rosen, D. Intracellular transport mechnaims of signal transducers. Ann. Rev. Physiol. 2002, 64, 407–429. [Google Scholar] [CrossRef]
- Ron, D.; Chen, C.-H.; Caldwell, J.; Jamieson, L.; Orr, E.; Mochley-Rosen, D. Cloning of an intracellular receptor for protein kinase C: A homolog of the b subunit of G proteins. Proc. Natl. Acad. Sci. U. S. A. 1994, 91, 839–843. [Google Scholar] [CrossRef]
- Daniels, C.C.; Quackenbush, S.L. Colorado State University: Fort Collins, CO, USA, Unpublished work. 2009.
- Boya, P.; Pauleau, A.L.; Poncet, D.; Gonzalez-Polo, R.A.; Zamzami, N.; Kroemer, G. Viral proteins targeting mitochondria: controlling cell death. Biochim. Biophys. Acta 2004, 1659, 178–189. [Google Scholar] [CrossRef]
- Brewster, C.D.; Quackenbush, S.L. Colorado State University: Fort Collins, CO, USA, Unpublished work. 2006.






© 2010 by the authors. licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Rovnak, J.; Quackenbush, S.L. Walleye Dermal Sarcoma Virus: Molecular Biology and Oncogenesis. Viruses 2010, 2, 1984-1999. https://doi.org/10.3390/v2091984
Rovnak J, Quackenbush SL. Walleye Dermal Sarcoma Virus: Molecular Biology and Oncogenesis. Viruses. 2010; 2(9):1984-1999. https://doi.org/10.3390/v2091984
Chicago/Turabian StyleRovnak, Joel, and Sandra L. Quackenbush. 2010. "Walleye Dermal Sarcoma Virus: Molecular Biology and Oncogenesis" Viruses 2, no. 9: 1984-1999. https://doi.org/10.3390/v2091984
APA StyleRovnak, J., & Quackenbush, S. L. (2010). Walleye Dermal Sarcoma Virus: Molecular Biology and Oncogenesis. Viruses, 2(9), 1984-1999. https://doi.org/10.3390/v2091984