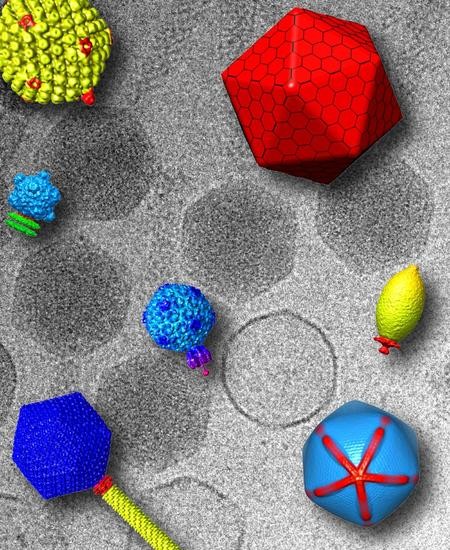
Breaking Symmetry in Viral Icosahedral Capsids as Seen through the Lenses of X-ray Crystallography and Cryo-Electron Microscopy
Abstract
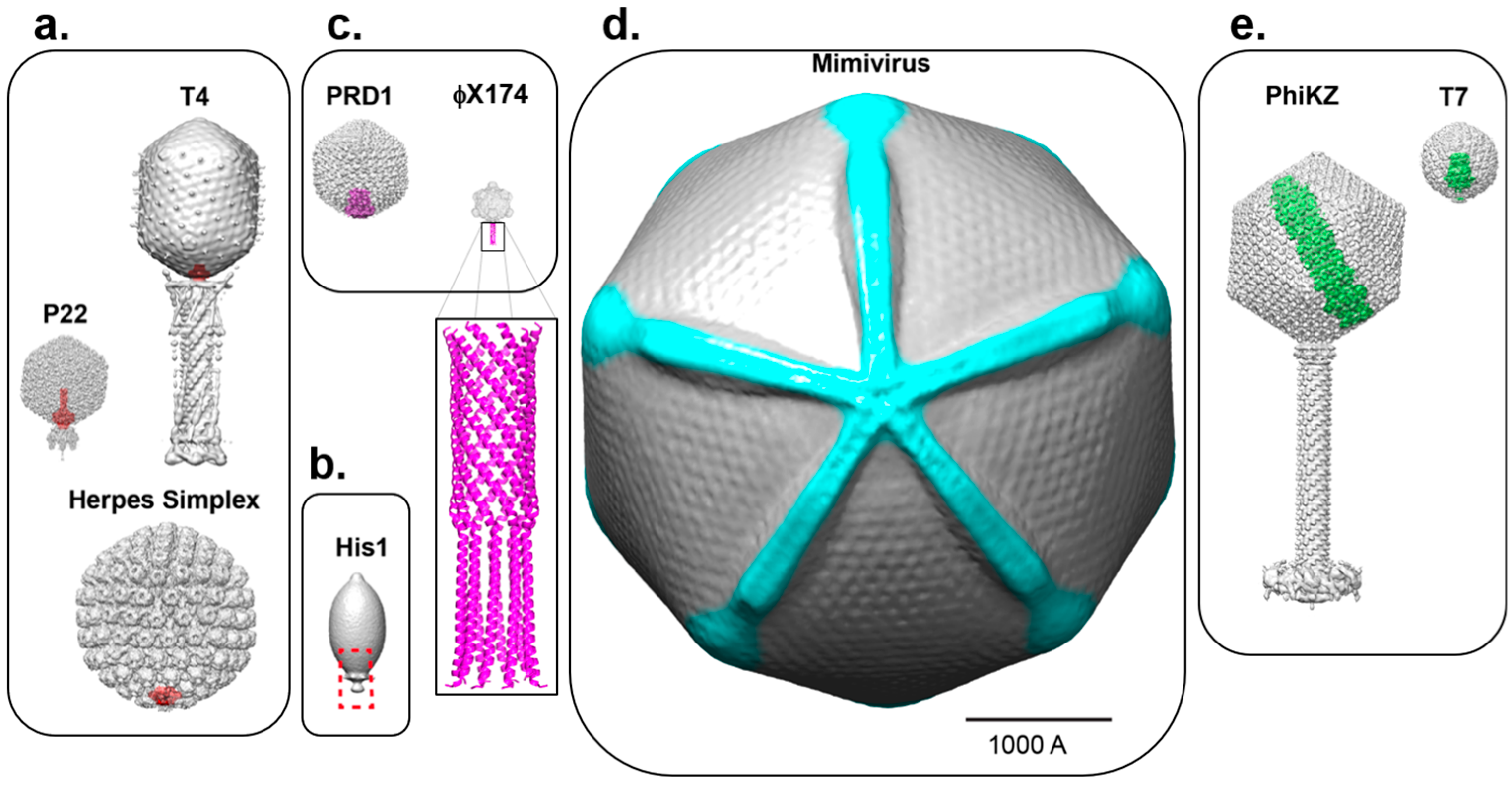
:1. Principles of Icosahedral Virus Assembly
2. Asymmetric Cryo-EM Reconstructions Reveal the Portal Protein Occupies a Unique 5-Fold Vertex
3. Cryo-Electron Tomography Identifies a Portal Protein in Herpesviruses
4. The Challenge of Visualizing Symmetry Mismatches at the Portal Vertex in Genome Packaging Motors
5. Unique Capsid Vertices that Break Icosahedral Symmetry without a Portal Protein
6. Bubblegrams and Cryo-ET Reveal Asymmetrically Organized Proteins inside Icosahedral Capsids
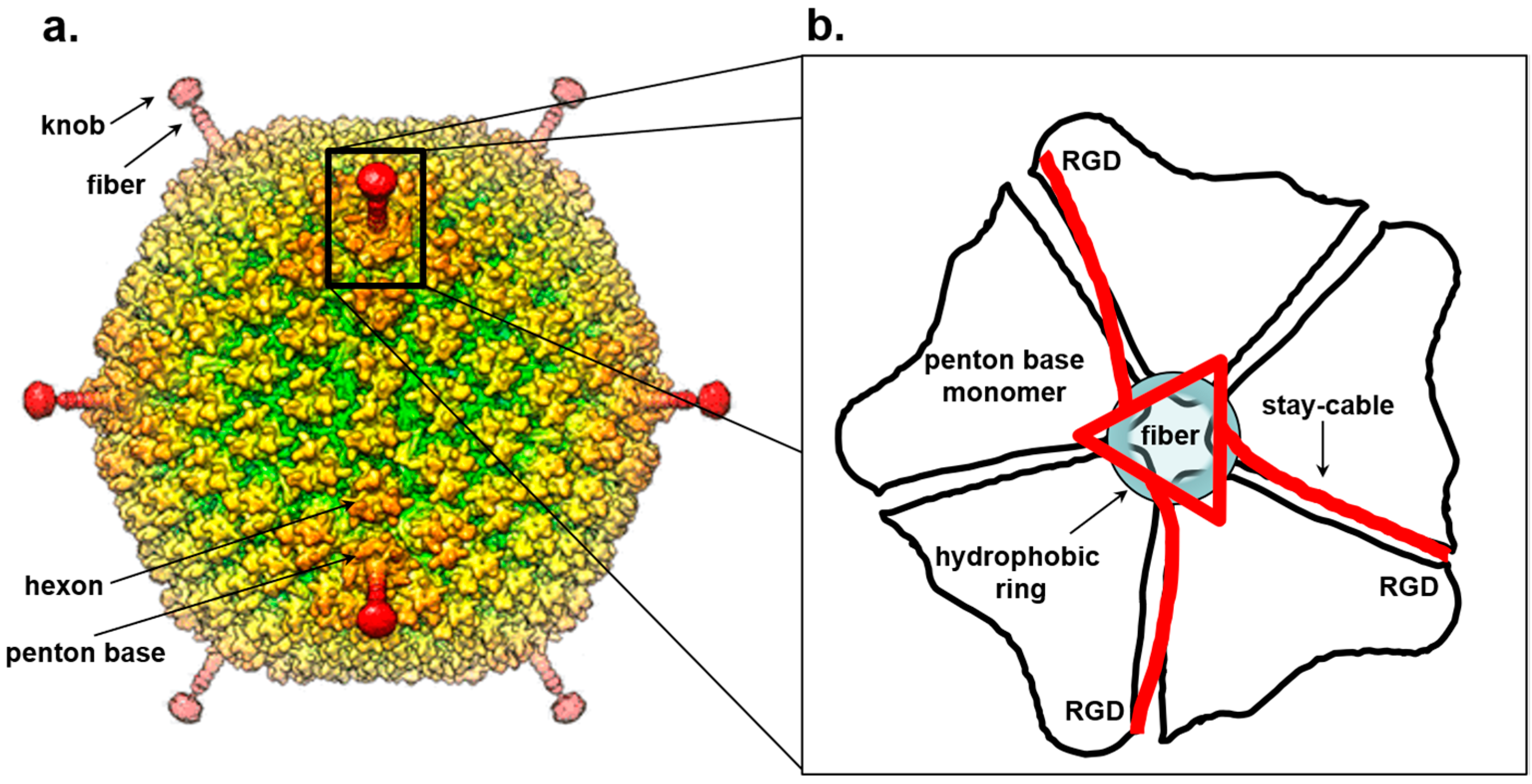
7. Trimeric Fiber-Penton Base Interaction in the Adenovirus Lineage
8. Conclusive Remarks: The Evolution of Structural Methods to “Observe” Asymmetric Features in Icosahedral Capsids
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rossmann, M.G. Structure of viruses: A short history. Q. Rev. Biophys. 2013, 46, 133–180. [Google Scholar] [CrossRef] [PubMed]
- Prasad, B.V.; Schmid, M.F. Principles of virus structural organization. Adv. Exp. Med. Biol. 2012, 726, 17–47. [Google Scholar] [PubMed]
- Rochal, S.B.; Konevtsova, O.V.; Lorman, V.L. Static and dynamic hidden symmetries of icosahedral viral capsids. Nanoscale 2017, 9, 12449–12460. [Google Scholar] [CrossRef] [PubMed]
- Suhanovsky, M.M.; Teschke, C.M. Nature’s favorite building block: Deciphering folding and capsid assembly of proteins with the HK97-fold. Virology 2015, 479–480, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Katen, S.; Zlotnick, A. The thermodynamics of virus capsid assembly. Methods Enzymol. 2009, 455, 395–417. [Google Scholar] [PubMed]
- Dykeman, E.C.; Grayson, N.E.; Toropova, K.; Ranson, N.A.; Stockley, P.G.; Twarock, R. Simple rules for efficient assembly predict the layout of a packaged viral RNA. J. Mol. Biol. 2011, 408, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Caspar, D.L.; Klug, A. Physical principles in the construction of regular viruses. Cold Spring Harb. Symp. Quant. Biol. 1962, 27, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, R.M.; Ravantti, J.J.; Bamford, D.H. Nucleic and Amino Acid Sequences Support Structure-Based Viral Classification. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [PubMed]
- Bamford, D.H.; Grimes, J.M.; Stuart, D.I. What does structure tell us about virus evolution? Curr. Opin. Struct. Biol. 2005, 15, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Padilla-Meier, G.P.; Gilcrease, E.B.; Weigele, P.R.; Cortines, J.R.; Siegel, M.; Leavitt, J.C.; Teschke, C.M.; Casjens, S.R. Unraveling the role of the C-terminal helix turn helix of the coat-binding domain of bacteriophage P22 scaffolding protein. J. Biol. Chem. 2012, 287, 33766–33780. [Google Scholar] [CrossRef] [PubMed]
- Morais, M.C.; Kanamaru, S.; Badasso, M.O.; Koti, J.S.; Owen, B.A.; McMurray, C.T.; Anderson, D.L.; Rossmann, M.G. Bacteriophage phi29 scaffolding protein gp7 before and after prohead assembly. Nat. Struct. Biol. 2003, 10, 572–576. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Parker, M.H.; Weigele, P.; Casjens, S.; Prevelige, P.E., Jr.; Krishna, N.R. Structure of the coat protein-binding domain of the scaffolding protein from a double-stranded DNA virus. J. Mol. Biol. 2000, 297, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Wikoff, W.R.; Liljas, L.; Duda, R.L.; Tsuruta, H.; Hendrix, R.W.; Johnson, J.E. Topologically linked protein rings in the bacteriophage HK97 capsid. Science 2000, 289, 2129–2133. [Google Scholar] [CrossRef] [PubMed]
- Pietila, M.K.; Laurinmaki, P.; Russell, D.A.; Ko, C.C.; Jacobs-Sera, D.; Hendrix, R.W.; Bamford, D.H.; Butcher, S.J. Structure of the archaeal head-tailed virus HSTV-1 completes the HK97 fold story. Proc. Natl. Acad. Sci. USA 2013, 110, 10604–10609. [Google Scholar] [CrossRef] [PubMed]
- Atanasova, N.S.; Bamford, D.H.; Oksanen, H.M. Haloarchaeal virus morphotypes. Biochimie 2015, 118, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.L.; Jiang, W.; Rixon, F.J.; Chiu, W. Common ancestry of herpesviruses and tailed DNA bacteriophages. J. Virol. 2005, 79, 14967–14970. [Google Scholar] [CrossRef] [PubMed]
- Huet, A.; Makhov, A.M.; Huffman, J.B.; Vos, M.; Homa, F.L.; Conway, J.F. Extensive subunit contacts underpin herpesvirus capsid stability and interior-to-exterior allostery. Nat. Struct. Mol. Biol. 2016, 23, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Jih, J.; Jiang, J.; Zhou, Z.H. Atomic structure of the human cytomegalovirus capsid with its securing tegument layer of pp150. Science 2017, 356. [Google Scholar] [CrossRef] [PubMed]
- Serwer, P.; Hayes, S.J.; Thomas, J.A.; Hardies, S.C. Propagating the missing bacteriophages: A large bacteriophage in a new class. Virol. J. 2007, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.; Huet, A.; Lopez, C.A.; Toropova, K.; Pope, W.H.; Duda, R.L.; Hendrix, R.W.; Conway, J.F. Capsids and Genomes of Jumbo-Sized Bacteriophages Reveal the Evolutionary Reach of the HK97 Fold. mBio 2017, 8, e01579-17. [Google Scholar] [CrossRef] [PubMed]
- Hendrix, R.W. Jumbo bacteriophages. Curr. Top. Microbiol. Immunol. 2009, 328, 229–240. [Google Scholar] [PubMed]
- Baker, T.S.; Olson, N.H.; Fuller, S.D. Adding the third dimension to virus life cycles: Three-dimensional reconstruction of icosahedral viruses from cryo-electron micrographs. Microbiol. Mol. Biol. Rev. 1999, 63, 862–922. [Google Scholar] [CrossRef] [PubMed]
- Binshtein, E.; Ohi, M.D. Cryo-electron microscopy and the amazing race to atomic resolution. Biochemistry 2015, 54, 3133–3141. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.E. Confessions of an icosahedral virus crystallographer. Microscopy 2013, 62, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Doerschuk, P.C.; Gong, Y.; Xu, N.; Domitrovic, T.; Johnson, J.E. Virus particle dynamics derived from CryoEM studies. Curr. Opin. Virol. 2016, 18, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Tang, L. Atomic cryo-EM structures of viruses. Curr. Opin. Struct. Biol. 2017, 46, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Temple, L.; Lewis, L. Phage on the stage. Bacteriophage 2015, 5, e1062589. [Google Scholar] [CrossRef] [PubMed]
- Jordan, T.C.; Burnett, S.H.; Carson, S.; Caruso, S.M.; Clase, K.; DeJong, R.J.; Dennehy, J.J.; Denver, D.R.; Dunbar, D.; Elgin, S.C.; et al. A broadly implementable research course in phage discovery and genomics for first-year undergraduate students. mBio 2014, 5, e01051-13. [Google Scholar] [CrossRef] [PubMed]
- Doore, S.M.; Schrad, J.R.; Dean, W.F.; Dover, J.A.; Parent, K.N. Shigella phages isolated during a dysentery outbreak reveal unique structures and broad species diversity. J. Virol. 2018, in press. [Google Scholar]
- Hendrix, R.W. Symmetry mismatch and DNA packaging in large bacteriophages. Proc. Natl. Acad. Sci. USA 1978, 75, 4779–4783. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, A.; Olia, A.S.; Cingolani, G. Architecture of viral genome-delivery molecular machines. Curr. Opin. Struct. Biol. 2014, 25, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Orlova, E.V.; Dube, P.; Beckmann, E.; Zemlin, F.; Lurz, R.; Trautner, T.A.; Tavares, P.; van Heel, M. Structure of the 13-fold symmetric portal protein of bacteriophage SPP1. Nat. Struct. Biol. 1999, 6, 842–846. [Google Scholar] [PubMed]
- Cingolani, G.; Moore, S.D.; Prevelige, P.E., Jr.; Johnson, J.E. Preliminary crystallographic analysis of the bacteriophage P22 portal protein. J. Struct. Biol. 2002, 139, 46–54. [Google Scholar] [CrossRef]
- Trus, B.L.; Cheng, N.; Newcomb, W.W.; Homa, F.L.; Brown, J.C.; Steven, A.C. Structure and polymorphism of the UL6 portal protein of herpes simplex virus type 1. J. Virol. 2004, 78, 12668–12671. [Google Scholar] [CrossRef] [PubMed]
- Lorenzen, K.; Olia, A.S.; Uetrecht, C.; Cingolani, G.; Heck, A.J. Determination of stoichiometry and conformational changes in the first step of the P22 tail assembly. J. Mol. Biol. 2008, 379, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Cerritelli, M.E.; Conway, J.F.; Cheng, N.; Trus, B.L.; Steven, A.C. Molecular mechanisms in bacteriophage T7 procapsid assembly, maturation, and DNA containment. Adv. Protein Chem. 2003, 64, 301–323. [Google Scholar] [PubMed]
- Motwani, T.; Lokareddy, R.K.; Dunbar, C.A.; Cortines, J.R.; Jarrold, M.F.; Cingolani, G.; Teschke, C.M. A viral scaffolding protein triggers portal ring oligomerization and incorporation during procapsid assembly. Sci. Adv. 2017, 3, e1700423. [Google Scholar] [CrossRef] [PubMed]
- Simpson, A.A.; Leiman, P.G.; Tao, Y.; He, Y.; Badasso, M.O.; Jardine, P.J.; Anderson, D.L.; Rossmann, M.G. Structure determination of the head-tail connector of bacteriophage phi29. Acta Crystallogr. D Biol. Crystallogr. 2001, 57, 1260–1269. [Google Scholar] [CrossRef] [PubMed]
- Simpson, A.A.; Tao, Y.; Leiman, P.G.; Badasso, M.O.; He, Y.; Jardine, P.J.; Olson, N.H.; Morais, M.C.; Grimes, S.; Anderson, D.L.; et al. Structure of the bacteriophage phi29 DNA packaging motor. Nature 2000, 408, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Guasch, A.; Pous, J.; Ibarra, B.; Gomis-Ruth, F.X.; Valpuesta, J.M.; Sousa, N.; Carrascosa, J.L.; Coll, M. Detailed architecture of a DNA translocating machine: The high-resolution structure of the bacteriophage phi29 connector particle. J. Mol. Biol. 2002, 315, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Agirrezabala, X.; Martin-Benito, J.; Valle, M.; Gonzalez, J.M.; Valencia, A.; Valpuesta, J.M.; Carrascosa, J.L. Structure of the connector of bacteriophage T7 at 8A resolution: Structural homologies of a basic component of a DNA translocating machinery. J. Mol. Biol. 2005, 347, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Cuervo, A.; Pulido-Cid, M.; Chagoyen, M.; Arranz, R.; Gonzalez-Garcia, V.A.; Garcia-Doval, C.; Caston, J.R.; Valpuesta, J.M.; van Raaij, M.J.; Martin-Benito, J.; et al. Structural characterization of the bacteriophage T7 tail machinery. J. Biol. Chem. 2013, 288, 26290–26299. [Google Scholar] [CrossRef] [PubMed]
- Lebedev, A.A.; Krause, M.H.; Isidro, A.L.; Vagin, A.A.; Orlova, E.V.; Turner, J.; Dodson, E.J.; Tavares, P.; Antson, A.A. Structural framework for DNA translocation via the viral portal protein. EMBO J. 2007, 26, 1984–1994. [Google Scholar] [CrossRef] [PubMed]
- Orlova, E.V.; Gowen, B.; Droge, A.; Stiege, A.; Weise, F.; Lurz, R.; van Heel, M.; Tavares, P. Structure of a viral DNA gatekeeper at 10 A resolution by cryo-electron microscopy. EMBO J. 2003, 22, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Chaban, Y.; Lurz, R.; Brasiles, S.; Cornilleau, C.; Karreman, M.; Zinn-Justin, S.; Tavares, P.; Orlova, E.V. Structural rearrangements in the phage head-to-tail interface during assembly and infection. Proc. Natl. Acad. Sci. USA 2015, 112, 7009–7014. [Google Scholar] [CrossRef] [PubMed]
- Olia, A.S.; Prevelige, P.E., Jr.; Johnson, J.E.; Cingolani, G. Three-dimensional structure of a viral genome-delivery portal vertex. Nat. Struct. Mol. Biol. 2011, 18, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Olia, A.S.; Gonen, M.; Andrews, S.; Cingolani, G.; Gonen, T. A conformational switch in bacteriophage P22 portal protein primes genome injection. Mol. Cell 2008, 29, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Marion, W.R.; Cingolani, G.; Prevelige, P.E.; Johnson, J.E. Three-dimensional structure of the bacteriophage P22 tail machine. EMBO J. 2005, 24, 2087–2095. [Google Scholar] [CrossRef] [PubMed]
- Lander, G.C.; Khayat, R.; Li, R.; Prevelige, P.E.; Potter, C.S.; Carragher, B.; Johnson, J.E. The P22 tail machine at subnanometer resolution reveals the architecture of an infection conduit. Structure 2009, 17, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhang, X.; Gao, S.; Rao, P.A.; Padilla-Sanchez, V.; Chen, Z.; Sun, S.; Xiang, Y.; Subramaniam, S.; Rao, V.B.; et al. Cryo-EM structure of the bacteriophage T4 portal protein assembly at near-atomic resolution. Nat. Commun. 2015, 6, 7548. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Sinkovits, R.S.; Baker, T.S. Three-dimensional asymmetric reconstruction of tailed bacteriophage. Methods Enzymol. 2010, 482, 185–210. [Google Scholar] [PubMed]
- Tang, J.; Lander, G.C.; Olia, A.; Li, R.; Casjens, S.; Prevelige, P., Jr.; Cingolani, G.; Baker, T.S.; Johnson, J.E. Peering down the barrel of a bacteriophage portal: The genome packaging and release valve in P22. Structure 2011, 19, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.H.; Baker, M.L.; Hryc, C.F.; DiMaio, F.; Jakana, J.; Wu, W.; Dougherty, M.; Haase-Pettingell, C.; Schmid, M.F.; Jiang, W.; et al. Structural basis for scaffolding-mediated assembly and maturation of a dsDNA virus. Proc. Natl. Acad. Sci. USA 2011, 108, 1355–1360. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Olson, N.; Jardine, P.J.; Grimes, S.; Anderson, D.L.; Baker, T.S. DNA poised for release in bacteriophage phi29. Structure 2008, 16, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Saha, M.; Zhao, W.; Jardine, P.J.; Zhang, W.; Grimes, S.; Morais, M.C. Insights into the structure and assembly of the bacteriophage 29 double-stranded DNA packaging motor. J. Virol. 2014, 88, 3986–3996. [Google Scholar] [CrossRef] [PubMed]
- Lander, G.C.; Tang, L.; Casjens, S.R.; Gilcrease, E.B.; Prevelige, P.; Poliakov, A.; Potter, C.S.; Carragher, B.; Johnson, J.E. The Structure of an Infectious P22 Virion Shows the Signal for Headful DNA Packaging. Science 2006, 312, 1791–1795. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Weigele, P.; King, J.; Chiu, W.; Jiang, W. Cryo-EM asymmetric reconstruction of bacteriophage P22 reveals organization of its DNA packaging and infecting machinery. Structure 2006, 14, 1073–1082. [Google Scholar] [CrossRef] [PubMed]
- Agirrezabala, X.; Martin-Benito, J.; Caston, J.R.; Miranda, R.; Valpuesta, J.M.; Carrascosa, J.L. Maturation of phage T7 involves structural modification of both shell and inner core components. EMBO J. 2005, 24, 3820–3829. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Q.; Murata, K.; Baker, M.L.; Sullivan, M.B.; Fu, C.; Dougherty, M.T.; Schmid, M.F.; Osburne, M.S.; Chisholm, S.W.; et al. Structural changes in a marine podovirus associated with release of its genome into Prochlorococcus. Nat. Struct. Mol. Biol. 2010, 17, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Fu, C.; Raytcheva, D.; Flanagan, J.; Khant, H.A.; Liu, X.; Rochat, R.H.; Haase-Pettingell, C.; Piret, J.; Ludtke, S.J.; et al. Visualizing virus assembly intermediates inside marine cyanobacteria. Nature 2013, 502, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Chang, J.; Jakana, J.; Weigele, P.; King, J.; Chiu, W. Structure of epsilon15 bacteriophage reveals genome organization and DNA packaging/injection apparatus. Nature 2006, 439, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hardies, S.C.; Fokine, A.; Klose, T.; Jiang, W.; Cho, B.C.; Rossmann, M.G. Structure of the Marine Siphovirus TW1: Evolution of Capsid-Stabilizing Proteins and Tail Spikes. Structure 2017, 356. [Google Scholar] [CrossRef] [PubMed]
- Parent, K.N.; Gilcrease, E.B.; Casjens, S.R.; Baker, T.S. Structural evolution of the P22-like phages: Comparison of Sf6 and P22 procapsid and virion architectures. Virology 2012, 427, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Parent, K.N.; Tang, J.; Cardone, G.; Gilcrease, E.B.; Janssen, M.E.; Olson, N.H.; Casjens, S.R.; Baker, T.S. Three-dimensional reconstructions of the bacteriophage CUS-3 virion reveal a conserved coat protein I-domain but a distinct tailspike receptor-binding domain. Virology 2014, 464–465, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Pintilie, G.; Chen, D.H.; Haase-Pettingell, C.A.; King, J.A.; Chiu, W. Resolution and Probabilistic Models of Components in CryoEM Maps of Mature P22 Bacteriophage. Biophys. J. 2016, 110, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Casjens, S.; Wyckoff, E.; Hayden, M.; Sampson, L.; Eppler, K.; Randall, S.; Moreno, E.T.; Serwer, P. Bacteriophage P22 portal protein is part of the gauge that regulates packing density of intravirion DNA. J. Mol. Biol. 1992, 224, 1055–1074. [Google Scholar] [CrossRef]
- Lokareddy, R.K.; Sankhala, R.S.; Roy, A.; Afonine, P.V.; Motwani, T.; Teschke, C.M.; Parent, K.N.; Cingolani, G. Portal protein functions akin to a DNA-sensor that couples genome-packaging to icosahedral capsid maturation. Nat. Commun. 2017, 8, 14310. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Zhao, Z.; Haak, J.; Wang, S.; Wu, D.; Meng, B.; Weitao, T. Common mechanisms of DNA translocation motors in bacteria and viruses using one-way revolution mechanism without rotation. Biotechnol. Adv. 2014, 32, 853–872. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Schwartz, C.; Haak, J.; Zhao, Z. Discovery of a new motion mechanism of biomotors similar to the earth revolving around the sun without rotation. Virology 2013, 446, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ji, Z.; Yan, E.; Haque, F.; Guo, P. Three-step channel conformational changes common to DNA packaging motors of bacterial viruses T3, T4, SPP1, and Phi29. Virology 2017, 500, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Baumann, R.G.; Mullaney, J.; Black, L.W. Portal fusion protein constraints on function in DNA packaging of bacteriophage T4. Mol. Microbiol. 2006, 61, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Hugel, T.; Michaelis, J.; Hetherington, C.L.; Jardine, P.J.; Grimes, S.; Walter, J.M.; Falk, W.; Anderson, D.L.; Bustamante, C. Experimental test of connector rotation during DNA packaging into bacteriophage phi29 capsids. PLoS Biol. 2007, 5, e59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, S.; Kondabagil, K.; Draper, B.; Alam, T.I.; Bowman, V.D.; Zhang, Z.; Hegde, S.; Fokine, A.; Rossmann, M.G.; Rao, V.B. The structure of the phage T4 DNA packaging motor suggests a mechanism dependent on electrostatic forces. Cell 2008, 135, 1251–1262. [Google Scholar] [CrossRef] [PubMed]
- Dauden, M.I.; Martin-Benito, J.; Sanchez-Ferrero, J.C.; Pulido-Cid, M.; Valpuesta, J.M.; Carrascosa, J.L. Large terminase conformational change induced by connector binding in bacteriophage T7. J. Biol. Chem. 2013, 288, 16998–17007. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Saha, M.; Reyes-Aldrete, E.; Sherman, M.B.; Woodson, M.; Atz, R.; Grimes, S.; Jardine, P.J.; Morais, M.C. Structural and Molecular Basis for Coordination in a Viral DNA Packaging Motor. Cell Rep. 2016, 14, 2017–2029. [Google Scholar] [CrossRef] [PubMed]
- XOlia, A.S.; Al-Bassam, J.; Winn-Stapley, D.A.; Joss, L.; Casjens, S.R.; Cingolani, G. Binding-induced stabilization and assembly of the phage P22 tail accessory factor gp4. J. Mol. Biol. 2006, 363, 558–576. [Google Scholar]
- Lhuillier, S.; Gallopin, M.; Gilquin, B.; Brasiles, S.; Lancelot, N.; Letellier, G.; Gilles, M.; Dethan, G.; Orlova, E.V.; Couprie, J.; et al. Structure of bacteriophage SPP1 head-to-tail connection reveals mechanism for viral DNA gating. Proc. Natl. Acad. Sci. USA 2009, 106, 8507–8512. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, K.L.; Yee, A.A.; Arrowsmith, C.H.; Gold, M.; Davidson, A.R. The solution structure of the bacteriophage lambda head-tail joining protein, gpFII. J. Mol. Biol. 2002, 318, 1395–1404. [Google Scholar] [CrossRef]
- Olia, A.S.; Bhardwaj, A.; Joss, L.; Casjens, S.; Cingolani, G. Role of gene 10 protein in the hierarchical assembly of the bacteriophage P22 portal vertex structure. Biochemistry 2007, 46, 8776–8784. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, A.; Olia, A.S.; Walker-Kopp, N.; Cingolani, G. Domain organization and polarity of tail needle GP26 in the portal vertex structure of bacteriophage P22. J. Mol. Biol. 2007, 371, 374–387. [Google Scholar] [CrossRef] [PubMed]
- Newcomb, W.W.; Juhas, R.M.; Thomsen, D.R.; Homa, F.L.; Burch, A.D.; Weller, S.K.; Brown, J.C. The UL6 gene product forms the portal for entry of DNA into the herpes simplex virus capsid. J. Virol. 2001, 75, 10923–10932. [Google Scholar] [CrossRef] [PubMed]
- Homa, F.L.; Brown, J.C. Capsid assembly and DNA packaging in herpes simplex virus. Rev. Med. Virol. 1997, 7, 107–122. [Google Scholar] [CrossRef]
- Mocarski, E.S., Jr. Comparative Analysis of Herpesvirus-Common Proteins; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Dittmer, A.; Bogner, E. Analysis of the quaternary structure of the putative HCMV portal protein PUL104. Biochemistry 2005, 44, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Howard, A.J.; Sherman, D.M.; Visalli, M.A.; Burnside, D.M.; Visalli, R.J. The Varicella-zoster virus ORF54 gene product encodes the capsid portal protein, pORF54. Virus Res. 2012, 167, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Visalli, R.J.; Howard, A.J. Non-axial view of the varicella-zoster virus portal protein reveals conserved crown, wing and clip architecture. Intervirology 2014, 57, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Cardone, G.; Winkler, D.C.; Trus, B.L.; Cheng, N.; Heuser, J.E.; Newcomb, W.W.; Brown, J.C.; Steven, A.C. Visualization of the herpes simplex virus portal in situ by cryo-electron tomography. Virology 2007, 361, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.T.; Schmid, M.F.; Rixon, F.J.; Chiu, W. Electron cryotomography reveals the portal in the herpesvirus capsid. J. Virol. 2007, 81, 2065–2068. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.; O’Connor, C.M.; Kedes, D.H.; Zhou, Z.H. Direct visualization of the putative portal in the Kaposi’s sarcoma-associated herpesvirus capsid by cryoelectron tomography. J. Virol. 2007, 81, 3640–3644. [Google Scholar] [CrossRef] [PubMed]
- Rochat, R.H.; Liu, X.; Murata, K.; Nagayama, K.; Rixon, F.J.; Chiu, W. Seeing the portal in herpes simplex virus type 1 B capsids. J. Virol. 2011, 85, 1871–1874. [Google Scholar] [CrossRef] [PubMed]
- Schmid, M.F.; Hecksel, C.W.; Rochat, R.H.; Bhella, D.; Chiu, W.; Rixon, F.J. A tail-like assembly at the portal vertex in intact herpes simplex type-1 virions. PLoS Pathog. 2012, 8, e1002961. [Google Scholar] [CrossRef] [PubMed]
- Beilstein, F.; Higgs, M.R.; Stow, N.D. Mutational analysis of the herpes simplex virus type 1 DNA packaging protein UL33. J. Virol. 2009, 83, 8938–8945. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Christensen, T.E.; Kamau, Y.N.; Tang, L. Structures of the phage Sf6 large terminase provide new insights into DNA translocation and cleavage. Proc. Natl. Acad. Sci. USA 2013, 110, 8075–8080. [Google Scholar] [CrossRef] [PubMed]
- Smits, C.; Chechik, M.; Kovalevskiy, O.V.; Shevtsov, M.B.; Foster, A.W.; Alonso, J.C.; Antson, A.A. Structural basis for the nuclease activity of a bacteriophage large terminase. EMBO Rep. 2009, 10, 592–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, A.; Cingolani, G. Structure of p22 headful packaging nuclease. J. Biol. Chem. 2012, 287, 28196–28205. [Google Scholar] [CrossRef] [PubMed]
- Nadal, M.; Mas, P.J.; Blanco, A.G.; Arnan, C.; Sola, M.; Hart, D.J.; Coll, M. Structure and inhibition of herpesvirus DNA packaging terminase nuclease domain. Proc. Natl. Acad. Sci. USA 2010, 107, 16078–16083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selvarajan Sigamani, S.; Zhao, H.; Kamau, Y.N.; Baines, J.D.; Tang, L. The structure of the herpes simplex virus DNA-packaging terminase pUL15 nuclease domain suggests an evolutionary lineage among eukaryotic and prokaryotic viruses. J. Virol. 2013, 87, 7140–7148. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.X.; Erickson, S.; Anderson, D. A small viral RNA is required for in vitro packaging of bacteriophage phi 29 DNA. Science 1987, 236, 690–694. [Google Scholar] [CrossRef] [PubMed]
- Ray, K.; Ma, J.; Oram, M.; Lakowicz, J.R.; Black, L.W. Single-molecule and FRET fluorescence correlation spectroscopy analyses of phage DNA packaging: Colocalization of packaged phage T4 DNA ends within the capsid. J. Mol. Biol. 2010, 395, 1102–1113. [Google Scholar] [CrossRef] [PubMed]
- Morita, M.; Tasaka, M.; Fujisawa, H. Structural and functional domains of the large subunit of the bacteriophage T3 DNA packaging enzyme: Importance of the C-terminal region in prohead binding. J. Mol. Biol. 1995, 245, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Yeo, A.; Feiss, M. Specific interaction of terminase, the DNA packaging enzyme of bacteriophage lambda, with the portal protein of the prohead. J. Mol. Biol. 1995, 245, 141–150. [Google Scholar] [CrossRef] [PubMed]
- McNulty, R.; Lokareddy, R.K.; Roy, A.; Yang, Y.; Lander, G.C.; Heck, A.J.; Johnson, J.E.; Cingolani, G. Architecture of the Complex Formed by Large and Small Terminase Subunits from Bacteriophage P22. J. Mol. Biol. 2015, 427, 3285–3299. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Bhardwaj, A.; Datta, P.; Lander, G.C.; Cingolani, G. Small terminase couples viral DNA binding to genome-packaging ATPase activity. Structure 2012, 20, 1403–1413. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Bhardwaj, A.; Cingolani, G. Crystallization of the Nonameric Small Terminase Subunit of Bacteriophage P22. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2011, F67, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Nemecek, D.; Gilcrease, E.B.; Kang, S.; Prevelige, P.E., Jr.; Casjens, S.; Thomas, G.J., Jr. Subunit conformations and assembly states of a DNA-translocating motor: The terminase of bacteriophage P22. J. Mol. Biol. 2007, 374, 817–836. [Google Scholar] [CrossRef] [PubMed]
- Buttner, C.R.; Chechik, M.; Ortiz-Lombardia, M.; Smits, C.; Ebong, I.O.; Chechik, V.; Jeschke, G.; Dykeman, E.; Benini, S.; Robinson, C.V.; et al. Structural basis for DNA recognition and loading into a viral packaging motor. Proc. Natl. Acad. Sci. USA 2012, 109, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Finch, C.J.; Sequeira, R.D.; Johnson, B.A.; Johnson, J.E.; Casjens, S.R.; Tang, L. Crystal structure of the DNA-recognition component of the bacterial virus Sf6 genome-packaging machine. Proc. Natl. Acad. Sci. USA 2010, 107, 1971–1976. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Gao, S.; Kondabagil, K.; Xiang, Y.; Rossmann, M.G.; Rao, V.B. Structure and function of the small terminase component of the DNA packaging machine in T4-like bacteriophages. Proc. Natl. Acad. Sci. USA 2012, 109, 817–822. [Google Scholar] [CrossRef] [PubMed]
- De Beer, T.; Fang, J.; Ortega, M.; Yang, Q.; Maes, L.; Duffy, C.; Berton, N.; Sippy, J.; Overduin, M.; Feiss, M.; et al. Insights into specific DNA recognition during the assembly of a viral genome packaging machine. Mol. Cell 2002, 9, 981–991. [Google Scholar] [CrossRef]
- Zhao, H.; Kamau, Y.N.; Christensen, T.E.; Tang, L. Structural and functional studies of the phage Sf6 terminase small subunit reveal a DNA-spooling device facilitated by structural plasticity. J. Mol. Biol. 2012, 423, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Heming, J.D.; Huffman, J.B.; Jones, L.M.; Homa, F.L. Isolation and characterization of the herpes simplex virus 1 terminase complex. J. Virol. 2014, 88, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Maluf, N.K.; Yang, Q.; Catalano, C.E. Self-association properties of the bacteriophage lambda terminase holoenzyme: Implications for the DNA packaging motor. J. Mol. Biol. 2005, 347, 523–542. [Google Scholar] [CrossRef] [PubMed]
- Maluf, N.K.; Gaussier, H.; Bogner, E.; Feiss, M.; Catalano, C.E. Assembly of bacteriophage lambda terminase into a viral DNA maturation and packaging machine. Biochemistry 2006, 45, 15259–15268. [Google Scholar] [CrossRef] [PubMed]
- Stedman, K.M.; DeYoung, M.; Saha, M.; Sherman, M.B.; Morais, M.C. Structural insights into the architecture of the hyperthermophilic Fusellovirus SSV1. Virology 2015, 474, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.; Pietila, M.K.; Fu, C.J.; Schmid, M.F.; Bamford, D.H.; Chiu, W. Lemon-shaped halo archaeal virus His1 with uniform tail but variable capsid structure. Proc. Natl. Acad. Sci. USA 2015, 112, 2449–2454. [Google Scholar] [CrossRef] [PubMed]
- Doore, S.M.; Fane, B.A. The microviridae: Diversity, assembly, and experimental evolution. Virology 2016, 491, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Wichman, H.A.; Brown, C.J. Experimental evolution of viruses: Microviridae as a model system. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 2495–2501. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.R.; Lee, K.H.; Wichman, H.A.; Ytreberg, F.M. Changing folding and binding stability in a viral coat protein: A comparison between substitutions accessible through mutation and those fixed by natural selection. PLoS ONE 2014, 9, e112988. [Google Scholar] [CrossRef] [PubMed]
- Bull, J.J.; Badgett, M.R.; Wichman, H.A. Big-benefit mutations in a bacteriophage inhibited with heat. Mol. Biol. Evol. 2000, 17, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Young, L.N.; Hockenberry, A.M.; Fane, B.A. Mutations in the N terminus of the oX174 DNA pilot protein H confer defects in both assembly and host cell attachment. J. Virol. 2014, 88, 1787–1794. [Google Scholar] [CrossRef] [PubMed]
- Pepin, K.M.; Domsic, J.; McKenna, R. Genomic evolution in a virus under specific selection for host recognition. Infect. Genet. Evol. 2008, 8, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Young, L.N.; Zhang, X.; Boudko, S.P.; Fokine, A.; Zbornik, E.; Roznowski, A.P.; Molineux, I.J.; Rossmann, M.G.; Fane, B.A. Icosahedral bacteriophage PhiX174 forms a tail for DNA transport during infection. Nature 2014, 505, 432–435. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Roznowski, A.P.; Tokuda, J.M.; Klose, T.; Mauney, A.; Pollack, L.; Fane, B.A.; Rossmann, M.G. Structural changes of tailless bacteriophage PhiX174 during penetration of bacterial cell walls. Proc. Natl. Acad. Sci. USA 2017, 114, 13708–13713. [Google Scholar] [CrossRef] [PubMed]
- Roznowski, A.P.; Fane, B.A. Structure-Function Analysis of the varphiX174 DNA-Piloting Protein Using Length-Altering Mutations. J. Virol. 2016, 90, 7956–7966. [Google Scholar] [CrossRef] [PubMed]
- Peralta, B.; Gil-Carton, D.; Castano-Diez, D.; Bertin, A.; Boulogne, C.; Oksanen, H.M.; Bamford, D.H.; Abrescia, N.G. Mechanism of membranous tunnelling nanotube formation in viral genome delivery. PLoS Biol. 2013, 11, e1001667. [Google Scholar] [CrossRef] [PubMed]
- Stromsten, N.J.; Bamford, D.H.; Bamford, J.K. The unique vertex of bacterial virus PRD1 is connected to the viral internal membrane. J. Virol. 2003, 77, 6314–6321. [Google Scholar] [CrossRef] [PubMed]
- Gowen, B.; Bamford, J.K.; Bamford, D.H.; Fuller, S.D. The tailless icosahedral membrane virus PRD1 localizes the proteins involved in genome packaging and injection at a unique vertex. J. Virol. 2003, 77, 7863–7871. [Google Scholar] [CrossRef] [PubMed]
- Jaatinen, S.T.; Viitanen, S.J.; Bamford, D.H.; Bamford, J.K. Integral membrane protein P16 of bacteriophage PRD1 stabilizes the adsorption vertex structure. J. Virol. 2004, 78, 9790–9797. [Google Scholar] [CrossRef] [PubMed]
- Assis, F.L.; Bajrai, L.; Abrahao, J.S.; Kroon, E.G.; Dornas, F.P.; Andrade, K.R.; Boratto, P.V.; Pilotto, M.R.; Robert, C.; Benamar, S.; et al. Pan-Genome Analysis of Brazilian Lineage A Amoebal Mimiviruses. Viruses 2015, 7, 3483–3499. [Google Scholar] [CrossRef] [PubMed]
- La Scola, B.; Audic, S.; Robert, C.; Jungang, L.; de Lamballerie, X.; Drancourt, M.; Birtles, R.; Claverie, J.M.; Raoult, D. A giant virus in amoebae. Science 2003, 299, 2033. [Google Scholar] [CrossRef] [PubMed]
- Philippe, N.; Legendre, M.; Doutre, G.; Coute, Y.; Poirot, O.; Lescot, M.; Arslan, D.; Seltzer, V.; Bertaux, L.; Bruley, C.; et al. Pandoraviruses: Amoeba viruses with genomes up to 2.5 Mb reaching that of parasitic eukaryotes. Science 2013, 341, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Klose, T.; Kuznetsov, Y.G.; Xiao, C.; Sun, S.; McPherson, A.; Rossmann, M.G. The three-dimensional structure of Mimivirus. Intervirology 2010, 53, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Schrad, J.R.; Young, E.J.; Abrahao, J.S.; Cortines, J.R.; Parent, K.N. Microscopic Characterization of the Brazilian Giant Samba Virus. Viruses 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Abergel, C.; Legendre, M.; Claverie, J.M. The rapidly expanding universe of giant viruses: Mimivirus, Pandoravirus, Pithovirus and Mollivirus. FEMS Microbiol. Rev. 2015, 39, 779–796. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Kuznetsov, Y.G.; Sun, S.; Hafenstein, S.L.; Kostyuchenko, V.A.; Chipman, P.R.; Suzan-Monti, M.; Raoult, D.; McPherson, A.; Rossmann, M.G. Structural studies of the giant mimivirus. PLoS Biol. 2009, 7, e1000092. [Google Scholar] [CrossRef] [PubMed]
- Campos, R.K.; Boratto, P.V.; Assis, F.L.; Aguiar, E.R.; Silva, L.C.; Albarnaz, J.D.; Dornas, F.P.; Trindade, G.S.; Ferreira, P.P.; Marques, J.T.; et al. Samba virus: A novel mimivirus from a giant rain forest, the Brazilian Amazon. Virol. J. 2014, 11, 95. [Google Scholar] [CrossRef] [PubMed]
- Zauberman, N.; Mutsafi, Y.; Halevy, D.B.; Shimoni, E.; Klein, E.; Xiao, C.; Sun, S.; Minsky, A. Distinct DNA exit and packaging portals in the virus Acanthamoeba polyphaga mimivirus. PLoS Biol. 2008, 6, e114. [Google Scholar] [CrossRef] [PubMed]
- Legendre, M.; Bartoli, J.; Shmakova, L.; Jeudy, S.; Labadie, K.; Adrait, A.; Lescot, M.; Poirot, O.; Bertaux, L.; Bruley, C.; et al. Thirty-thousand-year-old distant relative of giant icosahedral DNA viruses with a pandoravirus morphology. Proc. Natl. Acad. Sci. USA 2014, 111, 4274–4279. [Google Scholar] [CrossRef] [PubMed]
- Andreani, J.; Aherfi, S.; Bou Khalil, J.Y.; Di Pinto, F.; Bitam, I.; Raoult, D.; Colson, P.; La Scola, B. Cedratvirus, a Double-Cork Structured Giant Virus, is a Distant Relative of Pithoviruses. Viruses 2016, 8, 300. [Google Scholar] [CrossRef] [PubMed]
- Legendre, M.; Lartigue, A.; Bertaux, L.; Jeudy, S.; Bartoli, J.; Lescot, M.; Alempic, J.M.; Ramus, C.; Bruley, C.; Labadie, K.; et al. In-depth study of Mollivirus sibericum, a new 30,000-y-old giant virus infecting Acanthamoeba. Proc. Natl. Acad. Sci. USA 2015, 112, E5327–E5335. [Google Scholar] [CrossRef] [PubMed]
- Wrigley, N.G. An electron microscope study of the structure of Sericesthis iridescent virus. J. Gen Virol. 1969, 5, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Ang, K.S.; Schaposnik, L.P. On the geometry of regular icosahedral capsids containing disymmetrons. J. Struct. Biol. 2017, 197, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Sinkovits, R.S.; Baker, T.S. A tale of two symmetrons: Rules for construction of icosahedral capsids from trisymmetrons and pentasymmetrons. J. Struct. Biol. 2009, 170, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Mutsafi, Y.; Zauberman, N.; Sabanay, I.; Minsky, A. Vaccinia-like cytoplasmic replication of the giant Mimivirus. Proc. Natl. Acad. Sci. USA 2010, 107, 5978–5982. [Google Scholar] [CrossRef] [PubMed]
- Suzan-Monti, M.; La Scola, B.; Barrassi, L.; Espinosa, L.; Raoult, D. Ultrastructural characterization of the giant volcano-like virus factory of Acanthamoeba polyphaga Mimivirus. PLoS ONE 2007, 2, e328. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, K.; Miyazaki, N.; Song, C.; Maia, F.; Reddy, H.K.N.; Abergel, C.; Claverie, J.M.; Hajdu, J.; Svenda, M.; Murata, K. Structural variability and complexity of the giant Pithovirus sibericum particle revealed by high-voltage electron cryo-tomography and energy-filtered electron cryo-microscopy. Sci. Rep. 2017, 7, 13291. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Rossmann, M.G. Structures of giant icosahedral eukaryotic dsDNA viruses. Curr. Opin. Virol. 2011, 1, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Krylov, V.N.; Smirnova, T.A.; Minenkova, I.B.; Plotnikova, T.G.; Zhazikov, I.Z.; Khrenova, E.A. Pseudomonas bacteriophage phi KZ contains an inner body in its capsid. Can. J. Microbiol. 1984, 30, 758–762. [Google Scholar] [CrossRef] [PubMed]
- Fokine, A.; Battisti, A.J.; Bowman, V.D.; Efimov, A.V.; Kurochkina, L.P.; Chipman, P.R.; Mesyanzhinov, V.V.; Rossmann, M.G. Cryo-EM study of the Pseudomonas bacteriophage phiKZ. Structure 2007, 15, 1099–1104. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Thomas, J.A.; Cheng, N.; Black, L.W.; Steven, A.C. Bubblegrams reveal the inner body of bacteriophage phiKZ. Science 2012, 335, 182. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, O.S.; Shaburova, O.V.; Pechnikova, E.V.; Shaytan, A.K.; Krylov, S.V.; Kiselev, N.A.; Krylov, V.N. Genome packaging in EL and Lin68, two giant phiKZ-like bacteriophages of P. aeruginosa. Virology 2014, 468–470, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Lupo, D.; Leptihn, S.; Nagler, G.; Haase, M. Molineux, I.J.; Kuhn, A. The T7 ejection nanomachine components gp15-gp16 form a spiral ring complex that binds DNA and a lipid membrane. Virology 2016, 486, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Raytcheva, D.A.; Haase-Pettingell, C.; Piret, J.M.; King, J.A. Intracellular assembly of cyanophage Syn5 proceeds through a scaffold-containing procapsid. J. Virol. 2011, 85, 2406–2415. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Liu, Z.; Vago, F.; Ren, Y.; Wu, W.; Wright, E.T.; Serwer, P.; Jiang, W. Visualization of uncorrelated, tandem symmetry mismatches in the internal genome packaging apparatus of bacteriophage T7. Proc. Natl. Acad. Sci. USA 2013, 110, 6811–6816. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.; Wu, W.; Watts, N.R.; Steven, A.C. Exploiting radiation damage to map proteins in nucleoprotein complexes: The internal structure of bacteriophage T7. J. Struct. Biol. 2014, 185, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.T.; Schmid, M.F.; Haase-Pettingell, C.; Weigele, P.R.; King, J.A.; Chiu, W. Visualizing the structural changes of bacteriophage Epsilon15 and its Salmonella host during infection. J. Mol. Biol. 2010, 402, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Margolin, W.; Molineux, I.J.; Liu, J. The bacteriophage T7 virion undergoes extensive structural remodeling during infection. Science 2013, 339, 576–579. [Google Scholar] [CrossRef] [PubMed]
- Murata, K.; Zhang, Q.; Gerardo Galaz-Montoya, J.; Fu, C.; Coleman, M.L.; Osburne, M.S.; Schmid, M.F.; Sullivan, M.B.; Chisholm, S.W.; Chiu, W. Visualizing Adsorption of Cyanophage P-SSP7 onto Marine Prochlorococcus. Sci. Rep. 2017, 7, 44176. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Leavitt, J.C.; Cheng, N.; Gilcrease, E.B.; Motwani, T.; Teschke, C.M.; Casjens, S.R.; Steven, A.C. Localization of the Houdinisome (Ejection Proteins) inside the Bacteriophage P22 Virion by Bubblegram Imaging. mBio 2016, 7, e01152-16. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, B.; Levine, M. Bacteriophage P22 virion protein which performs an essential early function. I. Analysis of 16-ts mutants. J. Virol. 1975, 16, 1536–1546. [Google Scholar] [PubMed]
- Hoffman, B.; Levine, M. Bacteriophage P22 virion protein which performs an essential early function. II. Characterization of the gene 16 function. J. Virol. 1975, 16, 1547–1559. [Google Scholar] [PubMed]
- Israel, V.; Woodworth-Gutai, M.; Levine, M. Inhibitory effect of bacteriophage P22 infection on host cell deoxyribonuclease activity. J. Virol. 1972, 9, 752–757. [Google Scholar] [PubMed]
- Perez, G.L.; Huynh, B.; Slater, M.; Maloy, S. Transport of phage P22 DNA across the cytoplasmic membrane. J. Bacteriol. 2009, 191, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Sdao, S.M.; Dover, J.A.; Porcek, N.B.; Knobler, C.M.; Gelbart, W.M.; Parent, K.N. Bacteriophage P22 ejects all of its internal proteins before its genome. Virology 2015, 485, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Hryc, C.F.; Chen, D.H.; Afonine, P.V.; Jakana, J.; Wang, Z.; Haase-Pettingell, C.; Jiang, W.; Adams, P.D.; King, J.A.; Schmid, M.F.; et al. Accurate model annotation of a near-atomic resolution cryo-EM map. Proc. Natl. Acad. Sci. USA 2017, 114, 3103–3108. [Google Scholar] [CrossRef] [PubMed]
- Cerritelli, M.E.; Cheng, N.; Rosenberg, A.H.; McPherson, C.E.; Booy, F.P.; Steven, A.C. Encapsidated conformation of bacteriophage T7 DNA. Cell 1997, 91, 271–280. [Google Scholar] [CrossRef]
- Cerritelli, M.E.; Trus, B.L.; Smith, C.S.; Cheng, N.; Conway, J.F.; Steven, A.C. A second symmetry mismatch at the portal vertex of bacteriophage T7: 8-fold symmetry in the procapsid core. J. Mol. Biol. 2003, 327, 1–6. [Google Scholar] [CrossRef]
- San Martin, C. Latest insights on adenovirus structure and assembly. Viruses 2012, 4, 847–877. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.S.; Natchiar, S.K.; Stewart, P.L.; Nemerow, G.R. Crystal structure of human adenovirus at 3.5 A resolution. Science 2010, 329, 1071–1075. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Jin, L.; Koh, S.B.; Atanasov, I.; Schein, S.; Wu, L.; Zhou, Z.H. Atomic structure of human adenovirus by cryo-EM reveals interactions among protein networks. Science 2010, 329, 1038–1043. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Veesler, D.; Campbell, M.G.; Barry, M.E.; Asturias, F.J.; Barry, M.A.; Reddy, V.S. Cryo-EM structure of human adenovirus D26 reveals the conservation of structural organization among human adenoviruses. Sci. Adv. 2017, 3, e1602670. [Google Scholar] [CrossRef] [PubMed]
- Zubieta, C.; Schoehn, G.; Chroboczek, J.; Cusack, S. The structure of the human adenovirus 2 penton. Mol. Cell 2005, 17, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Wu, E.; Pache, L.; Von Seggern, D.J.; Mullen, T.M.; Mikyas, Y.; Stewart, P.L.; Nemerow, G.R. Flexibility of the adenovirus fiber is required for efficient receptor interaction. J. Virol. 2003, 77, 7225–7235. [Google Scholar] [CrossRef] [PubMed]
- Nemerow, G.R.; Stewart, P.L.; Reddy, V.S. Structure of human adenovirus. Curr. Opin. Virol. 2012, 2, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Van Raaij, M.J.; Mitraki, A.; Lavigne, G.; Cusack, S. A triple beta-spiral in the adenovirus fibre shaft reveals a new structural motif for a fibrous protein. Nature 1999, 401, 935–938. [Google Scholar] [CrossRef] [PubMed]
- Bewley, M.C.; Springer, K.; Zhang, Y.B.; Freimuth, P.; Flanagan, J.M. Structural analysis of the mechanism of adenovirus binding to its human cellular receptor, CAR. Science 1999, 286, 1579–1583. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Dong, X.; Wu, X.; Wen, B.; Ji, G.; Cheng, L.; Liu, H. Conserved fiber-penton base interaction revealed by nearly atomic resolution cryo-electron microscopy of the structure of adenovirus provides insight into receptor interaction. J. Virol. 2012, 86, 12322–12329. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wu, L.; Zhou, Z.H. Model of the trimeric fiber and its interactions with the pentameric penton base of human adenovirus by cryo-electron microscopy. J. Mol. Biol. 2011, 406, 764–774. [Google Scholar] [CrossRef] [PubMed]
- Wickham, T.J.; Mathias, P.; Cheresh, D.A.; Nemerow, G.R. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell 1993, 73, 309–319. [Google Scholar] [CrossRef]
- Mathias, P.; Wickham, T.; Moore, M.; Nemerow, G. Multiple adenovirus serotypes use alpha v integrins for infection. J. Virol. 1994, 68, 6811–6814. [Google Scholar] [PubMed]
- Schoehn, G.; Fender, P.; Chroboczek, J.; Hewat, E.A. Adenovirus 3 penton dodecahedron exhibits structural changes of the base on fibre binding. EMBO J. 1996, 15, 6841–6866. [Google Scholar] [PubMed]
- Lindert, S.; Silvestry, M.; Mullen, T.M.; Nemerow, G.R.; Stewart, P.L. Cryo-electron microscopy structure of an adenovirus-integrin complex indicates conformational changes in both penton base and integrin. J. Virol. 2009, 83, 11491–11501. [Google Scholar] [CrossRef] [PubMed]
- Rydman, P.S.; Caldentey, J.; Butcher, S.J.; Fuller, S.D.; Rutten, T.; Bamford, D.H. Bacteriophage PRD1 contains a labile receptor-binding structure at each vertex. J. Mol. Biol. 1999, 291, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Merckel, M.C.; Huiskonen, J.T.; Bamford, D.H.; Goldman, A.; Tuma, R. The structure of the bacteriophage PRD1 spike sheds light on the evolution of viral capsid architecture. Mol. Cell 2005, 18, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, A.; Molineux, I.J.; Casjens, S.R.; Cingolani, G. Atomic structure of bacteriophage Sf6 tail needle knob. J. Biol. Chem. 2011, 286, 30867–30877. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Benson, S.D.; Butcher, S.J.; Bamford, D.H.; Burnett, R.M. The receptor binding protein P2 of PRD1, a virus targeting antibiotic-resistant bacteria, has a novel fold suggesting multiple functions. Structure 2003, 11, 309–322. [Google Scholar] [CrossRef]
- Abrescia, N.G.; Cockburn, J.J.; Grimes, J.M.; Sutton, G.C.; Diprose, J.M.; Butcher, S.J.; Fuller, S.D.; San Martin, C.; Burnett, R.M.; Stuart, D.I.; et al. Insights into assembly from structural analysis of bacteriophage PRD1. Nature 2004, 432, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Huiskonen, J.T.; Manole, V.; Butcher, S.J. Tale of two spikes in bacteriophage PRD1. Proc. Natl. Acad. Sci. USA 2007, 104, 6666–6671. [Google Scholar] [CrossRef] [PubMed]
- Hess, M.; Cuzange, A.; Ruigrok, R.W.; Chroboczek, J.; Jacrot, B. The avian adenovirus penton: Two fibres and one base. J. Mol. Biol. 1995, 252, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.C.; Olson, A.J.; Schutt, C.E.; Winkler, F.K.; Bricogne, G. Tomato bushy stunt virus at 2.9 A resolution. Nature 1978, 276, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Fuller, S.D. The T = 4 envelope of Sindbis virus is organized by interactions with a complementary T = 3 capsid. Cell 1987, 48, 923–934. [Google Scholar] [CrossRef]
- Guo, F.; Jiang, W. Single particle cryo-electron microscopy and 3-D reconstruction of viruses. Methods Mol. Biol. 2014, 1117, 401–443. [Google Scholar] [PubMed]
- Yan, X.; Cardone, G.; Zhang, X.; Zhou, Z.H.; Baker, T.S. Single particle analysis integrated with microscopy: A high-throughput approach for reconstructing icosahedral particles. J. Struct. Biol. 2014, 186, 8–18. [Google Scholar] [CrossRef] [PubMed]

| Virus | Protomer M.W. (kDa) | Accession Number(s) | Cryo-EM | X-ray | ||
|---|---|---|---|---|---|---|
| EMDB | PDB | |||||
| Bacteriophages | P22 (MV) | 82.7 | 5049, 5050, 5051, 1482, 1483 | 5JJ3, 4V4K | +++ | +++ |
| P22 (PC) | 82.7 | 5375 * | 5JJ1 | +++ | +++ | |
| Phi29 | 35.9 | 1IJG, 1JNB, 1H5W, 1FOU | +++ | |||
| T7 | 59.1 | 1231, 2356, 2717, 5690 | 3J4A | +++ | ||
| SPP1 | 57.3 | 2993, 2994 1021 | 2JES | +++ | +++ | |
| HK97-like | 32.9 | 3KDR | +++ | |||
| G20C | 49.7 | 4ZJN | +++ | |||
| T4 | 61.0 | 6324 | 3JA7 | +++ | ||
| p2 | 39.1 | 2463 | +++ | |||
| Herpes | HSV-1 | 74.2 | 5260 ** 5261 *** | +++ | ||
| Virus | EMDB Accession Number(s) | Cryo-EM | Cryo-ET | Year | |
|---|---|---|---|---|---|
| Podoviridae | Phi29 | 1506, 5010 | +++ | 2009 | |
| Phi29 | 1419, 1420 | +++ | 2008 | ||
| Phi29 | 6560 | +++ | 2016 | ||
| CUS-3 | 5946 | +++ | 2014 | ||
| Sf6 | 5730 | +++ | 2013 | ||
| T7 | 5566–5573 | +++ | 2013 | ||
| T7 | 5534–5537 | +++ | 2013 | ||
| C1 | 5446 | +++ | 2012 | ||
| P22 | 1119 | +++ | 2005 | ||
| P22 | 1220 | +++ | 2006 | ||
| P22 | 1222 | +++ | 2006 | ||
| P22 | 1827 | +++ | 2011 | ||
| P22 | 5348, 5231 | +++ | 2011 | ||
| P22 | 8258–6261 | +++ | 2016 | ||
| P22 | 8005 | +++ | 2016 | ||
| P-SSP7 | 1707 | +++ | 2010 | ||
| P-SSP7 | 6427 | +++ | 2016 | ||
| P-SSP7 | 1714, 1715 | +++ | 2010 | ||
| P-SSP7 | 3131 | +++ | TBP | ||
| Syn5 | 5743–5746 | +++ | 2013 | ||
| ε15 | 1175 | +++ | 2005 | ||
| ε15 | 5203, 5204 | +++ | 2010 | ||
| ε15 | 5207–5209 | +++ | 2010 | ||
| ε15 | 5216–5219 | +++ | 2010 | ||
| BPP-1 | 1619 | +++ | 2010 | ||
| N4 | 1475 | +++ | 2009 | ||
| PRD-1 | 3548–3550 | +++ | 2017 | ||
| PRD-1 | 2438–2440 | +++ | 2013 | ||
| K1E | 1336 | +++ | 2007 | ||
| K1-5 | 1337 | +++ | 2007 | ||
| Myo | PhiKZ | 1415 | +++ | 2007 | |
| T4 | 1572, 1573 | +++ | 2008 | ||
| T4 | 6323 | +++ | 2015 | ||
| T4 | 2774, 6078–6083 | +++ | 2015 | ||
| Sipho | P2 | 2463, 2464 | +++ | 2013 | |
| Araucaria | 2335–2338 | +++ | 2013 | ||
| 1358 | 2820 | +++ | 2016 | ||
| TW1 | 7070, 8854, 8867, 8868 | +++ | 2017 | ||
| ssDNA | ΦX174 | 7033, 8862 | +++ | 2017 |
| Virus | EMDB Accession Number(s) | Cryo-EM | Cryo-ET | Year | |
|---|---|---|---|---|---|
| Archeal | APBV1 * | 3857–3859 | +++ | 2017 | |
| His1 * | 6220–6222 | +++ | 2015 | ||
| Eukaryotic | HSV-1 | 5452, 5453 | +++ | 2012 | |
| HSV-1 | 5255, 5260, 5261 | +++ | 2011 | ||
| HSV-1 | 1305–1308 | +++ | 2007 | ||
| KSHV | 1320 | +++ | 2007 | ||
| Faustovirus | 8144, 8145 | +++ | 2016 | ||
| PBCV-1 | 1597 | +++ | 2009 | ||
| PBCV-1 | 5384 | +++ | 2012 | ||
| CroV ** | 8748 | +++ | 2017 | ||
| Mimivirus ** | 5039 | +++ | 2009 | ||
| Samba virus ** | 8599 | +++ | 2017 | ||
| Chilo Iridescent Virus ** | 1580 | +++ | 2009 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parent, K.N.; Schrad, J.R.; Cingolani, G. Breaking Symmetry in Viral Icosahedral Capsids as Seen through the Lenses of X-ray Crystallography and Cryo-Electron Microscopy. Viruses 2018, 10, 67. https://doi.org/10.3390/v10020067
Parent KN, Schrad JR, Cingolani G. Breaking Symmetry in Viral Icosahedral Capsids as Seen through the Lenses of X-ray Crystallography and Cryo-Electron Microscopy. Viruses. 2018; 10(2):67. https://doi.org/10.3390/v10020067
Chicago/Turabian StyleParent, Kristin N., Jason R. Schrad, and Gino Cingolani. 2018. "Breaking Symmetry in Viral Icosahedral Capsids as Seen through the Lenses of X-ray Crystallography and Cryo-Electron Microscopy" Viruses 10, no. 2: 67. https://doi.org/10.3390/v10020067
APA StyleParent, K. N., Schrad, J. R., & Cingolani, G. (2018). Breaking Symmetry in Viral Icosahedral Capsids as Seen through the Lenses of X-ray Crystallography and Cryo-Electron Microscopy. Viruses, 10(2), 67. https://doi.org/10.3390/v10020067