Antiviral Activity of 4'-thioIDU and Thymidine Analogs against Orthopoxviruses
Abstract
1. Introduction: Activity of Thymidine Analogs against the Orthopoxviruses
2. Molecular Targets of Thymidine Analogs in the Orthopoxviruses
3. Antiviral Activity and Mechanism of Action of (N)-MCT
4. Thymidine Analogs with Large Substituents at the 5 Position
5. Inhibition of Orthopoxvirus Replication with 4'-Thio Pyrimidine Analogs
6. Conclusions
Acknowledgements
References and Notes
- Kaufman, H.E.; Nesburn, A.B.; Maloney, E.D. Cure of vaccinia infection by 5-iodo-2'-deoxyuridine. Virology 1962, 18, 567–569. [Google Scholar] [CrossRef]
- Prusoff, W.H. Synthesis and biological activities of iododeoxyuridine, an analog of thymidine. Biochim. Biophys. Acta 1959, 32, 295–296. [Google Scholar] [CrossRef] [PubMed]
- Calabresi, P.; Mc, C.R.; Welch, A.D. Suppression of infections resulting from a deoxyribonucleic acid virus (vaccinia) by systemic adminstration of 5-iodo-2'-deoxyuridine. Nature 1963, 197, 767–769. [Google Scholar] [CrossRef] [PubMed]
- Loddo, B.; Muntoni, S.; Ferrari, W. Effect of 5-iodo-2'-deoxyuridine on vaccinia virus, in vitro. Nature 1963, 198, 510. [Google Scholar] [CrossRef] [PubMed]
- Prusoff, W.H.; Bakhle, Y.S.; McCrea, J.F. Incorporation of 5-Iodo-2'-Deoxyuridine into the deoxyribonucleic acid of vaccinia virus. Nature 1963, 199, 1310–1311. [Google Scholar] [CrossRef]
- Chen, M.S.; Ward, D.C.; Prusoff, W.H. Specific herpes simplex virus-induced incorporation of 5-iodo-5'-amino-2',5'-dideoxyuridine into deoxyribonucleic acid. J. Biol. Chem. 1976, 251, 4833–4838. [Google Scholar] [CrossRef]
- Chen, M.S.; Prusoff, W.H. Phosphorylation of 5-iodo-5'-amino-2',5',dideoxyuridine by herpes simplex virus type 1 encoded thymidine kinase. J. Biol. Chem. 1979, 254, 10449–10452. [Google Scholar] [CrossRef]
- Descamps, J.; De Clercq, E. Specific phosphorylation of E-5-(2-iodovinyl)-2'-deoxyuridine by herpes simplex virus-infected cells. J. Biol. Chem. 1981, 256, 5973–5976. [Google Scholar] [CrossRef]
- De Clercq, E. (E)-5-(2-bromovinyl)-2'-deoxyuridine (BVDU). Med. Res. Rev. 2005, 25, 1–20. [Google Scholar] [CrossRef]
- Furman, P.A.; De Miranda, P.; St Clair, M.H.; Elion, G.B. Metabolism of acyclovir in virus-infected and uninfected cells. Antimicrob. Agents Chemother. 1981, 20, 518–524. [Google Scholar] [CrossRef]
- Kern, E.R. In vitro activity of potential anti-poxvirus agents. Antivir. Res. 2003, 57, 35–40. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, E. Vaccinia virus inhibitors as a paradigm for the chemotherapy of poxvirus infections. Clin. Microbiol. Rev. 2001, 14, 382–397. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, E. Antiviral and antitumor activities of 5-substituted 2'-deoxyuridines. Methods Find. Exp. Clin. Pharmacol. 1980, 2, 253–267. [Google Scholar] [PubMed]
- Neyts, J.; Verbeken, E.; De Clercq, E. Effect of 5-iodo-2'-deoxyuridine on vaccinia virus (orthopoxvirus) infections in mice. Antimicrob. Agents Chemother. 2002, 46, 2842–2847. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, E.; Luczak, M.; Shugar, D.; Torrence, P.F.; Waters, J.A.; Witkop, B. Effect of cytosine, arabinoside, iododeoxyuridine, ethyldeoxyuridine, thiocyanatodeoxyuridine, and ribavirin on tail lesion formation in mice infected with vaccinia virus. Proc. Soc. Exp. Biol. Med. 1976, 151, 487–490. [Google Scholar] [CrossRef]
- El Omari, K.; Solaroli, N.; Karlsson, A.; Balzarini, J.; Stammers, D.K. Structure of vaccinia virus thymidine kinase in complex with dTTP: Insights for drug design. BMC Struct. Biol. 2006, 6, 22. [Google Scholar] [CrossRef]
- Prichard, M.N.; Keith, K.A.; Johnson, M.P.; Harden, E.A.; McBrayer, A.; Luo, M.; Qiu, S.; Chattopadhyay, D.; Fan, X.; Torrence, P.F.; Kern, E.R. Selective phosphorylation of antiviral drugs by vaccinia virus thymidine kinase. Antimicrob. Agents Chemother. 2007, 51, 1795–1803. [Google Scholar] [CrossRef]
- Marquez, V.E.; Hughes, S.H.; Sei, S.; Agbaria, R. The history of N-methanocarbathymidine: The investigation of a conformational concept leads to the discovery of a potent and selective nucleoside antiviral agent. Antivir. Res. 2006, 71, 268–275. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, X.; Zhou, L.; Keith, K.A.; Kern, E.R.; Torrence, P.F. Assembling a smallpox biodefense by interrogating 5-substituted pyrimidine nucleoside chemical space. Antivir. Res. 2006, 71, 201–205. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, X.; Zhou, L.; Keith, K.A.; Kern, E.R.; Torrence, P.F. 5-(Dimethoxymethyl)-2'-deoxyuridine: A novel gem diether nucleoside with anti-orthopoxvirus activity. J. Med. Chem. 2006, 49, 3377–3382. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, X.; Zhou, L.; Keith, K.A.; Kern, E.R.; Torrence, P.F. A pyrimidine-pyrazolone nucleoside chimera with potent in vitro anti-orthopoxvirus activity. Bioorg. Medicinal Chem. Letter. 2006, 16, 3224–3228. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, X.; Zhou, L.; Keith, K.A.; Prichard, M.N.; Kern, E.R.; Torrence, P.F. Toward orthopoxvirus countermeasures: A novel heteromorphic nucleoside of unusual structure. J. Med. Chem. 2006, 49, 4052–4054. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rahim, S.G.; Trivedi, N.; Bogunovic-Batchelor, M.V.; Hardy, G.W.; Mills, G.; Selway, J.W.; Snowden, W.; Littler, E.; Coe, P.L.; Basnak, I.; Whale, R.F.; Walker, R.T. Synthesis and anti-herpes virus activity of 2'-deoxy-4'-thiopyrimidine nucleosides. J. Med. Chem. 1996, 39, 789–795. [Google Scholar] [CrossRef]
- Secrist, J.A., 3rd; Tiwari, K.N.; Riordan, J.M.; Montgomery, J.A. Synthesis and biological activity of 2'-deoxy-4'-thio pyrimidine nucleosides. J. Med. Chem. 1991, 34, 2361–2366. [Google Scholar] [CrossRef] [PubMed]
- Kern, E.R.; Prichard, M.N.; Quenelle, D.C.; Keith, K.A.; Tiwari, K.N.; Maddry, J.A.; Secrist, J.A., 3rd. Activities of certain 5-substituted 4'-thiopyrimidine nucleosides against orthopoxvirus infections. Antimicrob. Agents Chemother. 2009, 53, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Jasamai, M.; Balzarini, J.; Simons, C. 6-Azathymidine-4'-thionucleosides: Synthesis and antiviral evaluation. J. Enzyme. Inhib. Med. Chem. 2008, 23, 56–61. [Google Scholar] [CrossRef]
- Gammon, D.B.; Snoeck, R.; Fiten, P.; Krecmerova, M.; Holy, A.; De Clercq, E.; Opdenakker, G.; Evans, D.H.; Andrei, G. Mechanism of antiviral drug resistance of vaccinia virus: Identification of residues in the viral DNA polymerase conferring differential resistance to antipoxvirus drugs. J. Virol. 2008, 82, 12520–12534. [Google Scholar] [CrossRef]
- Andrei, G.; Gammon, D.B.; Fiten, P.; De Clercq, E.; Opdenakker, G.; Snoeck, R.; Evans, D.H. Cidofovir resistance in vaccinia virus is linked to diminished virulence in mice. J. Virol. 2006, 80, 9391–9401. [Google Scholar] [CrossRef]
- Smee, D.F.; Wandersee, M.K.; Bailey, K.W.; Hostetler, K.Y.; Holy, A.; Sidwell, R.W. Characterization and treatment of cidofovir-resistant vaccinia (WR strain) virus infections in cell culture and in mice. Antivir. Chem. Chemother. 2005, 16, 203–211. [Google Scholar] [CrossRef]
- Becker, M.N.; Obraztsova, M.; Kern, E.R.; Quenelle, D.C.; Keith, K.A.; Prichard, M.N.; Luo, M.; Moyer, R.W. Isolation and characterization of cidofovir resistant vaccinia viruses. Virol. J. 2008, 5, 58. [Google Scholar] [CrossRef]
- Prichard, M.N.; Kern, E.R. Orthopoxvirus targets for the development of antiviral therapies. Curr. Drug Targets Infect. Disord. 2005, 5, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Prichard, M.N.; Kern, E.R. Antiviral targets in orthopoxviruses. In Antiviral Research: Strategies in Antiviral Drug Discovery; LaFemina, R.L., Ed.; ASM Press: Washington, DC, USA, 2009; pp. 167–186. [Google Scholar]
- Kit, S.; Piekarski, L.J.; Dubbs, D.R. Induction of thymidine kinase by vaccinia-infected mouse fibroblasts. J. Mol. Biol. 1963, 6, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Prichard, M.N.; Williams, A.D.; Keith, K.A.; Harden, E.A.; Kern, E.R. Distinct thymidine kinases encoded by cowpox virus and herpes simplex virus contribute significantly to the differential antiviral activity of nucleoside analogs. Antivir. Res. 2006, 71, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Lefkowitz, E.J.; Wang, C.; Upton, C. Poxviruses: Past, present and future. Virus Res. 2006, 117, 105–118. [Google Scholar] [CrossRef]
- Black, M.E.; Hruby, D.E. Site-directed mutagenesis of a conserved domain in vaccinia virus thymidine kinase. Evidence for a potential role in magnesium binding. J. Biol. Chem. 1992, 267, 6801–6806. [Google Scholar] [CrossRef]
- Hruby, D.E.; Maki, R.A.; Miller, D.B.; Ball, L.A. Fine structure analysis and nucleotide sequence of the vaccinia virus thymidine kinase gene. Proc. Natl. Acad. Sci. U. S. A. 1983, 80, 3411–3415. [Google Scholar] [CrossRef]
- Black, M.E.; Hruby, D.E. A single amino acid substitution abolishes feedback inhibition of vaccinia virus thymidine kinase. J. Biol. Chem. 1992, 267, 9743–9748. [Google Scholar] [CrossRef]
- Solaroli, N.; Johansson, M.; Balzarini, J.; Karlsson, A. Substrate specificity of three viral thymidine kinases (TK): Vaccinia virus TK, feline herpesvirus TK, and canine herpesvirus TK. Nucleosides Nucleotides Nucleic Acids 2006, 25, 1189–1192. [Google Scholar] [CrossRef]
- Prichard, M.N.; Keith, K.A.; Quenelle, D.C.; Kern, E.R. Activity and mechanism of action of N-methanocarbathymidine against herpesvirus and orthopoxvirus infections. Antimicrob. Agents Chemother. 2006, 50, 1336–1341. [Google Scholar] [CrossRef]
- Smee, D.F.; Humphreys, D.E.; Hurst, B.L.; Barnard, D.L. Antiviral activities and phosphorylation of 5-halo-2'-deoxyuridines and N-methanocarbathymidine in cells infected with vaccinia virus. Antivir. Chem. Chemother. 2008, 19, 15–24. [Google Scholar] [CrossRef]
- Harden, E.A.; Keith, K.A.; Daily, S.; Tiwari, K.; Maddry, J.; Secrist, J.; Kern, E.R.; Prichard, M.N. Mutation of the thymidine kinases encoded by herpes simplex virus or vaccinia virus can confer resistance to 5-iodo-4'-thio-2'-deoxyuridine. Antivir. Res. 2009, 82, A48. [Google Scholar] [CrossRef]
- Birringer, M.S.; Claus, M.T.; Folkers, G.; Kloer, D.P.; Schulz, G.E.; Scapozza, L. Structure of a type II thymidine kinase with bound dTTP. FEBS Lett. 2005, 579, 1376–1382. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.J.; Johnston, L.H.; De Carlos, A.; Smith, G.L. Vaccinia virus encodes an active thymidylate kinase that complements a cdc8 mutant of Saccharomyces cerevisiae. J. Biol. Chem. 1991, 266, 20103–20109. [Google Scholar] [CrossRef]
- Caillat, C.; Topalis, D.; Agrofoglio, L.A.; Pochet, S.; Balzarini, J.; Deville-Bonne, D.; Meyer, P. Crystal structure of poxvirus thymidylate kinase: An unexpected dimerization has implications for antiviral therapy. Proc. Natl. Acad. Sci. U. S. A. 2008, 105, 16900–16905. [Google Scholar] [CrossRef]
- Topalis, D.; Collinet, B.; Gasse, C.; Dugue, L.; Balzarini, J.; Pochet, S.; Deville-Bonne, D. Substrate specificity of vaccinia virus thymidylate kinase. FEBS J. 2005, 272, 6254–6265. [Google Scholar] [CrossRef] [PubMed]
- Auvynet, C.; Topalis, D.; Caillat, C.; Munier-Lehmann, H.; Seclaman, E.; Balzarini, J.; Agrofoglio, L.A.; Kaminski, P.A.; Meyer, P.; Deville-Bonne, D.; El Amri, C. Phosphorylation of dGMP analogs by vaccinia virus TMP kinase and human GMP kinase. Biochem. Biophys. Res. Commun. 2009, 388, 6–11. [Google Scholar] [CrossRef]
- Goebel, S.J.; Johnson, G.P.; Perkus, M.E.; Davis, S.W.; Winslow, J.P.; Paoletti, E. The complete DNA sequence of vaccinia virus. Virology 1990, 179, 247–266, 517–263. [Google Scholar] [CrossRef] [PubMed]
- Broyles, S.S. Vaccinia virus encodes a functional dUTPase. Virology 1993, 195, 863–865. [Google Scholar] [CrossRef]
- Roseman, N.A.; Evans, R.K.; Mayer, E.L.; Rossi, M.A.; Slabaugh, M.B. Purification and characterization of the vaccinia virus deoxyuridine triphosphatase expressed in Escherichia coli. J. Biol. Chem. 1996, 271, 23506–23511. [Google Scholar] [CrossRef]
- Prichard, M.N.; Kern, E.R.; Quenelle, D.C.; Keith, K.A.; Moyer, R.W.; Turner, P.C. Vaccinia virus lacking the deoxyuridine triphosphatase gene (F2L) replicates well in vitro and in vivo, but is hypersensitive to the antiviral drug (N)-methanocarbathymidine. Virol. J. 2008, 5, 39. [Google Scholar] [CrossRef]
- Keith, K.A.; Harden, E.A.; Gill, R.; Marquez, V.E.; Kern, E.R.; Prichard, M.N. Efficacy of N-methanocarbathymidine against herpes simplex virus is cell cycle dependent. Antivir. Res. 2010, 86, A58. [Google Scholar] [CrossRef]
- Smee, D.F.; Wandersee, M.K.; Bailey, K.W.; Wong, M.H.; Chu, C.K.; Gadthula, S.; Sidwell, R.W. Cell line dependency for antiviral activity and in vivo efficacy of N-methanocarbathymidine against orthopoxvirus infections in mice. Antivir. Res. 2007, 73, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Sauerbrei, A.; Meier, C.; Meerbach, A.; Schiel, M.; Helbig, B.; Wutzler, P. In vitro activity of cycloSal-nucleoside monophosphates and polyhydroxycarboxylates against orthopoxviruses. Antivir. Res. 2005, 67, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Schelling, P.; Claus, M.T.; Johner, R.; Marquez, V.E.; Schulz, G.E.; Scapozza, L. Biochemical and structural characterization of (South)-methanocarbathymidine that specifically inhibits growth of herpes simplex virus type 1 thymidine kinase-transduced osteosarcoma cells. J. Biol. Chem. 2004, 279, 32832–32838. [Google Scholar] [CrossRef]
- Martin, J.A.; Thomas, G.J.; Merrett, J.H.; Lambert, R.W.; Bushnell, D.J.; Dunsdon, S.J.; Freeman, A.C.; Hopkins, R.A.; Johns, I.R.; Keech, E.; Simmonite, H.; Kai-In, P.W.; Holland, M. The design, synthesis and properties of highly potent and selective inhibitors of herpes simplex virus types 1 and 2 thymidine kinase. Antivir. Chem. Chemother. 1998, 9, 1–8. [Google Scholar]
- Zalah, L.; Huleihel, M.; Manor, E.; Konson, A.; Ford, H., Jr.; Marquez, V.E.; Johns, D.G.; Agbaria, R. Metabolic pathways of N-methanocarbathymidine, a novel antiviral agent, in native and herpes simplex virus type 1 infected Vero cells. Antivir. Res. 2002, 55, 63–75. [Google Scholar] [CrossRef]
- Chen, M.S.; Walker, J.; Prusoff, W.H. Kinetic studies of herpes simplex virus type 1-encoded thymidine and thymidylate kinase, a multifunctional enzyme. J. Biol. Chem. 1979, 254, 10747–10753. [Google Scholar] [CrossRef]
- Prota, A.; Vogt, J.; Pilger, B.; Perozzo, R.; Wurth, C.; Marquez, V.E.; Russ, P.; Schulz, G.E.; Folkers, G.; Scapozza, L. Kinetics and crystal structure of the wild-type and the engineered Y101F mutant of Herpes simplex virus type 1 thymidine kinase interacting with (North)-methanocarba-thymidine. Biochemistry 2000, 39, 9597–9603. [Google Scholar] [CrossRef]
- Marquez, V.E.; Siddiqui, M.A.; Ezzitouni, A.; Russ, P.; Wang, J.; Wagner, R.W.; Matteucci, M.D. Nucleosides with a twist. Can fixed forms of sugar ring pucker influence biological activity in nucleosides and oligonucleotides? J. Med. Chem 1996, 39, 3739–3747. [Google Scholar] [CrossRef]
- Marquez, V.E.; Ben-Kasus, T.; Barchi, J.J., Jr.; Green, K.M.; Nicklaus, M.C.; Agbaria, R. Experimental and structural evidence that herpes 1 kinase and cellular DNA polymerase(s) discriminate on the basis of sugar pucker. J. Am. Chem. Soc. 2004, 126, 543–549. [Google Scholar] [CrossRef]
- Smee, D.F.; Hurst, B.L.; Wong, M.H.; Glazer, R.I.; Rahman, A.; Sidwell, R.W. Efficacy of N-methanocarbathymidine in treating mice infected intranasally with the IHD and WR strains of vaccinia virus. Antivir. Res. 2007, 76, 124–129. [Google Scholar] [CrossRef] [PubMed][Green Version]
- De Clercq, E.; Descamps, J.; Huang, G.F.; Torrence, P.F. 5-Nitro-2'-deoxyuridine and 5-nitro-2'-deoxyuridine 5'-monophosphate: Antiviral activity and inhibition of thymidylate synthetase in vivo. Mol. Pharmacol. 1978, 14, 422–430. [Google Scholar] [PubMed]
- Quenelle, D.C. University of Alabama at Birmingham, Birmingham, AL, USA. Personal Communication. 2010. [Google Scholar]
- Prichard, M.N.; Quenelle, D.C.; Hartline, C.B.; Harden, E.A.; Jefferson, G.; Frederick, S.L.; Daily, S.L.; Whitley, R.J.; Tiwari, K.N.; Maddry, J.A.; Secrist, J.A., 3rd; Kern, E.R. Inhibition of herpesvirus replication by 5-substituted 4'-thiopyrimidine nucleosides. Antimicrob. Agents Chemother. 2009, 53, 5251–5258. [Google Scholar] [CrossRef]
- Taupin, P. BrdU immunohistochemistry for studying adult neurogenesis: Paradigms, pitfalls, limitations, and validation. Brain Res. Rev. 2007, 53, 198–214. [Google Scholar] [CrossRef] [PubMed]
- Smee, D.F.; Sidwell, R.W. Anti-cowpox virus activities of certain adenosine analogs, arabinofuranosyl nucleosides, and 2'-fluoro-arabinofuranosyl nucleosides. Nucleosides Nucleotides Nucleic Acids 2004, 23, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Keith, K.A.; Sanders, S.; Tiwari, K.; Maddry, J.; Secrist, J.; Jordan, R.; Hruby, D.; Lanier, R.; Painter, G.; Kern, E.R.; Prichard, M.N. Combinations of 5-iodo-4'-thio-2'-deoxyuridine and ST-246 or CMX001 synergistically inhibit orthopoxvirus replication in vitro. Antivir. Res. 2009, 82, A33–A34. [Google Scholar] [CrossRef]


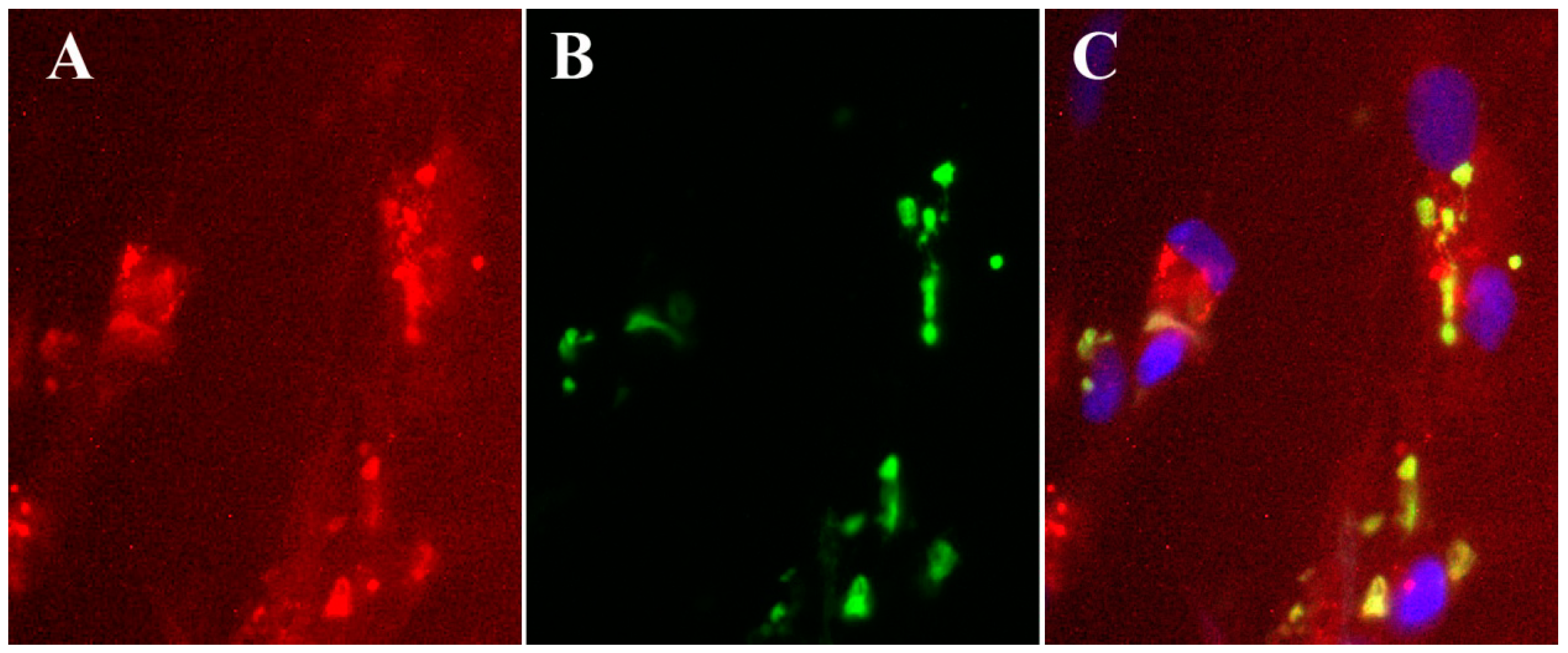
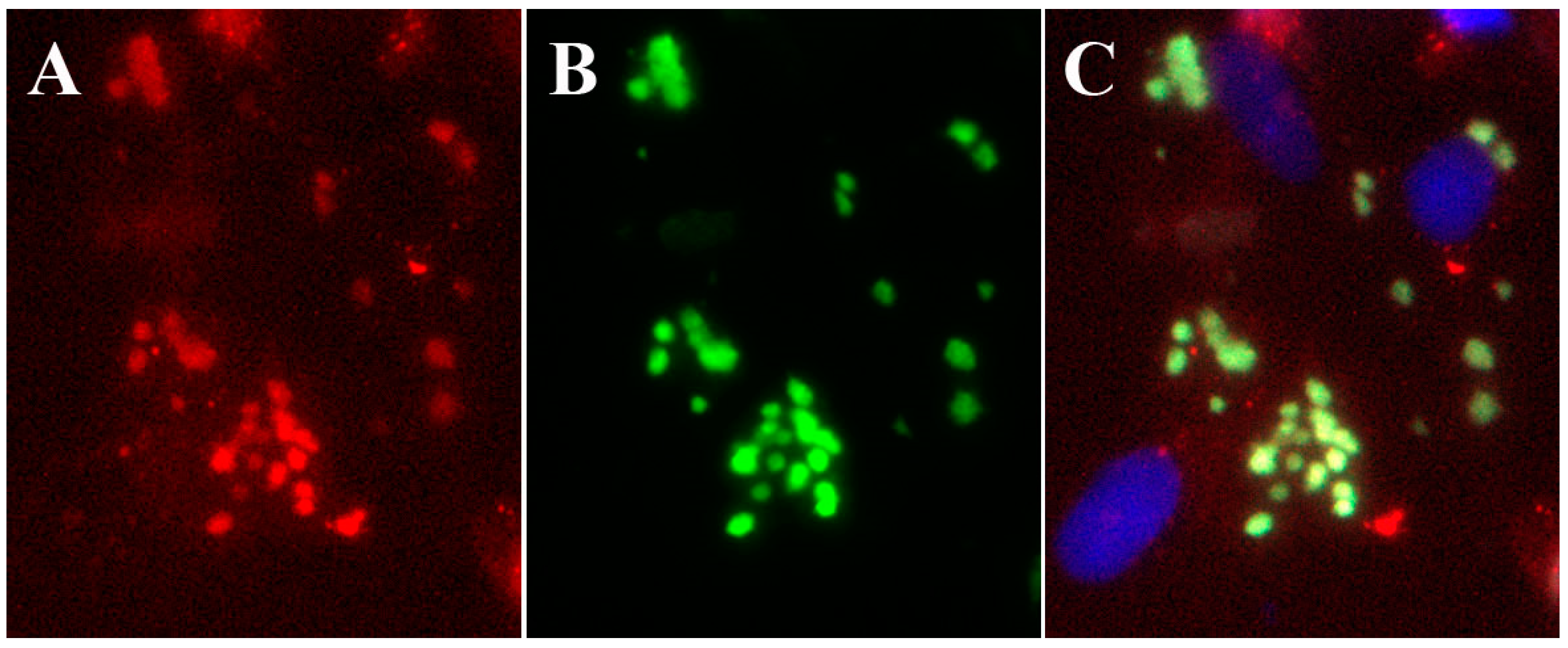
| Compound | Vaccinia virus (EC50, µM)b | Cowpox virus (EC50, µM) | Cytotoxicity (CC50, µM) |
|---|---|---|---|
| cidofovir | 19 ± 11 | 29 ± 6.1 | >317 ± 0 |
| idoxuridine | 8.4 ± 3.3 | 3.7 ± 2.7 | >100 ± 0 |
| fialuridine | 1.5 ± 0.4 | 0.2 ± 0.1 | >100 ± 0 |
| (N)-MCT | 0.6 ± 0.1 | 1.5 ± 1.2 | >100 ± 0 |
| 5-iodo-4’-thio-2’-deoxyuridine (4′-thioIDU) | 0.5 ± 0.2 | 0.1 ± 0.04 | >100 ± 0 |
| 1-(2-deoxy, 4'thio-β-D-ribofuranosyl)-thymidine | 0.03 ± 0.01 | 0.02 ± 0.01 | 29 ± 4.0 |
| 4-thio-β-D-arabinofuranosyl)-cytidine | 0.3 ± 0.2 | 1.6 ± 0.8 | 53 ± 6.4 |
| 1-(4-thio-β-D-arabinofuranosyl)-5-fluoro cytidine | 0.1 ± 0.01 | 0.4 ± 0.1 | 4.6 ± 1.1 |
| 5-iodo-4-thio-3’,5’-di-O-acetyl-2’-deoxyuridine | 0.9 ± 0.3 | 0.3 ± 0.2 | >80 ± 28 |
| 5-bromo-4’-thio-2’-deoxyuridine | 0.1 ± 0.02 | 0.05 ± 0.04 | >100 ± 0 |
| 5-trifluoromethyl-2’-deoxy-4’-thiouridine | 0.1 ± 0.004 | 0.1 ± 0.01 | >100 ± 0 |
| Treatmentb | Mortality | P-value | MDDc | P-value | |
|---|---|---|---|---|---|
| Number | Percent | ||||
| Vaccinia virus | |||||
| vehicle | 15/15 | 100 | - | 7.9 | - |
| CDV | |||||
| 15 mg/kg | 0/15 | 0 | <0.001 | - | - |
| (N)-MCT | |||||
| 50 mg/kg | 0/15 | 0 | <0.001 | - | - |
| 16.7 mg/kg | 2/15 | 13 | <0.001 | 7.5 | NSd |
| 5.6 mg/kg | 12/15 | 80 | NS | 8.2 | NS |
| Cowpox virus | |||||
| vehicle | 15/15 | 100 | - | 9.6 | - |
| CDV | |||||
| 15 mg/kg | 0/15 | 0 | <0.001 | - | - |
| (N)-MCT | |||||
| 50 mg/kg | 2/15 | 13 | <0.001 | 7.5 | NS |
| 16.7 mg/kg | 3/15 | 20 | <0.001 | 13.3 | 0.01 |
| 5.6 mg/kg | 6/15 | 40 | <0.001 | 14.0 | 0.05 |
| Treatmentb | Mortality | P-value | MDD + SDc | P-value | |
|---|---|---|---|---|---|
| Number | Percent | ||||
| Vehicle + 3 days | 14/15 | 93 | 11.9 ± 2.2 | ||
| CDV + 3 days | |||||
| 15 mg/kg | 0/14 | 0 | <0.001 | ||
| 4′-thioIDU + 3 days | |||||
| 15 mg/kg | 1/15 | 7 | <0.001 | 14.0 | NSc |
| 5 mg/kg | 0/15 | 0 | <0.001 | ||
| 1.5 mg/kg | 2/15 | 13 | <0.001 | 14.0 ± 5.7 | NSc |
| Vehicle + 4 days | 15/15 | 100 | 11.7 ± 2.3 | NS | |
| CDV + 4 days | |||||
| 15 mg/kg | 0/15 | 0 | <0.001 | ||
| 4′-thioIDU + 4 days | |||||
| 5 mg/kg | 4/15 | 27 | <0.001 | 13.3 ± 1.3 | <0.05 |
| Vehicle + 5 days | 12/15 | 80 | 14.6 ± 3.9 | ||
| CDV + 5 days | |||||
| 15 mg/kg | 4/15 | 27 | 0.01 | 10.5 ± 1.9 | 0.07 |
| 4′-thioIDU + 5 days | |||||
| 5 mg/kg | 13/15 | 87 | NSc | 9.8 ± 1.3 | <0.001 |
| Compound | WR (EC50, µM)b | CDVR 15 (EC50, µM) | VV911 (ST-246R) (EC50, µM) | VVTK::luc TK deficient (EC50, µM) |
|---|---|---|---|---|
| 4′-thioIDU | 0.04 ± 0.02 | 0.04 ± 0.01 | 0.02 ± 0.003 | 0.3 ± 0.01 |
| CDV | 11 ± 1.5 | 62 ± 34 | 33 ± 5.0 | 9.4 ± 0.1 |
| ST-246 | 0.07 ± 0.01 | NDc | >20 ± 0 | NDb |
| IDU | 2.8 ± 0.3 | 1.0 ± 0.1 | 2.4 ± 0.4 | 7.1 ± 4.3 |
© 2010 by the authors. licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Prichard, M.N.; Kern, E.R. Antiviral Activity of 4'-thioIDU and Thymidine Analogs against Orthopoxviruses. Viruses 2010, 2, 1968-1983. https://doi.org/10.3390/v2091968
Prichard MN, Kern ER. Antiviral Activity of 4'-thioIDU and Thymidine Analogs against Orthopoxviruses. Viruses. 2010; 2(9):1968-1983. https://doi.org/10.3390/v2091968
Chicago/Turabian StylePrichard, Mark N., and Earl R. Kern. 2010. "Antiviral Activity of 4'-thioIDU and Thymidine Analogs against Orthopoxviruses" Viruses 2, no. 9: 1968-1983. https://doi.org/10.3390/v2091968
APA StylePrichard, M. N., & Kern, E. R. (2010). Antiviral Activity of 4'-thioIDU and Thymidine Analogs against Orthopoxviruses. Viruses, 2(9), 1968-1983. https://doi.org/10.3390/v2091968




