The Defense Response of Nicotiana benthamiana to Peanut Stunt Virus Infection in the Presence of Symptom Exacerbating Satellite RNA
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Generation of Infectious PSV-G Transcripts
2.2. RNA Extraction and Detection of Viral and satRNA in the Plants
2.3. Assessment of Virus Accumulation in the Plants and Validation of the Gene Expression for Chosen Transcripts by RT-qPCR
2.4. Transcriptome Profiling with Species-Specific Microarrays
2.5. Data Acquisition and Statistical Analysis
2.6. Functional Annotation of Differentially Expressed Genes and Pathway Analysis
3. Results
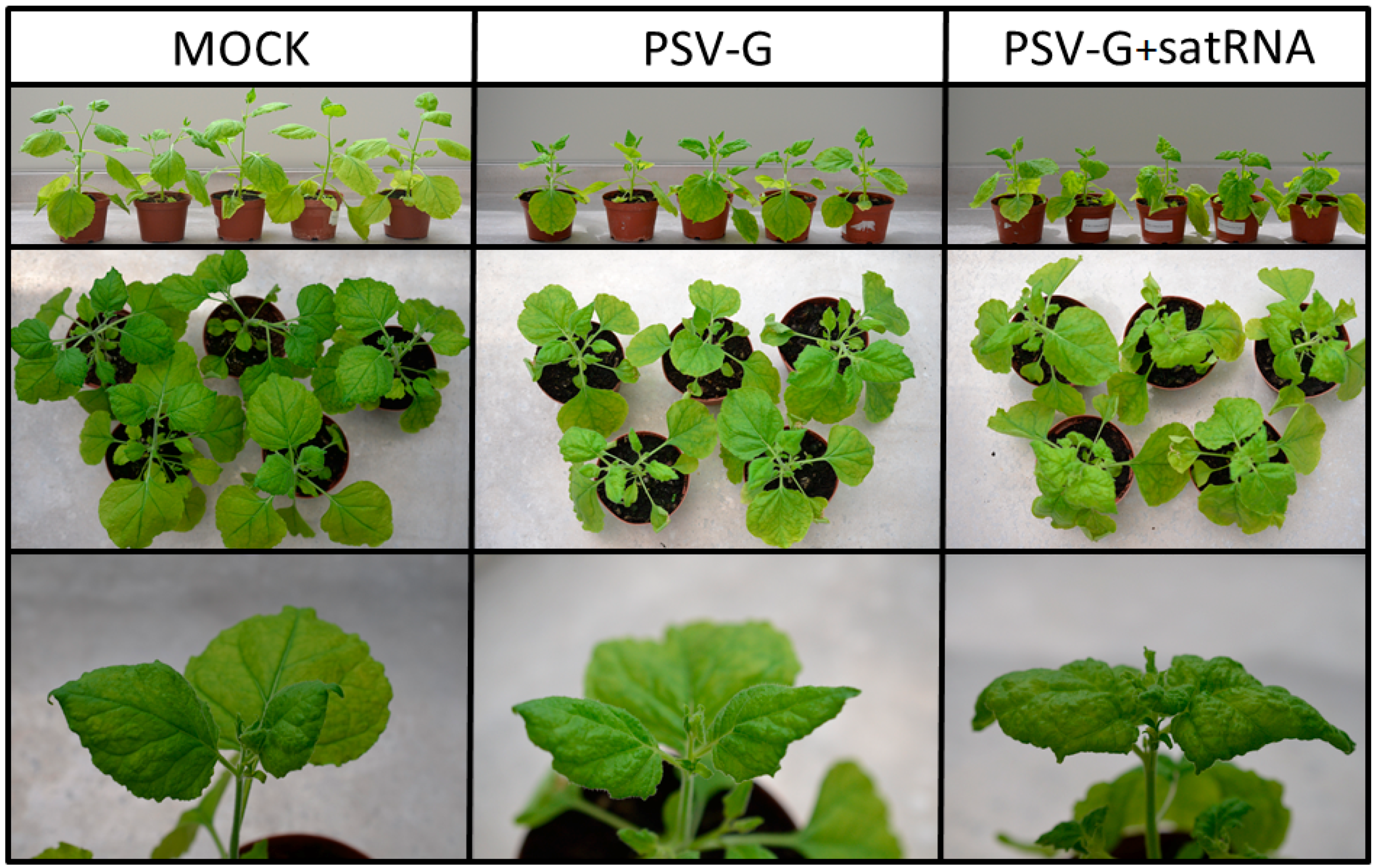
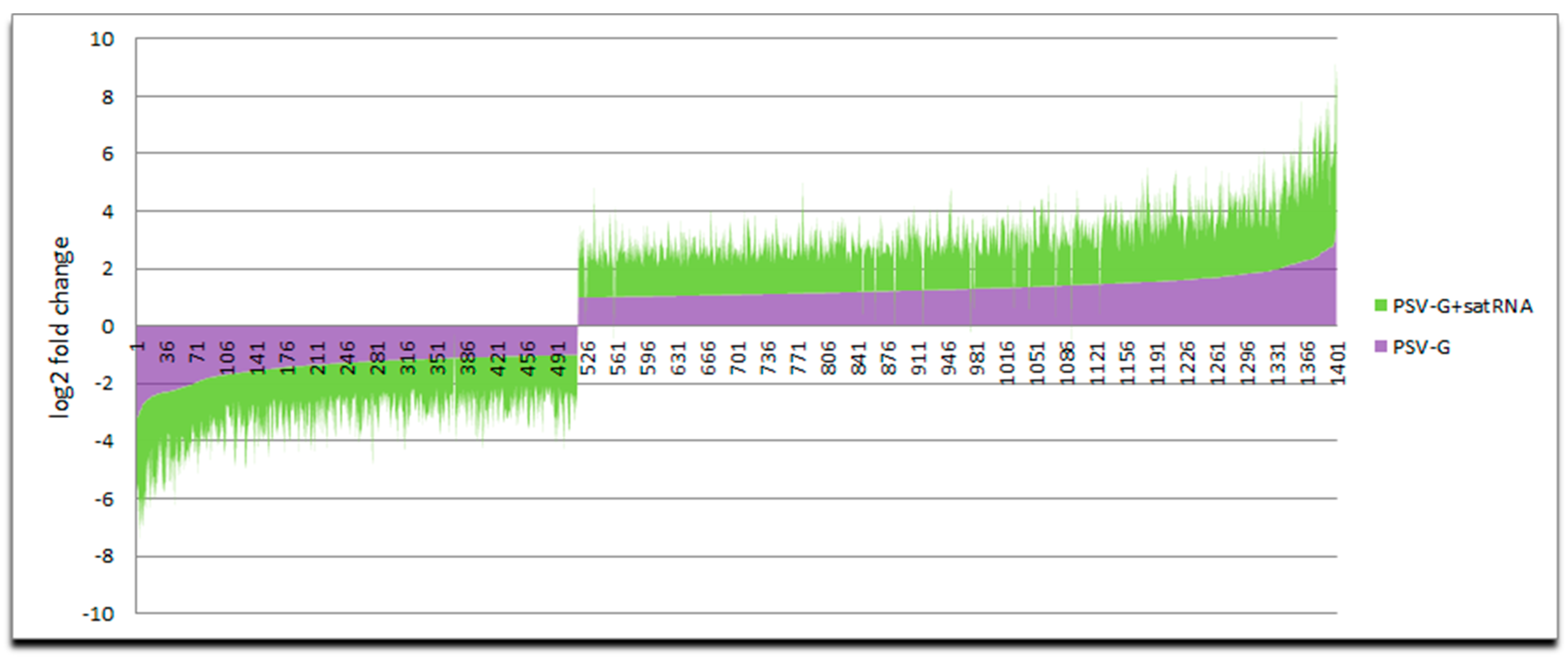
3.1. PSV-G Induces Strong Plant Defense but Addition of satRNA Exacerbates Symptoms Expression and Enhances Transcriptional Reprogramming in Infected Plant
3.2. Functional Categorization of PSV and satRNA Responsive Genes Indicates Deep Changes in Primary Metabolism and Stress Response in Infected Plants
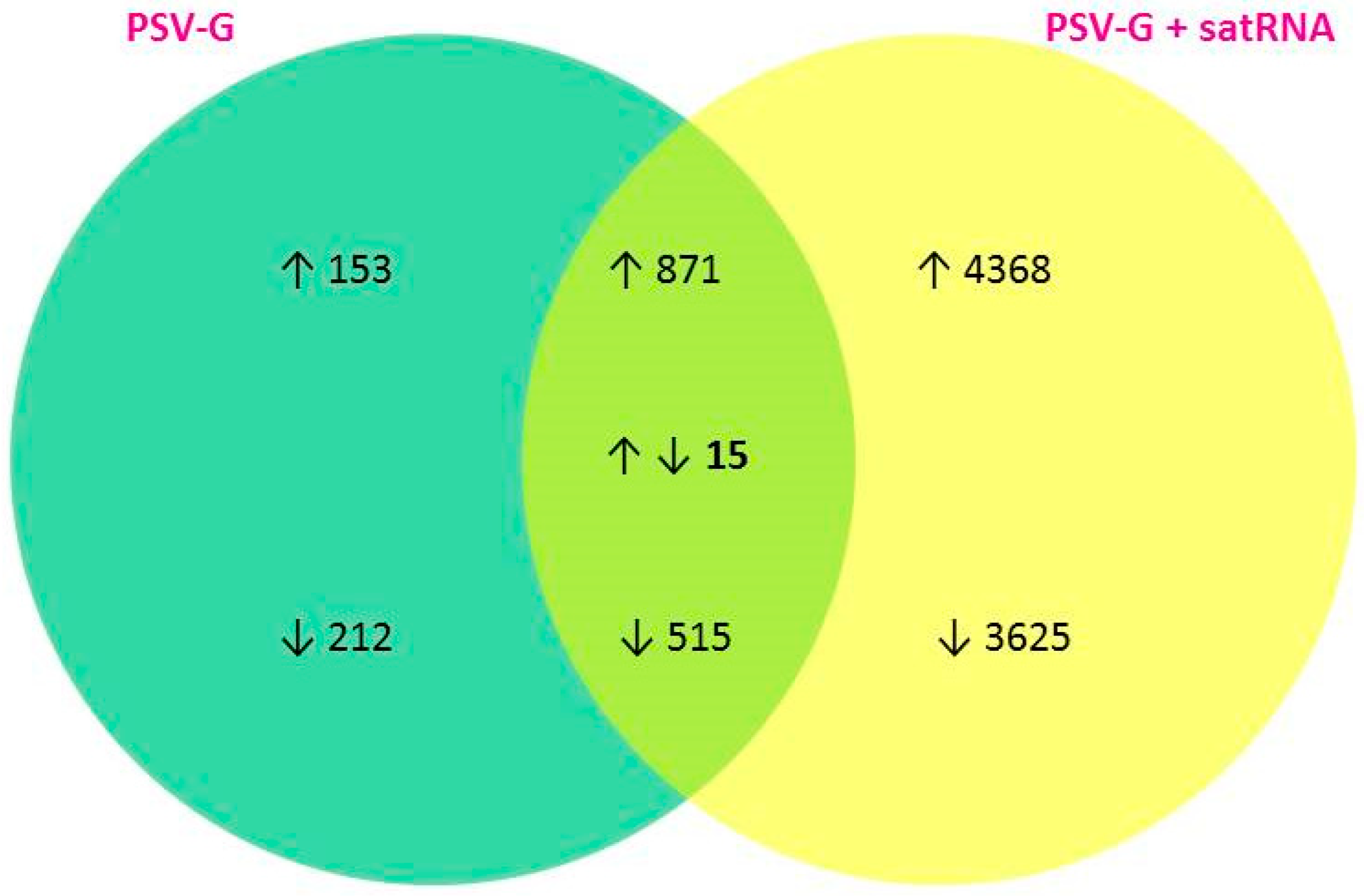
3.3. Up-Regulated and Down-Regulated Plant Transcripts upon PSV Infection or PSV + satRNA Co-Infection
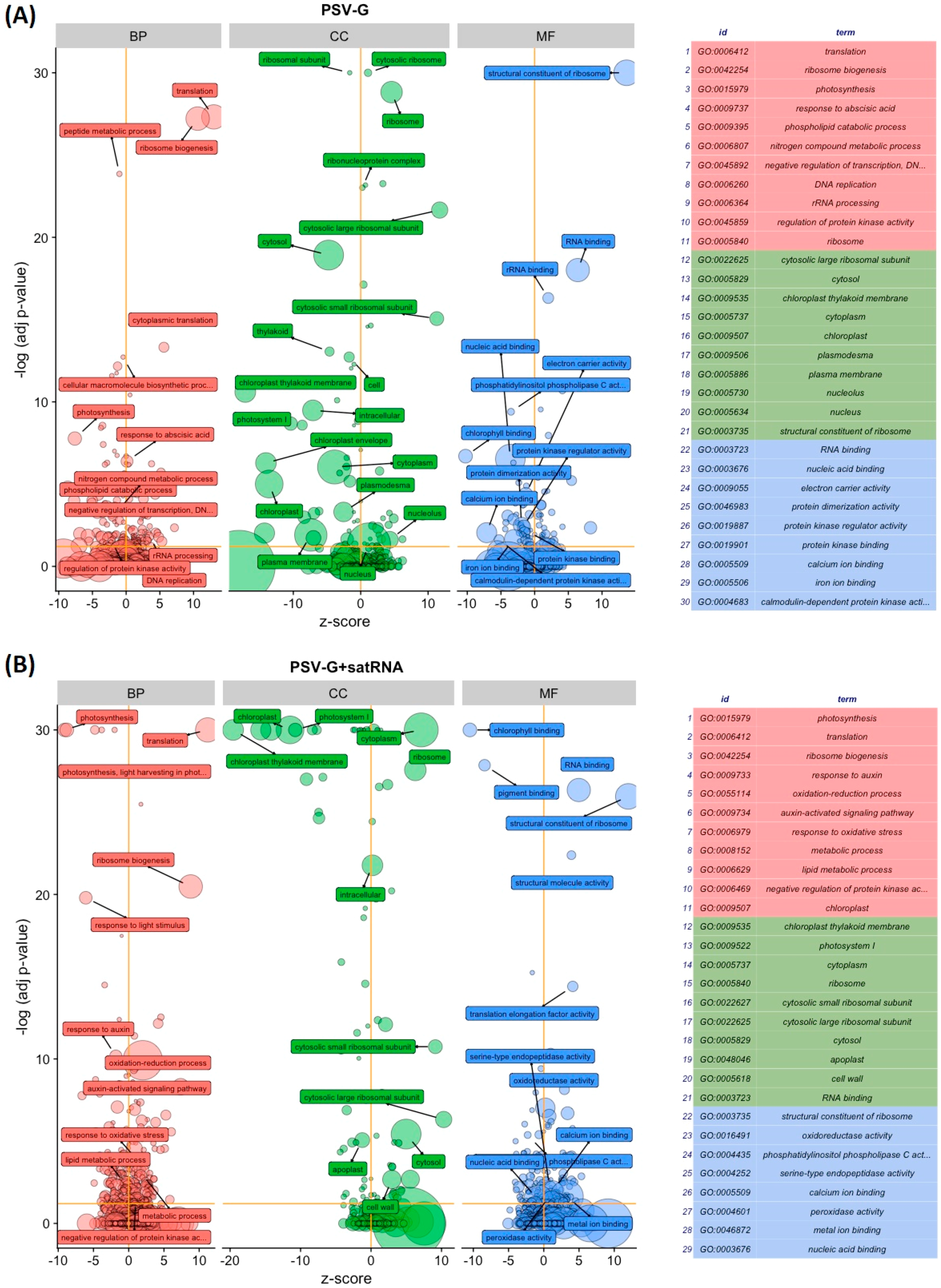
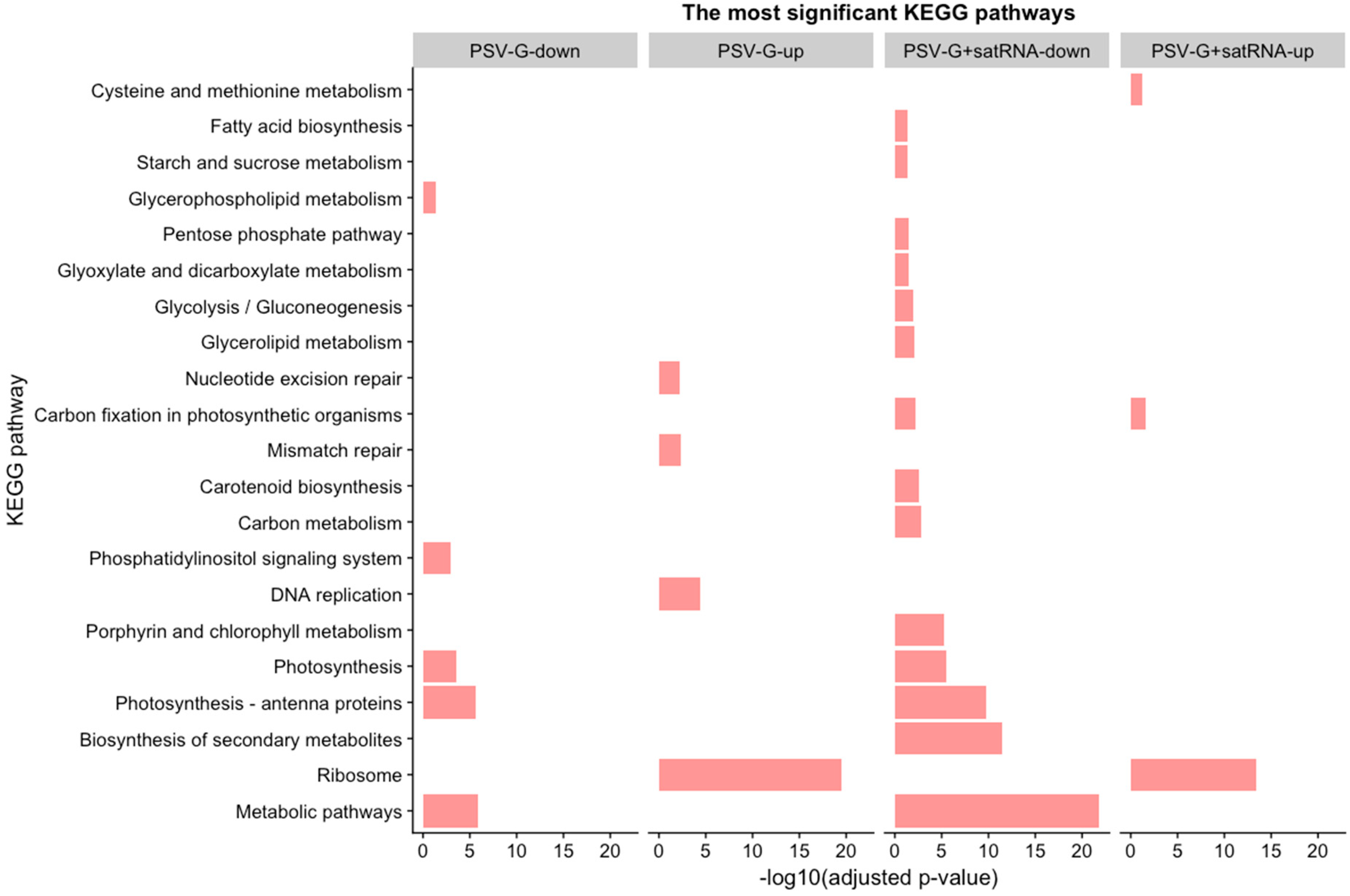
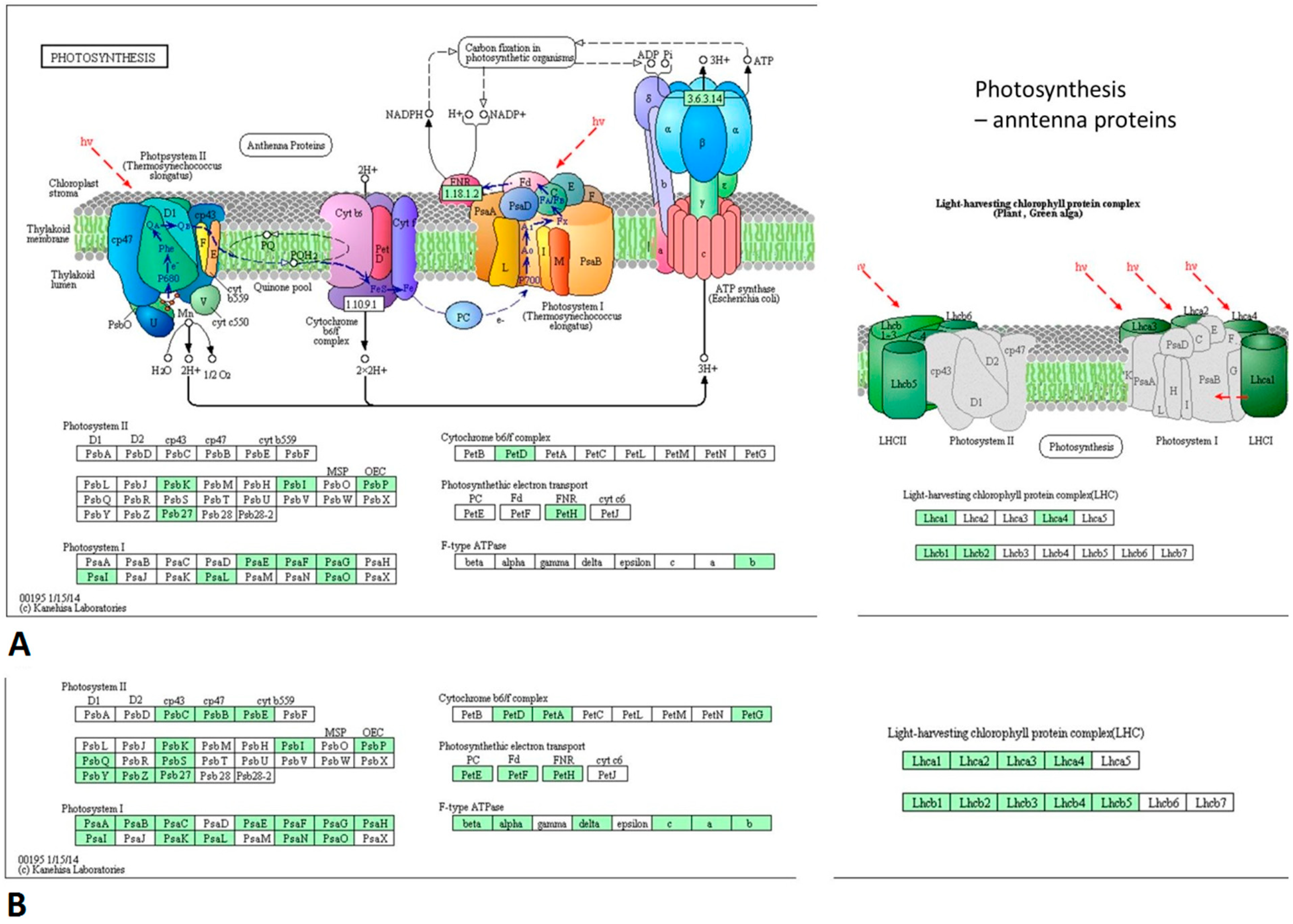
3.4. PSV-Infected Plants Show Down-Regulation of Genes Involved in Photosynthesis and Up-Regulation of Genes Associated with Ribosome
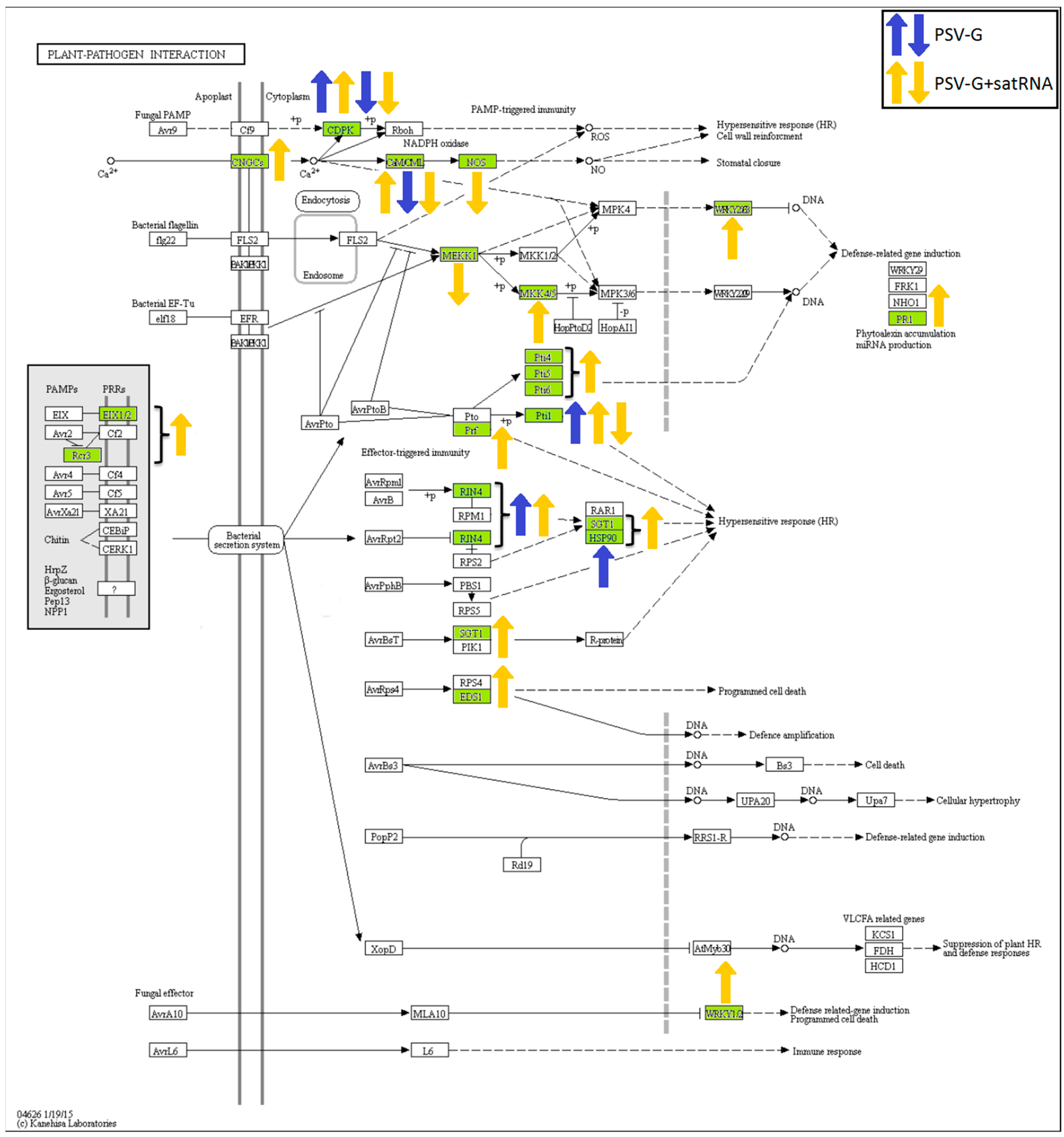
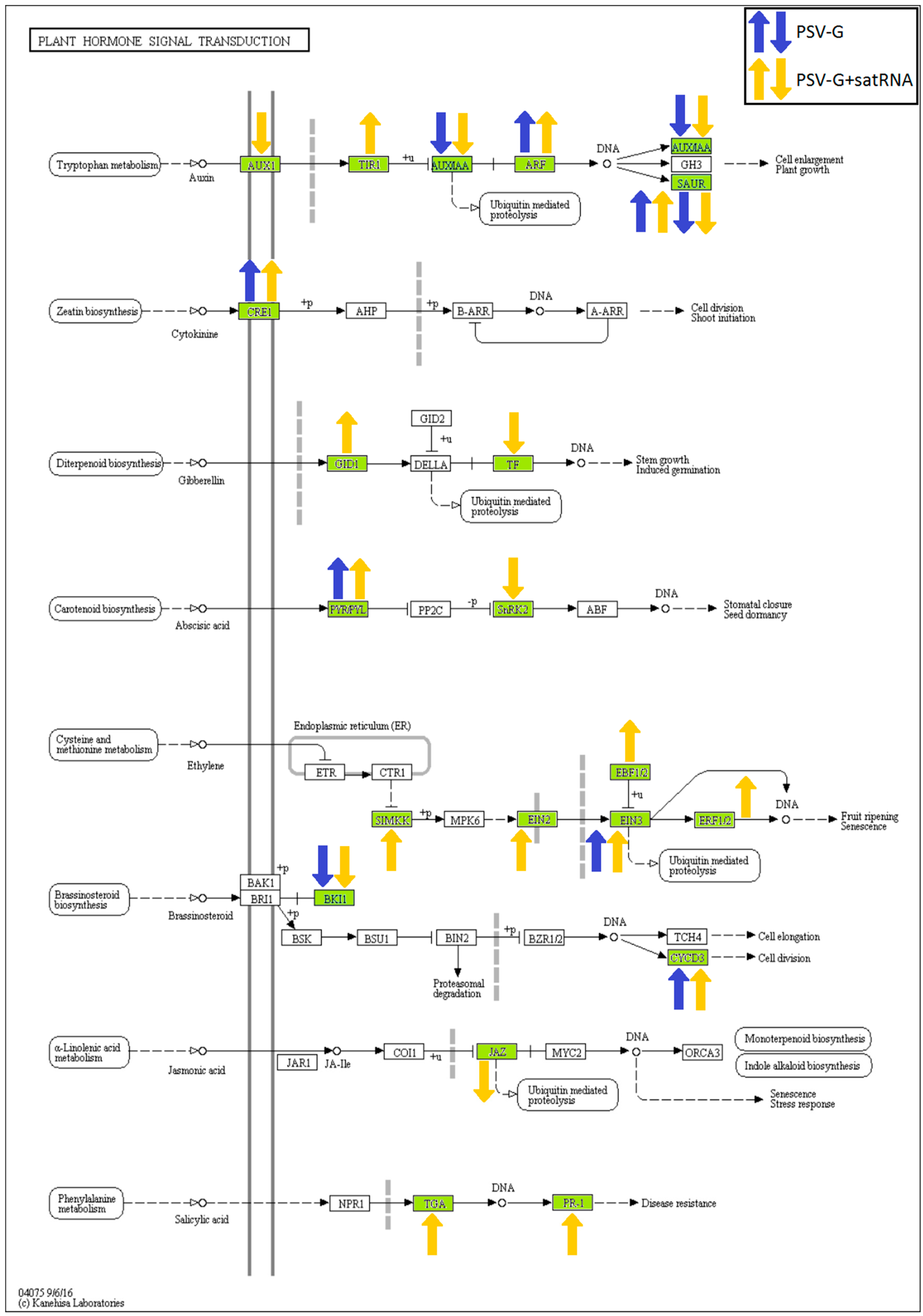
3.5. Processes Associated with Plant–Pathogen Interaction and Plant Defense
3.6. Posttranscriptional Gene Silencing (PTGS)
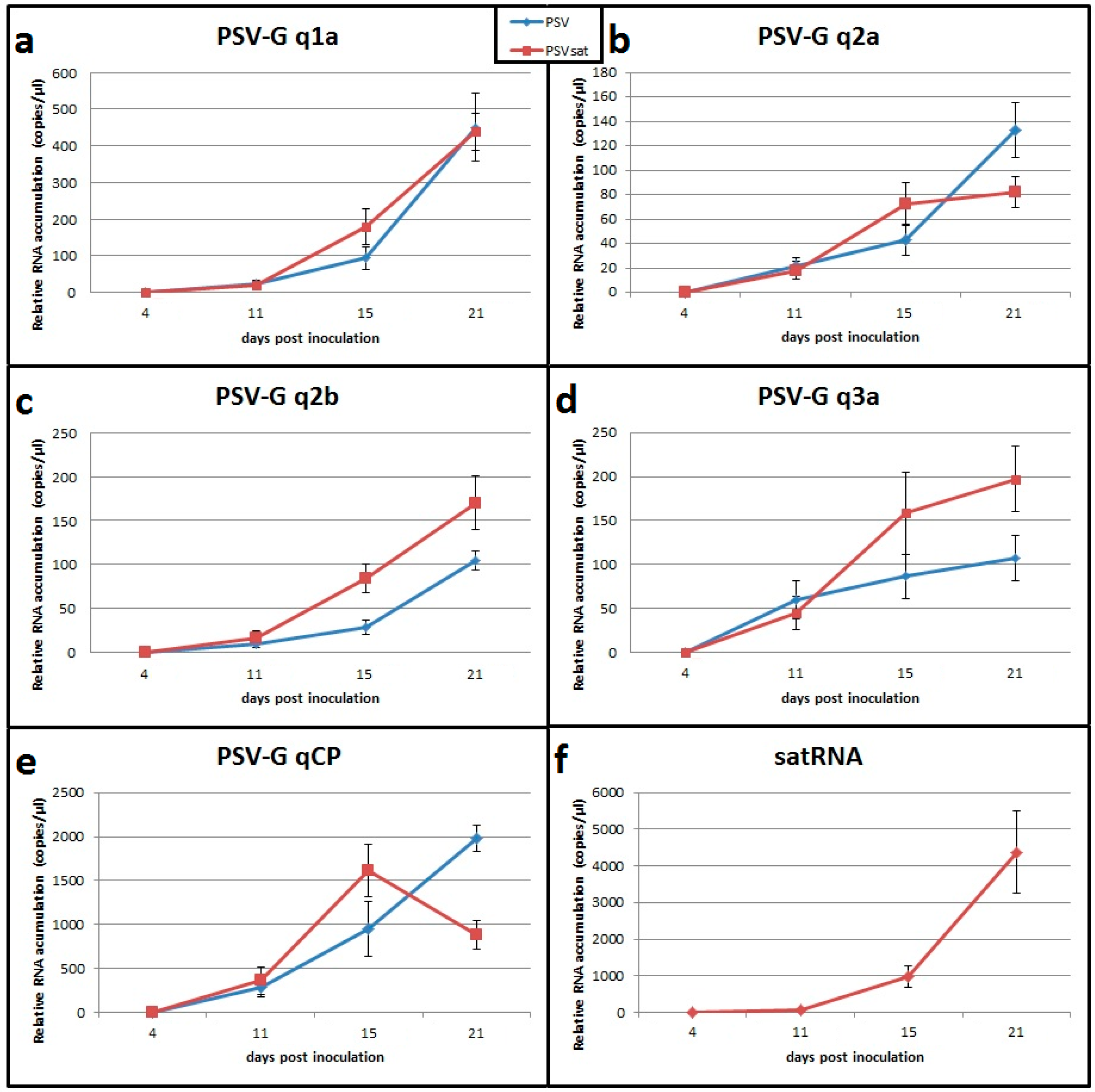
3.7. Validation of Transcriptomic Data by RT-qPCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kiss, L.; Balázs, E.; Salánki, K. Characterisation of black locust isolates of Peanut stunt virus (PSV) from the Pannon ecoregion show the frequent occurrence of the fourth taxonomic PSV subgroup. Eur. J. Plant Pathol. 2009, 125, 671. [Google Scholar] [CrossRef]
- Saruta, M.; Takada, Y.; Kikuchi, A.; Yamada, T.; Komatsu, K.; Sayama, T.; Ishimoto, M.; Okabe, A. Screening and genetic analysis of resistance to peanut stunt virus in soybean: Identification of the putative Rpsv1 resistance gene. Breed. Sci. 2012, 61, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Pourrahim, R.; Farzadfar, S. Analysis of coat protein of Peanut stunt virus subgroup II isolates from alfalfa fields in West Iran. J. Phytopathol. 2014, 162, 527–531. [Google Scholar] [CrossRef]
- Obrepalska-Steplowska, A.; Budziszewska, M.; Pospieszny, H. Complete nucleotide sequence of a Polish strain of Peanut stunt virus (PSV-P) that is related to but not a typical member of subgroup I. Acta Biochim. Pol. 2008, 55, 731–739. [Google Scholar] [PubMed]
- Mink, G.; Silbernagel, M.; Saksena, K. Host range, purification, and properties of the western strain of peanut stunt virus. Phytopathology 1969, 59, 1625–1631. [Google Scholar] [PubMed]
- Netsu, O.; Hiratsuka, K.; Kuwata, S.; Hibi, T.; Ugaki, M.; Suzuki, M. Peanut stunt virus 2B cistron plays a role in viral local and systemic accumulation and virulence in Nicotiana benthamiana. Arch. Virol. 2008, 153, 1731–1735. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.-W.; Anderson, B.J.; Haase, H.R.; Symons, R.H. New overlapping gene encoded by the cucumber mosaic virus genome. Virology 1994, 198, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Schwinghamer, M.W.; Symons, R.H. Fractionation of cucumber mosaic virus RNA and its translation in a wheat embryo cell-free system. Virology 1975, 63, 252–262. [Google Scholar] [CrossRef]
- Obrepalska-Steplowska, A.; Nowaczyk, K.; Budziszewska, M.; Czerwoniec, A.; Pospieszny, H. The sequence and model structure analysis of three Polish peanut stunt virus strains. Virus Genes 2008, 36, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Naidu, R.; Collins, G.; Ghabrial, S. Symptom-modulating properties of peanut stunt virus satellite RNA sequence variants. Mol. Plant Microbe Interact. 1990, 4, 268–275. [Google Scholar] [CrossRef]
- Naidu, R.; Collins, G.; Ghabrial, S. Peanut stunt virus satellite RNA: Analysis of sequences that affect symptom attenuation in tobacco. Virology 1992, 189, 668–677. [Google Scholar] [CrossRef]
- Obrępalska-Stęplowska, A.; Budziszewska, M.; Wieczorek, P.; Czerwoniec, A. Analysis of two strains of Peanut stunt virus: satRNA-associated and satRNA free. Virus Genes 2012, 44, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Obrepalska-Steplowska, A. Rola satRNA w patogenezie szczepow wirusa karlowatosci orzecha ziemnego (PSV) wystepujacych w Polsce. Rozpr. Nauk. Inst. Ochr. Roślin 2010, 22, 1–110. [Google Scholar]
- Palukaitis, P. Satellite RNAs and satellite viruses. Mol. Plant Microbe Interact. 2016, 29, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Pelczyk, M.; Obrepalska-Steplowska, A.; Pospieszny, H. Subviral molecules of RNA associated with plant ss (+) RNA viruses. Postepy Biochem. 2005, 52, 212–221. [Google Scholar]
- Xu, P.; Blancaflor, E.B.; Roossinck, M.J. In spite of induced multiple defense responses, tomato plants infected with Cucumber mosaic virus and D satellite RNA succumb to systemic necrosis. Mol. Plant Microbe Interact. 2003, 16, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Lai, L.; Lin, R.; Jin, C.; Chen, J. Differential effects of Cucumber mosaic virus satellite RNAs in the perturbation of microRNA-regulated gene expression in tomato. Mol. Biol. Rep. 2012, 39, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Xi, D.-H.; Huang, Q.-R.; Xu, M.-Y.; Yuan, S.; Wang, S.-D.; Jia, S.-D.; Cao, S.; Zhou, Z.-L.; Lin, H.-H. Effect of two satellite RNAs on Nicotiana glutinosa infected with Cucumber mosaic virus (CMV). Physiol. Mol. Plant Pathol. 2009, 74, 184–190. [Google Scholar] [CrossRef]
- Obrępalska-Stęplowska, A.; Wieczorek, P.; Budziszewska, M.; Jeszke, A.; Renaut, J. How can plant virus satellite RNAs alter the effects of plant virus infection? A study of the changes in the Nicotiana benthamiana proteome after infection by Peanut stunt virus in the presence or absence of its satellite RNA. Proteomics 2013, 13, 2162–2175. [Google Scholar] [CrossRef] [PubMed]
- Shimura, H.; Pantaleo, V.; Ishihara, T.; Myojo, N.; Inaba, J.-I.; Sueda, K.; Burgyán, J.; Masuta, C. A viral satellite RNA induces yellow symptoms on tobacco by targeting a gene involved in chlorophyll biosynthesis using the RNA silencing machinery. PLoS Pathog. 2011, 7, e1002021. [Google Scholar] [CrossRef] [PubMed]
- Palukaitis, P. Pathogenicity regulation by satellite RNAs of cucumber mosaic virus: Minor nucleotide sequence changes alter host responses. Mol. Plant Microbe Interact. 1988, 1, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Jaegle, M.; Devic, M.; Longstaff, M.; Baulcombe, D. Cucumber mosaic virus satellite RNA (Y strain): Analysis of sequences which affect yellow mosaic symptoms on tobacco. J. Gen. Virol. 1990, 71, 1905–1912. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Simon, A.E. Fitness of a Turnip crinkle virus satellite RNA correlates with a sequence-nonspecific hairpin and flanking sequences that enhance replication and repress the accumulation of virions. J. Virol. 2003, 77, 7880–7889. [Google Scholar] [CrossRef] [PubMed]
- Obrępalska-Stęplowska, A.; Renaut, J.; Planchon, S.; Przybylska, A.; Wieczorek, P.; Barylski, J.; Palukaitis, P. Effect of temperature on the pathogenesis, accumulation of viral and satellite RNAs and on plant proteome in peanut stunt virus and satellite RNA-infected plants. Front. Plant Sci. 2015, 6, 903. [Google Scholar] [CrossRef] [PubMed]
- Baek, E.; Yoon, J.-Y.; Palukaitis, P. Validation of reference genes for quantifying changes in gene expression in virus-infected tobacco. Virology 2017, 510, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Rotenberg, D.; Thompson, T.S.; German, T.L.; Willis, D.K. Methods for effective real-time RT-PCR analysis of virus-induced gene silencing. J. Virol. Methods 2006, 138, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W.; Horgan, G.W.; Dempfle, L. Relative expression software tool (REST©) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002, 30, e36. [Google Scholar] [CrossRef] [PubMed]
- Goralski, M.; Sobieszczanska, P.; Obrepalska-Steplowska, A.; Swiercz, A.; Zmienko, A.; Figlerowicz, M. A gene expression microarray for Nicotiana benthamiana based on de novo transcriptome sequence assembly. Plant Methods 2016, 12, 28. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef] [PubMed]
- Barrett, T.; Wilhite, S.E.; Ledoux, P.; Evangelista, C.; Kim, I.F.; Tomashevsky, M.; Marshall, K.A.; Phillippy, K.H.; Sherman, P.M.; Holko, M. NCBI GEO: Archive for functional genomics data sets—Update. Nucleic Acids Res. 2012, 41, D991–D995. [Google Scholar] [CrossRef] [PubMed]
- Conesa, A.; Götz, S.; García-Gómez, J.M.; Terol, J.; Talón, M.; Robles, M. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 2005, 21, 3674–3676. [Google Scholar] [CrossRef] [PubMed]
- Hunter, S.; Apweiler, R.; Attwood, T.K.; Bairoch, A.; Bateman, A.; Binns, D.; Bork, P.; Das, U.; Daugherty, L.; Duquenne, L. InterPro: The integrative protein signature database. Nucleic Acids Res. 2009, 37, D211–D215. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.A. The logic of inductive inference. J. R. Stat. Soc. 1935, 98, 39–82. [Google Scholar] [CrossRef]
- Walter, W.; Sánchez-Cabo, F.; Ricote, M. GOplot: An R package for visually combining expression data with functional analysis. Bioinformatics 2015, 31, 2912–2914. [Google Scholar] [CrossRef] [PubMed]
- Moriya, Y.; Itoh, M.; Okuda, S.; Yoshizawa, A.C.; Kanehisa, M. KAAS: An automatic genome annotation and pathway reconstruction server. Nucleic Acids Res. 2007, 35, W182–W185. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Guo, Y.; Shyr, Y. KEGGprofile: An annotation and visualization package for multi-types and multi-groups expression data in KEGG pathway. R Package Version 2012, 1. [Google Scholar] [CrossRef]
- Kachitvichyanukul, V.; Schmeiser, B. Computer generation of hypergeometric random variates. J. Stat. Comput. Simul. 1985, 22, 127–145. [Google Scholar] [CrossRef]
- Nakasugi, K.; Crowhurst, R.N.; Bally, J.; Wood, C.C.; Hellens, R.P.; Waterhouse, P.M. De novo transcriptome sequence assembly and analysis of RNA silencing genes of Nicotiana benthamiana. PLoS ONE 2013, 8, e59534. [Google Scholar] [CrossRef] [PubMed]
- Nakasugi, K.; Crowhurst, R.; Bally, J.; Waterhouse, P. Combining transcriptome assemblies from multiple de novo assemblers in the allo-tetraploid plant Nicotiana benthamiana. PLoS ONE 2014, 9, e91776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, W.-N.; Duan, C.-G.; Fang, R.-X.; Zhou, X.-Y.; Guo, H.-S. Satellite RNA reduces expression of the 2b suppressor protein resulting in the attenuation of symptoms caused by Cucumber mosaic virus infection. Mol. Plant Pathol. 2011, 12, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Simon, A.E. Symptom attenuation by a satellite RNA in vivo is dependent on reduced levels of virus coat protein. Virology 1999, 259, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Sayama, H.; Sato, T.; Kominato, M.; Natsuaki, T.; Kaper, J. Field testing of a satellite-containing attenuated strain of cucumber mosaic virus for tomato protection in Japan. Phytopathology 1993, 83, 405–410. [Google Scholar] [CrossRef]
- Hsu, Y.-H.; Chen, H.-C.; Cheng, J.; Annamali, P.; Lin, B.-Y.; Wu, C.-T.; Yeh, W.-B.; Lin, N.-S. Crucial role of the 5′ conserved structure of bamboo mosaic virus satellite RNA in downregulation of helper viral RNA replication. J. Virol. 2006, 80, 2566–2574. [Google Scholar] [CrossRef] [PubMed]
- Gamage, S.M.W.; McGrath, D.J.; Persley, D.M.; Dietzgen, R.G. Transcriptome Analysis of Capsicum Chlorosis Virus-Induced Hypersensitive Resistance Response in Bell Capsicum. PLoS ONE 2016, 11, e0159085. [Google Scholar]
- Martin, K.; Singh, J.; Hill, J.H.; Whitham, S.A.; Cannon, S.B. Dynamic transcriptome profiling of Bean Common Mosaic Virus (BCMV) infection in Common Bean (Phaseolus vulgaris L.). BMC Genom. 2016, 17, 613. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Sun, H.; Wang, Y.; Zhang, Y.; Wang, X.; Li, D.; Yu, J.; Han, C. Deep sequencing–based transcriptome profiling reveals comprehensive insights into the responses of Nicotiana benthamiana to Beet necrotic yellow vein virus infections containing or lacking RNA4. PLoS ONE 2014, 9, e85284. [Google Scholar] [CrossRef] [PubMed]
- Sood, A.; Chauhan, R.S. Comparative NGS Transcriptomics Unravels Molecular Components Associated with Mosaic Virus Infection in a Bioenergy Plant Species, Jatropha curcas L. BioEnergy Res. 2017, 10, 129–145. [Google Scholar] [CrossRef]
- Mochizuki, T.; Ogata, Y.; Hirata, Y.; Ohki, S.T. Quantitative transcriptional changes associated with chlorosis severity in mosaic leaves of tobacco plants infected with Cucumber mosaic virus. Mol. Plant Pathol. 2014, 15, 242–254. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, D.; Gnanasekaran, P.; Kumar, R.K.; Kushwaha, N.K.; Sharma, V.K.; Yusuf, M.A.; Chakraborty, S. A geminivirus betasatellite damages the structural and functional integrity of chloroplasts leading to symptom formation and inhibition of photosynthesis. J. Exp. Bot. 2015, 66, 5881–5895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kangasjärvi, S.; Tikkanen, M.; Durian, G.; Aro, E.-M. Photosynthetic light reactions–An adjustable hub in basic production and plant immunity signaling. Plant Physiol. Biochem. 2014, 81, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, X.; Hong, Y.; Liu, Y. Chloroplast in plant-virus interaction. Front. Microbiol. 2016, 7, 1565. [Google Scholar] [CrossRef] [PubMed]
- Ganusova, E.E.; Rice, J.H.; Carlew, T.S.; Patel, A.; Perrodin-Njoku, E.; Hewezi, T.; Burch-Smith, T.M. Altered Expression of a Chloroplast Protein Affects the Outcome of Virus and Nematode Infection. Mol. Plant Microbe Interact. 2017, 30, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.; Folimonova, S.Y.; Cole, A.B.; Ballard, K.D.; Lei, Z.; Watson, B.S.; Sumner, L.W.; Nelson, R.S. Influence of host chloroplast proteins on Tobacco mosaic virus accumulation and intercellular movement. Plant Physiol. 2013, 161, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Withers, J.; Dong, X. Post-translational regulation of plant immunity. Curr. Opin. Plant Boil. 2017, 38, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Perazzolli, M.; Palmieri, M.C.; Matafora, V.; Bachi, A.; Pertot, I. Phosphoproteomic analysis of induced resistance reveals activation of signal transduction processes by beneficial and pathogenic interaction in grapevine. J. Plant Physiol. 2016, 195, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Herman, M.; Davidson, J.; Smart, C. Induction of plant defense gene expression by plant activators and Pseudomonas syringae pv. tomato in greenhouse-grown tomatoes. Phytopathology 2008, 98, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Tornero, P.; Gadea, J.; Conejero, V.; Vera, P. Two PR-1 genes from tomato are differentially regulated and reveal a novel mode of expression for a pathogenesis-related gene during the hypersensitive response and development. Mol. Plant Microbe Interact. 1997, 10, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Jo, Y.; Lian, S.; Jo, K.-M.; Chu, H.; Yoon, J.-Y.; Choi, S.-K.; Kim, K.-H.; Cho, W.K. Comparative analysis of chrysanthemum transcriptome in response to three RNA viruses: Cucumber mosaic virus, Tomato spotted wilt virus and Potato virus X. Plant Mol. Boil. 2015, 88, 233–248. [Google Scholar] [CrossRef] [PubMed]
- Naseem, M.; Kaltdorf, M.; Dandekar, T. The nexus between growth and defence signalling: Auxin and cytokinin modulate plant immune response pathways. J. Exp. Bot. 2015, 66, 4885–4896. [Google Scholar] [CrossRef] [PubMed]
- Collum, T.D.; Padmanabhan, M.S.; Hsieh, Y.-C.; Culver, J.N. Tobacco mosaic virus-directed reprogramming of auxin/indole acetic acid protein transcriptional responses enhances virus phloem loading. Proc. Natl. Acad. Sci. USA 2016, 113, E2740–E2749. [Google Scholar] [CrossRef] [PubMed]
- Scholthof, H.B.; Alvarado, V.Y.; Vega-Arreguin, J.C.; Ciomperlik, J.; Odokonyero, D.; Brosseau, C.; Jaubert, M.; Zamora, A.; Moffett, P. Identification of an ARGONAUTE for antiviral RNA silencing in Nicotiana benthamiana. Plant Physiol. 2011, 156, 1548–1555. [Google Scholar] [CrossRef] [PubMed]
- Harvey, J.J.; Lewsey, M.G.; Patel, K.; Westwood, J.; Heimstädt, S.; Carr, J.P.; Baulcombe, D.C. An antiviral defense role of AGO2 in plants. PLoS ONE 2011, 6, e14639. [Google Scholar] [CrossRef] [PubMed]
- Carbonell, A.; Carrington, J.C. Antiviral roles of plant ARGONAUTES. Curr. Opin. Plant Boil. 2015, 27, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, B.; Sanfaçon, H. Temperature-dependent symptom recovery in Nicotiana benthamiana plants infected with tomato ringspot virus is associated with reduced translation of viral RNA2 and requires ARGONAUTE 1. Virology 2014, 456, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Anjanappa, R.B.; Mehta, D.; Okoniewski, M.J.; Szabelska, A.; Gruissem, W.; Vanderschuren, H. Molecular insights into cassava brown streak virus susceptibility and resistance by profiling of the early host response. Mol. Plant Pathol. 2017, 19, 476–489. [Google Scholar] [CrossRef] [PubMed]
- Rakhshandehroo, F.; Takeshita, M.; Squires, J.; Palukaitis, P. The influence of RNA-dependent RNA polymerase 1 on potato virus Y infection and on other antiviral response genes. Mol. Plant Microbe Interact. 2009, 22, 1312–1318. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Shi, N.; Gu, M.; Zhang, H.; Li, B.; Shen, J.; Mohammed, A.; Ryabov, E.; Li, C.; Wang, H. Involvement of RDR6 in short-range intercellular RNA silencing in Nicotiana benthamiana. Sci. Rep. 2012, 2, 467. [Google Scholar] [CrossRef] [PubMed]
- Jones, L. Revealing micro-RNAs in plants. Trends Plant Sci. 2002, 7, 473–475. [Google Scholar] [CrossRef]
- Cordero, T.; Cerdán, L.; Carbonell, A.; Katsarou, K.; Kalantidis, K.; Daròs, J.-A. Dicer-Like 4 is involved in restricting the systemic movement of Zucchini yellow mosaic virus in Nicotiana benthamiana. Mol. Plant Microbe Interact. 2017, 30, 63–71. [Google Scholar] [CrossRef] [PubMed]
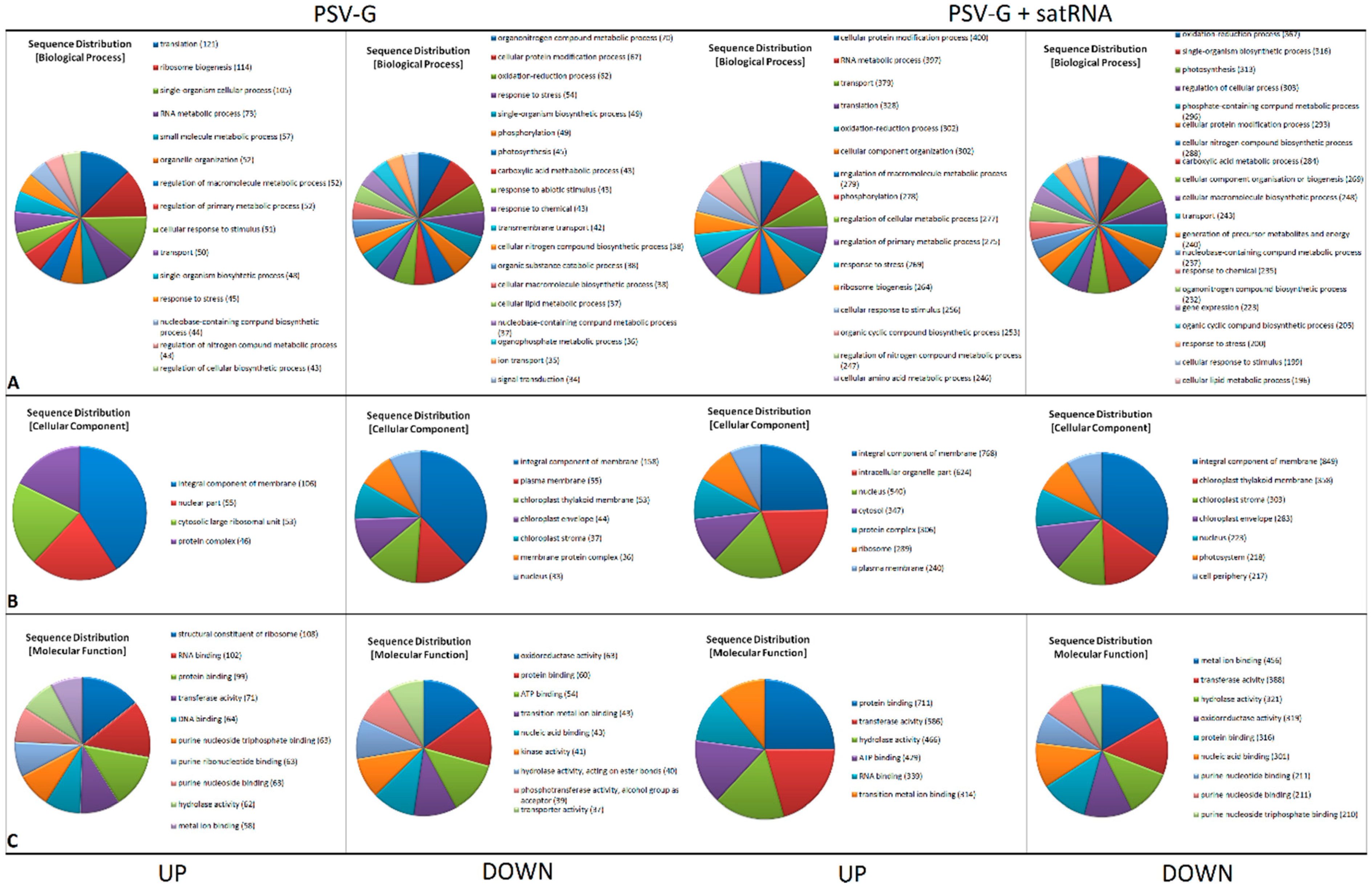
| Treatment Condition/Tendency | Number of DEGs | With Blast Hits | B2GO Annotated |
|---|---|---|---|
| PSV-G/up-regulated | 1024 | 856 (83%) | 611 (59%) |
| PSV-G/down-regulated | 727 | 578 (80%) | 445 (62%) |
| PSV-G + satRNA/up-regulated | 5239 | 4469 (85%) | 3453 (66%) |
| PSV-G + satRNA/down-regulated | 4140 | 3366 (81%) | 2559 (62%) |
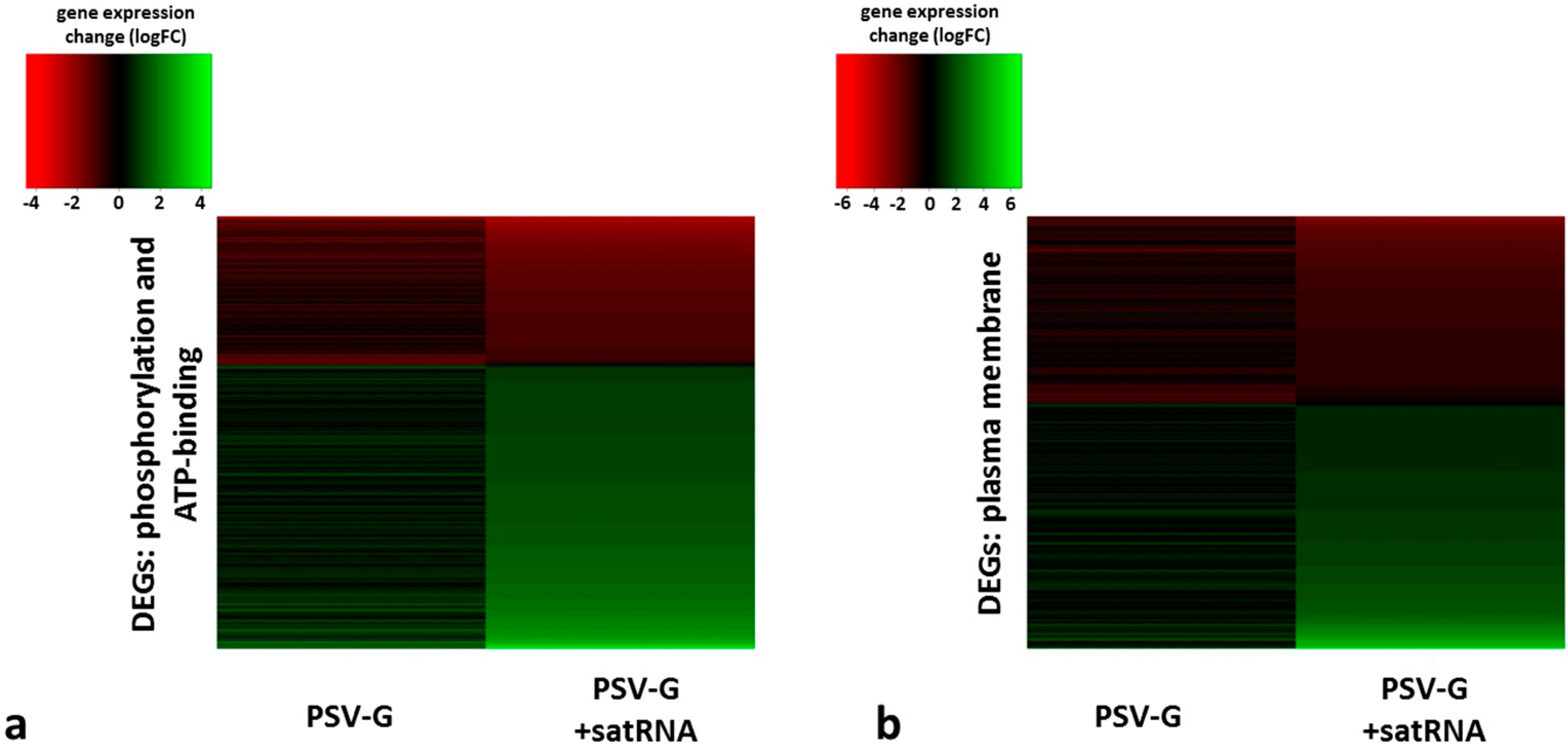
| Opposite Effects of PSV Infection in the Absence of Presence of satRNA (Number of Seqs) | ||
|---|---|---|
| PSV-G | PSV-G + satRNA | |
| phosphorylation (biological process) | ↓ (49) | ↑ (278) |
| plasma membrane (compartment distribution) | ↓ (55) | ↑ (240) |
| ATP binding (molecular function) | ↓ (54) | ↑ (429) |
| Log2 Change in Gene Expression for PSV-G | Log2 Change in Gene Expression for PSV-G + satRNA | p Value for PSV-G | p Value for PSV-G + satRNA | Function/Sequence Name/BlastN ID |
|---|---|---|---|---|
| 0.327 | 1.246 | 0.562778 | 0.000858 | Protein argonaute 5 Nbv3K625761138/XM_019408655.1 |
| 1.059 | 1.249 | 0.000215 | 1.00E−06 | Protein argonaute 1 (similar to) RC_Nbv3K685820100/XM_019369300.1 |
| 0.946 | 1.299 | 0.007104 | 3.70E−05 | Protein argonaute 1B Nbv3K785652119/XM_016590746.1 |
| 0.919 | 1.389 | 0.002047 | 1.00E−06 | Protein argonaute 1 (similar to) Nbv3K705831504/XM_019369300.1 |
| 1.019 | 1.42 | 0.003157 | 8.00E−06 | Protein argonaute 1-like (similar to) RC_Nbv3K705830082/XM_019369300.1 |
| 1.072 | 1.521 | 0.001496 | 2.00E−06 | Protein argonaute 1-like RC_Nbv3K625765725/XM_019369300.1 |
| 0.25 | 1.654 | 0.669212 | 2.50E−05 | Protein argonaute 5 RC_Nbv3K625760429/XM_009788807.1 |
| 1.192 | 1.736 | 0.003733 | 7.00E−06 | Protein argonaute 1A Nbv3K585682683/XM_016630483.1 |
| 1.473 | 1.958 | 2.60E-05 | 0 | Protein argonaute 1B Nbv3K685814294/KR942296.1 |
| 1.178 | 2.135 | 0.001783 | 1.00E−06 | Protein argonaute 1 Nbv3K665799914/XM_009771957.1 |
| 2.195 | 3.286 | 2.60E-05 | 0 | Protein argonaute 2 (similar to) RC_Nbv3K585706870/XM_016629769.1 |
| 0.286 | 1.283 | 0.353833 | 3.00E−06 | RNA-dependent RNA polymerase 6 (AtRDRP6) (similar to)/Nbv3K585707928/XM_019404979.1 |
| 0.83 | 2.252 | 0.020257 | 0 | RNA-dependent RNA polymerase 1 (AtRDRP1) (similar to)/Nbv3K585714562 |
| 0.812 | 2.276 | 0.155813 | 1.30E−05 | RNA-dependent RNA polymerase 1 (AtRDRP1) (putative) Nbv3K745620210/AY574374.1 |
| 0.421 | 1.093 | 0.206175 | 9.40E−05 | Endoribonuclease Dicer homolog 1 (similar to) RC_Nbv3K585722110/XM_009796481.1 |
| 0.752 | 1.314 | 0.036 | 5.30E−05 | Dicer-like protein 4 (putative) Nbv3K625768999/XM_009767633.1 |
| 0.623 | 1.419 | 0.015192 | 0 | Endoribonuclease Dicer homolog 1 (OsDCL1) (similar to) RC_Nbv3K585722111/XM_016616158.1 |
| 0.628 | 1.492 | 0.099327 | 2.00E−05 | Endoribonuclease Dicer homolog 4 (OsDCL4) (probable) RC_Nbv3K585724251/XM_009767634.1 |
| Gene | Results Obtained from RT-qPCR | Results Obtained from Transcriptomics Analysis | ||||
|---|---|---|---|---|---|---|
| Expression | Std. Error | p-Value | Expression | Std. Error | p-Value | |
| AGO2 | 2.761 | 0.449–16.281 | 0.002 | 4.594 | ||
| EF1delta | 7.347 | 3.358–16.188 | 0 | 4.317 | 0 | |
| Hsp17.3 | 19.034 | 5.997–49.014 | 0 | 6.821 | ||
| MBF1C | 4.089 | 0.901–18.471 | 0 | 5.242 | 0 | |
| PNO1 | 5.757 | 2.185–14.410 | 0 | 3.944 | ||
| PR1 | 2.178 | 0.607–7.063 | 0.006 | 4.347 | 0 | |
| PR2 | 1.222 | 0.337–4.482 | 0.46 | 5.502 | 0 | |
| ABCC | 0.148 | 0.093–0.241 | 0 | 0.177 | 0 | |
| IRT1 | 0.07 | 0.008–0.656 | 0 | 0.203 | 0 | |
| PAP1 | 0.065 | 0.024–0.161 | 0 | 0.189 | 0 | |
| PPCK | 0.431 | 0.264–0.701 | 0 | 0.308 | 0 | |
| Gene | Results Obtained from RT-qPCR | Results Obtained from Transcriptomics Analysis | ||||
|---|---|---|---|---|---|---|
| Expression | Std. Error | p-Value | Expression | Std. Error | p-Value | |
| AGO2 | 5.004 | 0.829–34.453 | 0 | 9.849 | 0 | |
| EF1delta | 2.607 | 1.113–5.838 | 0 | 3.732 | ||
| Hsp17.3 | 6.151 | 1.968–25.740 | 0 | 10.556 | ||
| MBF1C | 4.911 | 1.553–16.784 | 0 | 25.992 | ||
| PNO1 | 2.981 | 1.134–7.371 | 0 | 8.000 | 0 | |
| PR1 | 32.49 | 9.798–85.277 | 0 | 51.984 | 0 | |
| PR2 | 14.488 | 4.620–45.487 | 0 | 147.033 | ||
| ABCC | 0.206 | 0.145–0.313 | 0 | 0.330 | 0 | |
| IRT1 | 0.162 | 0.026–1.129 | 0 | 0.287 | 0 | |
| PAP1 | 0.048 | 0.026–0.084 | 0 | 0.144 | 0 | |
| PPCK | 0.268 | 0.198–0.408 | 0 | 0.353 | 0 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obrępalska-Stęplowska, A.; Zmienko, A.; Wrzesińska, B.; Goralski, M.; Figlerowicz, M.; Zyprych-Walczak, J.; Siatkowski, I.; Pospieszny, H. The Defense Response of Nicotiana benthamiana to Peanut Stunt Virus Infection in the Presence of Symptom Exacerbating Satellite RNA. Viruses 2018, 10, 449. https://doi.org/10.3390/v10090449
Obrępalska-Stęplowska A, Zmienko A, Wrzesińska B, Goralski M, Figlerowicz M, Zyprych-Walczak J, Siatkowski I, Pospieszny H. The Defense Response of Nicotiana benthamiana to Peanut Stunt Virus Infection in the Presence of Symptom Exacerbating Satellite RNA. Viruses. 2018; 10(9):449. https://doi.org/10.3390/v10090449
Chicago/Turabian StyleObrępalska-Stęplowska, Aleksandra, Agnieszka Zmienko, Barbara Wrzesińska, Michal Goralski, Marek Figlerowicz, Joanna Zyprych-Walczak, Idzi Siatkowski, and Henryk Pospieszny. 2018. "The Defense Response of Nicotiana benthamiana to Peanut Stunt Virus Infection in the Presence of Symptom Exacerbating Satellite RNA" Viruses 10, no. 9: 449. https://doi.org/10.3390/v10090449





