A Naturally Occurring Microhomology-Mediated Deletion of Three Genes in African Swine Fever Virus Isolated from Two Sardinian Wild Boars
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Sampling, Diagnostic Tests, and Virus Isolation
2.3. DNA Extraction, PCR Assay, and Sanger Sequencing
2.4. Full-Genome Sequencing and Assembly Analysis
2.5. Phylogenetic Analysis
3. Results
3.1. Isolation of 7212WB/19 and 7303WB/19 and Their Genomic Analysis
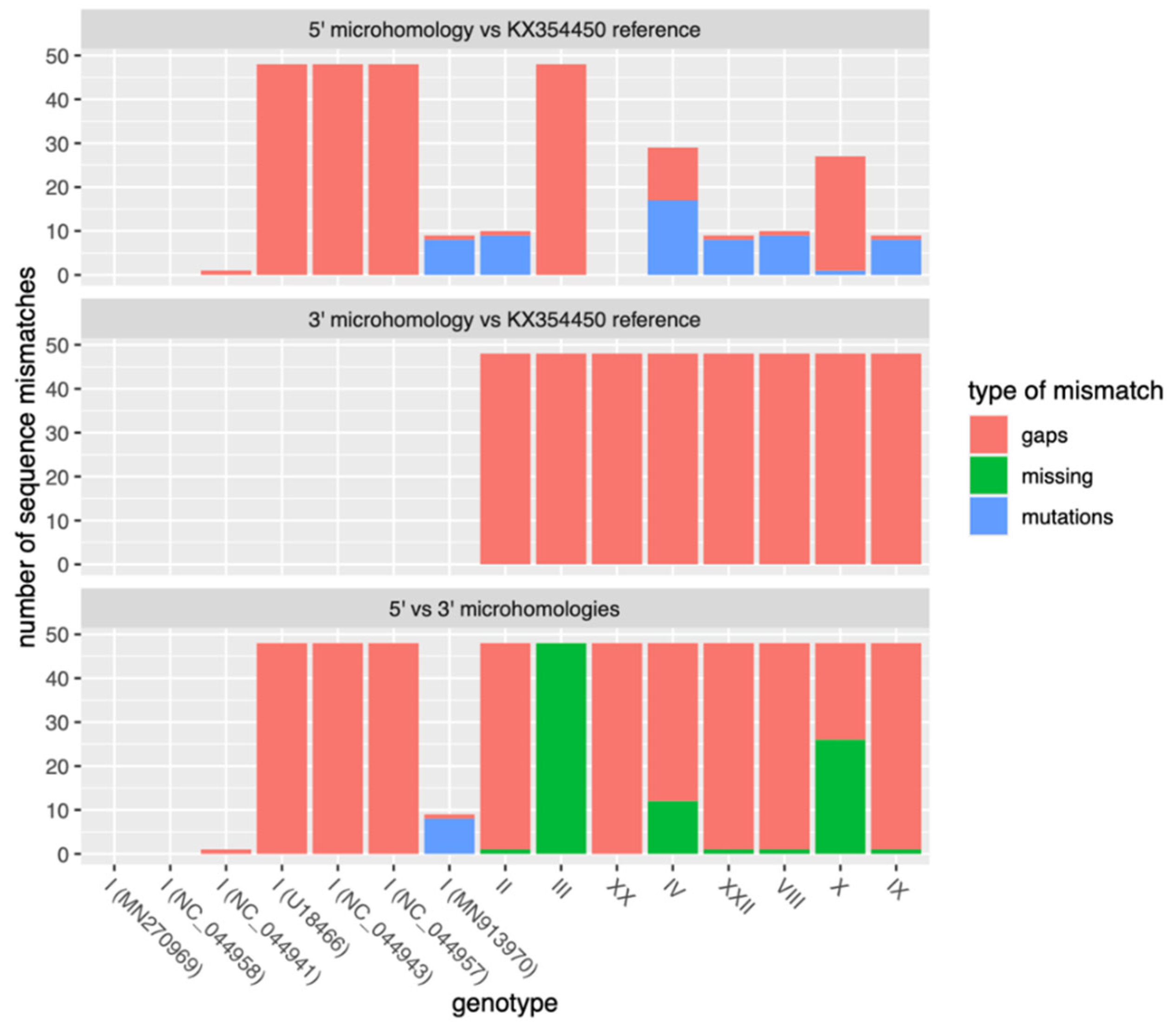
3.2. A Deletion with Respect to the Reference ASFV Isolate from Sardinia
3.3. Genetic Relatedness to other Sardinian ASFV Sequences
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dixon, L.K.; Chapman, D.A.; Netherton, C.L.; Upton, C. African swine fever virus replication and genomics. Virus Res. 2013, 173, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Penrith, M.-L.; Vosloo, W.; Jori, F.; Bastos, A. African swine fever virus eradication in Africa. Virus Res. 2013, 173, 228–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WOAH. WAHIS Interface. 2022. Available online: https://www.woah.org/en/what-we-do/animal-health-and-welfare/disease-data-collection/world-animal-health-information-system/ (accessed on 1 October 2022).
- Dixon, L.K.; Stahl, K.; Jori, F.; Vial, L.; Pfeiffer, D.U. African Swine Fever Epidemiology and Control. Annu. Rev. Anim. Biosci. 2020, 8, 221–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.; Sun, Y.; Huang, S.; Qiu, H.-J. Multifaceted Immune Responses to African Swine Fever Virus: Implications for Vaccine Development. Vet. Microbiol. 2020, 249, 108832. [Google Scholar] [CrossRef] [PubMed]
- Blome, S.; Franzke, K.; Beer, M. African swine fever—A review of current knowledge. Virus Res. 2020, 287, 198099. [Google Scholar] [CrossRef] [PubMed]
- Dixon, L.K.; Sun, H.; Roberts, H. African swine fever. Antivir. Res. 2019, 165, 34–41. [Google Scholar] [CrossRef]
- Kolbasov, D.; Titov, I.; Tsybanov, S.; Gogin, A.; Malogolovkin, A. African Swine Fever Virus, Siberia, Russia, 2017. Emerg. Infect. Dis. 2018, 24, 796–798. [Google Scholar] [CrossRef]
- Netherton, C.L.; Goatley, L.C.; Reis, A.L.; Portugal, R.; Nash, R.H.; Morgan, S.B.; Gault, L.; Nieto, R.; Norlin, V.; Gallardo, C.; et al. Identification and immunogenicity of African swine fever virus antigens. Front. Immunol. 2019, 10, 1318. [Google Scholar] [CrossRef] [Green Version]
- Njau, E.P.; Machuka, E.M.; Cleaveland, S.; Shirima, G.M.; Kusiluka, L.J.; Okoth, E.A.; Pelle, R. African Swine Fever Virus (ASFV): Biology, Genomics and Genotypes Circulating in Sub-Saharan Africa. Viruses 2021, 13, 2285. [Google Scholar] [CrossRef]
- Zhou, X.; Li, N.; Luo, Y.; Liu, Y.; Miao, F.; Chen, T.; Zhang, S.; Cao, P.; Li, X.; Tian, K.; et al. Emergence of African Swine Fever in China. Transbound. Emerg. Dis. 2018, 65, 1482–1484. [Google Scholar] [CrossRef]
- Sun, E.; Huang, L.; Zhang, X.; Zhang, J.; Shen, D.; Zhang, Z.; Wang, Z.; Huo, H.; Wang, W.; Huangfu, H.; et al. Genotype I African swine fever viruses emerged in domestic pigs in China and caused chronic infection. Emerg. Microbes Infect. 2021, 10, 2183–2193. [Google Scholar] [CrossRef]
- Iscaro, C.; Dondo, A.; Ruocco, L.; Masoero, L.; Giammarioli, M.; Zoppi, S.; Guberti, V.; Feliziani, F. Index case of new African Swine Fever incursion in mainland Italy. Transbound. Emerg. Dis. 2022, 69, 1707–1711. [Google Scholar] [CrossRef]
- Contini, A.; Cossu, P.; Firinu, A. African swine fever in Sardinia. In African Swine Fever, EUR 8466 EN, Pro CEC/FAO Research Seminar, Sassari, Sardinia, 23–25 September 1982; Wilkinson, P.J., Ed.; Commission of the European Communities: Brussels, Belgium, 1983; pp. 1–6. [Google Scholar]
- Rolesu, S.; Mandas, D.; Loi, F.; Oggiano, A.; Giudici, S.D.; Franzoni, G.; Guberti, V.; Cappai, S. African Swine Fever in Smallholder Sardinian Farms: Last 10 Years of Network Transmission Reconstruction and Analysis. Front. Vet. Sci. 2021, 8, 692448. [Google Scholar] [CrossRef]
- Fiori, M.S.; Sanna, D.; Scarpa, F.; Floris, M.; Di Nardo, A.; Ferretti, L.; Loi, F.; Cappai, S.; Sechi, A.M.; Angioi, P.P.; et al. A Deeper Insight into Evolutionary Patterns and Phylogenetic History of ASFV Epidemics in Sardinia (Italy) through Extensive Genomic Sequencing. Viruses 2021, 13, 1994. [Google Scholar] [CrossRef]
- Cappai, S.; Rolesu, S.; Feliziani, F.; Desini, P.; Guberti, V.; Loi, F. Standardized Methodology for Target Surveillance against African Swine Fever. Vaccines 2020, 8, 723. [Google Scholar] [CrossRef]
- Loi, F.; Cappai, S.; Laddomada, A.; Feliziani, F.; Oggiano, A.; Franzoni, G.; Rolesu, S.; Guberti, V. Mathematical Approach to Estimating the Main Epidemiological Parameters of African Swine Fever in Wild Boar. Vaccines 2020, 8, 521. [Google Scholar] [CrossRef]
- Vigário, J.D.; Terrinha, A.M.; Nunes, J.F.M. Antigenic relationships among strains of African swine fever virus. Arch. Gesamte Virusforsch. 1974, 45, 272–277. [Google Scholar] [CrossRef]
- Boinas, F.S.; Hutchings, G.H.; Dixon, L.K.; Wilkinson, P.J. Characterization of pathogenic and non-pathogenic African swine fever virus isolates from Ornithodoros erraticus inhabiting pig premises in Portugal. J. Gen. Virol. 2004, 85, 2177–2187. [Google Scholar] [CrossRef]
- Zani, L.; Forth, J.H.; Forth, L.; Nurmoja, I.; Leidenberger, S.; Henke, J.; Carlson, J.; Breidenstein, C.; Viltrop, A.; Höper, D.; et al. Deletion at the 5′-end of Estonian ASFV strains associated with an attenuated phenotype. Sci. Rep. 2018, 8, 6510. [Google Scholar] [CrossRef] [Green Version]
- Gallardo, C.; Soler, A.; Rodze, I.; Nieto, R.; Cano-Gómez, C.; Fernandez-Pinero, J.; Arias, M. Attenuated and non-haemadsorbing (non-HAD) genotype II African swine fever virus (ASFV) isolated in Europe, Latvia 2017. Transbound. Emerg. Dis. 2019, 66, 22. [Google Scholar] [CrossRef]
- Yanyan, Z. Identification of a natural variant of African swine fever virus in China. Chin. J. Vet. Med. 2021, 41, 199–207. [Google Scholar]
- Sun, E.; Zhang, Z.; Wang, Z.; He, X.; Zhang, X.; Wang, L.; Wang, W.; Huang, L.; Xi, F.; Huangfu, H.; et al. Emergence and prevalence of naturally occurring lower virulent African swine fever viruses in domestic pigs in China in 2020. Sci. China Life Sci. 2021, 64, 752–765. [Google Scholar] [CrossRef] [PubMed]
- World Organization for Animal Health (OIE). Manual of Diagnostic Tests and Vaccines for Terrestrial Animals 2019. Chapter 3.8.1—African Swine Fever (Infection with African Swine Fever Virus); OIE: Paris, France, 2019.
- King, D.P.; Reid, S.M.; Hutchings, G.H.; Grierson, S.S.; Wilkinson, P.J.; Dixon, L.K.; Bastos, A.D.; Drew, T.W. Development of a TaqMan® PCR assay with internal amplification control for the detection of African swine fever virus. J. Virol. Methods 2003, 107, 53–61. [Google Scholar] [CrossRef]
- Song, C.; Zhu, C.; Zhang, C.; Cui, S. Detection of porcine parvovirus using a taqman-based real-time pcr with primers and probe designed for the NS1 gene. Virol. J. 2010, 7, 353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Opriessnig, T.; Yu, S.; Gallup, J.M.; Evans, R.B.; Fenaux, M.; Pallares, F.; Thacker, E.L.; Brockus, C.W.; Ackermann, M.R.; Thomas, P.; et al. Effect of Vaccination with Selective Bacterins on Conventional Pigs Infected with Type 2 Porcine Circovirus. Vet. Pathol. 2003, 40, 521–529. [Google Scholar] [CrossRef] [Green Version]
- Sanna, G.; Dei Giudici, S.; Bacciu, D.; Angioi, P.P.; Giammarioli, M.; De Mia, G.M.; Oggiano, A. Improved strategy for molecular characterization of African swine fever virus from Sardinia, based on analysis of p30, CD2V and I73R/I329L variable regions. Transbound. and Emerg. Dis. 2017, 64, 1280–1286. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl. Acids. Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular Evolutionary Genetics Analysis Using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://support.illumina.com/sequencing/sequencing_software/bcl2fastq-conversion-software.html (accessed on 1 October 2022).
- Available online: https://github.com/FelixKrueger/TrimGalore (accessed on 1 October 2022).
- Groenen, M.A.M.; Archibald, A.L.; Uenishi, H.; Tuggle, C.K.; Takeuchi, Y.; Rothschild, M.F.; Rogel-Gaillard, C.; Park, C.; Milan, D.; Megens, H.-J.; et al. Analyses of pig genomes provide insight into porcine demography and evolution. Nature 2012, 491, 393–398. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows—Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef] [Green Version]
- Marco-Sola, S.; Sammeth, M.; Guigó, R.; Ribeca, P. The GEM mapper: Fast, accurate and versatile alignment by filtration. Nat. Methods 2012, 9, 1185–1188. [Google Scholar] [CrossRef]
- Available online: https://broadinstitute.github.io/picard (accessed on 1 October 2022).
- Li, H. A statistical framework for SNP calling, mutation discovery, association mapping and population genetical parameter estimation from sequencing data. Bioinformatics 2011, 27, 2987–2993. [Google Scholar] [CrossRef] [Green Version]
- Garrison, E.; Marth, G. Haplotype-Based Variant Detection from Short-Read Sequencing; Cornell University Press: Ithaca, NY, USA, 2012. [Google Scholar]
- Granberg, F.; Torresi, C.; Oggiano, A.; Malmberg, M.; Iscaro, C.; De Mia, G.M.; Belák, S. Complete Genome Sequence of an African Swine Fever Virus Isolate from Sardinia, Italy. Genome Announc. 2016, 4, e01220-16. [Google Scholar] [CrossRef] [Green Version]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [Green Version]
- Waterhouse, A.M.; Procter, J.B.; Martin, D.M.A.; Clamp, M.; Barton, G.J. Jalview Version 2—A multiple sequence alignment editor and analysis workbench. Bioinformatics 2009, 25, 1189–1191. [Google Scholar] [CrossRef] [Green Version]
- Robinson, J.T.; Thorvaldsdóttir, H.; Winckler, W.; Guttman, M.; Lander, E.S.; Getz, G.; Mesirov, J.P. Integrative genomics viewer. Nat. Biotechnol. 2011, 29, 24–26. [Google Scholar] [CrossRef] [Green Version]
- Tcherepanov, V.; Ehlers, A.; Upton, C. Genome Annotation Transfer Utility (GATU): Rapid annotation of viral genomes using a closely related reference genome. BMC Genomics 2006, 7, 150. [Google Scholar] [CrossRef]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef] [Green Version]
- Sagulenko, P.; Puller, V.; Neher, R.A. TreeTime: Maximum-likelihood phylodynamic analysis. Virus Evol. 2018, 4, vex042. [Google Scholar] [CrossRef] [Green Version]
- Cappai, S.; Baldi, I.; Desini, P.; Pintore, A.; Denurra, D.; Cherchi, M.; Rolesu, S.; Mandas, D.; Franzoni, G.; Fiori, M.S.; et al. Changes in Estimating the Wild Boar Carcasses Sampling Effort: Applying the EFSA ASF Exit Strategy by Means of the WBC-Counter Tool. Viruses 2022, 14, 1424. [Google Scholar] [CrossRef] [PubMed]
- Franzoni, G.; Giudici, S.D.; Loi, F.; Sanna, D.; Floris, M.; Fiori, M.; Sanna, M.L.; Madrau, P.; Scarpa, F.; Zinellu, S.; et al. African Swine Fever Circulation among Free-Ranging Pigs in Sardinia: Data from the Eradication Program. Vaccines 2020, 8, 549. [Google Scholar] [CrossRef] [PubMed]
- Torresi, C.; Fiori, M.S.; Bertolotti, L.; Floris, M.; Colitti, B.; Giammarioli, M.; Giudici, S.D.; Oggiano, A.; Malmberg, M.; De Mia, G.M.; et al. The evolution of African swine fever virus in Sardinia (1978 to 2014) as revealed by whole genome sequencing and comparative analysis. Transbound. Emerg. Dis. 2020, 67, 1971–1980. [Google Scholar] [CrossRef] [PubMed]
- Ottaviani, D.; LeCain, M.; Sheer, D. The role of microhomology in genomic structural variation. Trends Genet. 2014, 30, 85–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glover, L.; Jun, J.; Horn, D. Microhomology-mediated deletion and gene conversion in African trypanosomes. Nucleic Acids Res. 2011, 39, 1372–1380. [Google Scholar] [CrossRef] [Green Version]
- McVey, M.; Lee, S.E. MMEJ repair of double-strand breaks (director’s cut): Deleted sequences and alternative endings. Trends Genet. 2008, 24, 529–538. [Google Scholar] [CrossRef] [Green Version]
- Cappai, S.; Rolesu, S.; Coccollone, A.; Laddomada, A.; Loi, F. Evaluation of biological and socio-economic factors related to persistence of African swine fever in Sardinia. Prev. Vet. Med. 2018, 152, 1–11. [Google Scholar] [CrossRef]
- Loi, F.; Cappai, S.; Coccollone, A.; Rolesu, S. Standardized Risk Analysis Approach Aimed to Evaluate the Last African Swine Fever Eradication Program Performance, in Sardinia. Front. Vet. Sci. 2019, 6, 299. [Google Scholar] [CrossRef]
- Zhenzhong, W.; Chuanxiang, Q.; Shengqiang, G.; Jinming, L.; Yongxin, H.; Xiaoyue, Z.; Yan, L.; Naijun, H.; Xiaodong, W.; Zhiliang, W.; et al. Genetic variation and evolution of attenuated African swine fever virus strain isolated in the field: A review. Virus Res. 2022, 319, 198874. [Google Scholar] [CrossRef]
- Zhu, Z.; Chen, H.; Liu, L.; Cao, Y.; Jiang, T.; Zou, Y.; Peng, Y. Classification and characterization of multigene family proteins of African swine fever viruses. Brief. Bioinform. 2021, 22, bbaa380. [Google Scholar] [CrossRef]
- Rathakrishnan, A.; Connell, S.; Petrovan, V.; Moffat, K.; Goatley, L.C.; Jabbar, T.; Sánchez-Cordón, P.J.; Reis, A.L.; Dixon, L.K. Differential Effect of Deleting Members of African Swine Fever Virus Multigene Families 360 and 505 from the Genotype II Georgia 2007/1 Isolate on Virus Replication, Virulence, and Induction of Protection. J. Virol. 2022, 96, e0189921. [Google Scholar] [CrossRef]
- Zheng, X.; Nie, S.; Feng, W.-H. Regulation of antiviral immune response by African swine fever virus (ASFV). Virol. Sin. 2022, 37, 157–167. [Google Scholar] [CrossRef]
- Mazloum, A.; Zhukov, I.U.; Aronova, E.B.; Igolkin, A.S.; Vlasova, N.N. ASF virus replication features in the presence of recombinant proteins CD2v, pX69R and pE248R. Probl. Virol. 2019, 64, 193–200. [Google Scholar] [CrossRef] [Green Version]
- Ramirez-Medina, E.; Vuono, E.; Pruitt, S.; Rai, A.; Silva, E.; Zhu, J.; Velazquez-Salinas, L.; Gladue, D.P.; Borca, M.V. X69R Is a Non-Essential Gene That, When Deleted from African Swine Fever, Does Not Affect Virulence in Swine. Viruses 2020, 12, 918. [Google Scholar] [CrossRef]
- Ferretti, L.; Di Nardo, A.; Singer, B.; Lasecka-Dykes, L.; Logan, G.; Wright, C.F.; Pérez-Martín, E.; King, D.P.; Tuthill, T.J.; Ribeca, P. Within-Host Recombination in the Foot-and-Mouth Disease Virus Genome. Viruses 2018, 10, 221. [Google Scholar] [CrossRef] [Green Version]
- Ferretti, L.; Pérez-Martín, E.; Zhang, F.; Maree, F.; De Klerk-Lorist, L.-M.; Van Schalkwykc, L.; Juleff, N.D.; Charleston, B.; Ribeca, P. Correction: Pervasive within-host recombination and epistasis as major determinants of the molecular evolution of the foot-and-mouth disease virus capsid. PLoS Pathog. 2020, 16, e1009050. [Google Scholar] [CrossRef]
- Michaud, V.; Randriamparany, T.; Albina, E. Comprehensive Phylogenetic Reconstructions of African Swine Fever Virus: Proposal for a New Classification and Molecular Dating of the Virus. PLoS ONE 2013, 8, e69662. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Xiao, K.; Zhang, Z.; Yang, J.; Wang, R.; Shen, X.; Pan, J.; Irwin, D.M.; Chen, R.-A.; Shen, Y. The recombination hot spots and genetic diversity of the genomes of African swine fever viruses. J. Infect. 2020, 80, 121–142. [Google Scholar] [CrossRef]
- Nefedeva, M.; Titov, I.; Tsybanov, S.; Malogolovkin, A. Recombination shapes African swine fever virus serotype-specific locus evolution. Sci. Rep. 2020, 10, 18474. [Google Scholar] [CrossRef]



| Strain ID ° | Hunting Time (Year and Month) | Municipality (Province) | HMU § | Host Species | Genotype | Organ | Ct Value * | Malmquist Test | Elisa | IB # |
|---|---|---|---|---|---|---|---|---|---|---|
| 7303WB/19 | January 2019 | Pattada (Sassari) | Goceano-Gallura | Wild Boar | I | Lung | 36.2 | pos | pos | pos |
| 7212WB/19 | January 2019 | Lanusei (Nuoro) | Gennargentu-Ogliastra | Wild Boar | I | Spleen | 19.37 | pos | neg | neg |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiori, M.S.; Ferretti, L.; Di Nardo, A.; Zhao, L.; Zinellu, S.; Angioi, P.P.; Floris, M.; Sechi, A.M.; Denti, S.; Cappai, S.; et al. A Naturally Occurring Microhomology-Mediated Deletion of Three Genes in African Swine Fever Virus Isolated from Two Sardinian Wild Boars. Viruses 2022, 14, 2524. https://doi.org/10.3390/v14112524
Fiori MS, Ferretti L, Di Nardo A, Zhao L, Zinellu S, Angioi PP, Floris M, Sechi AM, Denti S, Cappai S, et al. A Naturally Occurring Microhomology-Mediated Deletion of Three Genes in African Swine Fever Virus Isolated from Two Sardinian Wild Boars. Viruses. 2022; 14(11):2524. https://doi.org/10.3390/v14112524
Chicago/Turabian StyleFiori, Mariangela Stefania, Luca Ferretti, Antonello Di Nardo, Lele Zhao, Susanna Zinellu, Pier Paolo Angioi, Matteo Floris, Anna Maria Sechi, Stefano Denti, Stefano Cappai, and et al. 2022. "A Naturally Occurring Microhomology-Mediated Deletion of Three Genes in African Swine Fever Virus Isolated from Two Sardinian Wild Boars" Viruses 14, no. 11: 2524. https://doi.org/10.3390/v14112524






