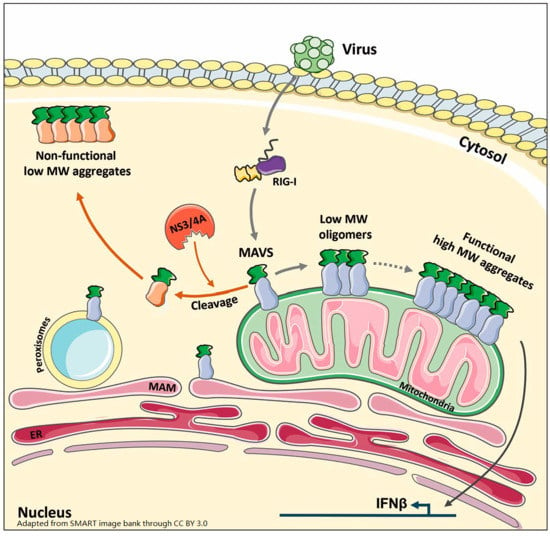
Virus Infection Triggers MAVS Polymers of Distinct Molecular Weight
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plasmids
2.2. Cell Culture
2.3. Plasmid Transfection
2.4. Virus Infection
2.5. Cell Lysis
2.6. SDD-AGE
2.7. Non-Reducing/Reducing SDS-PAGE
2.8. Immunoblot
3. Results
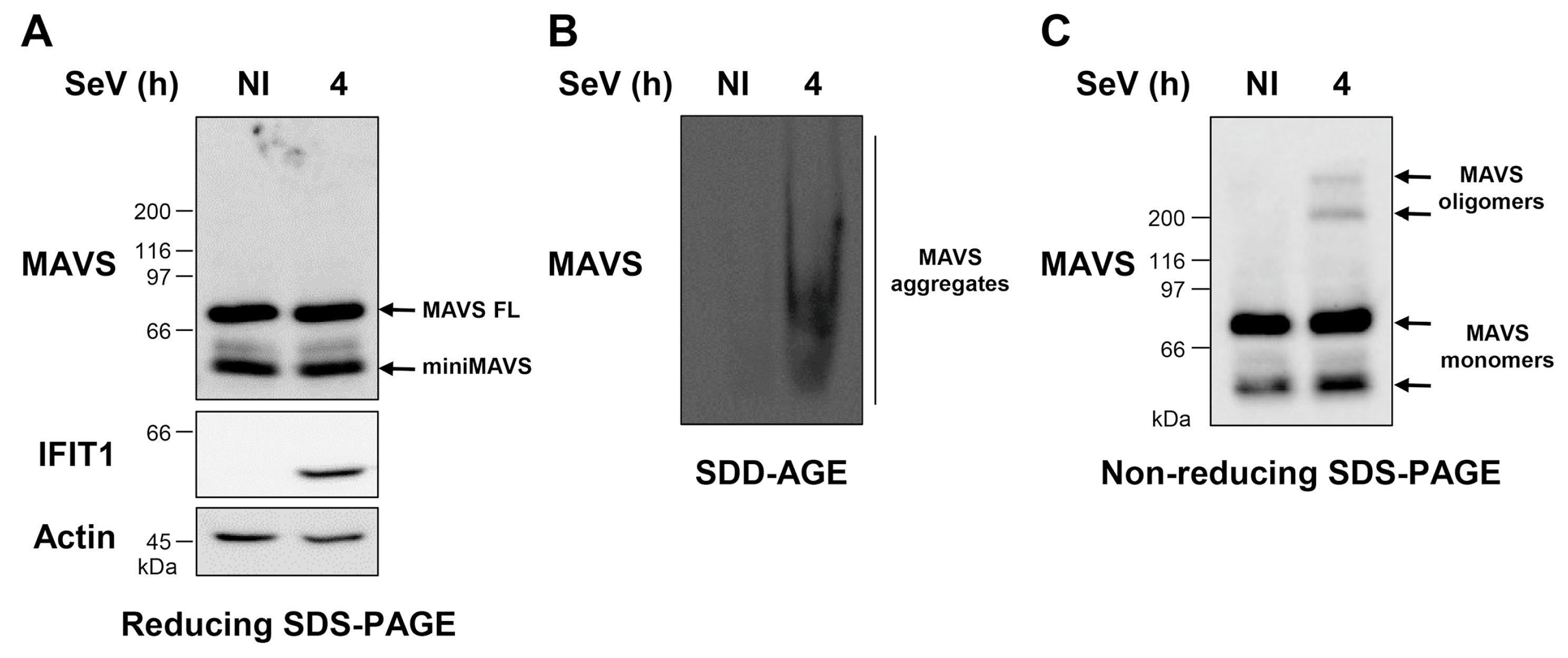
3.1. WCE Analysis by SDD-AGE and Non-Reducing SDS-PAGE Reveals MAVS Aggregates and Oligomers of Distinct MW Induced by SeV Infection
3.2. Activation of RIG-I Is Sufficient to Trigger MAVS Oligomerization and Aggregation
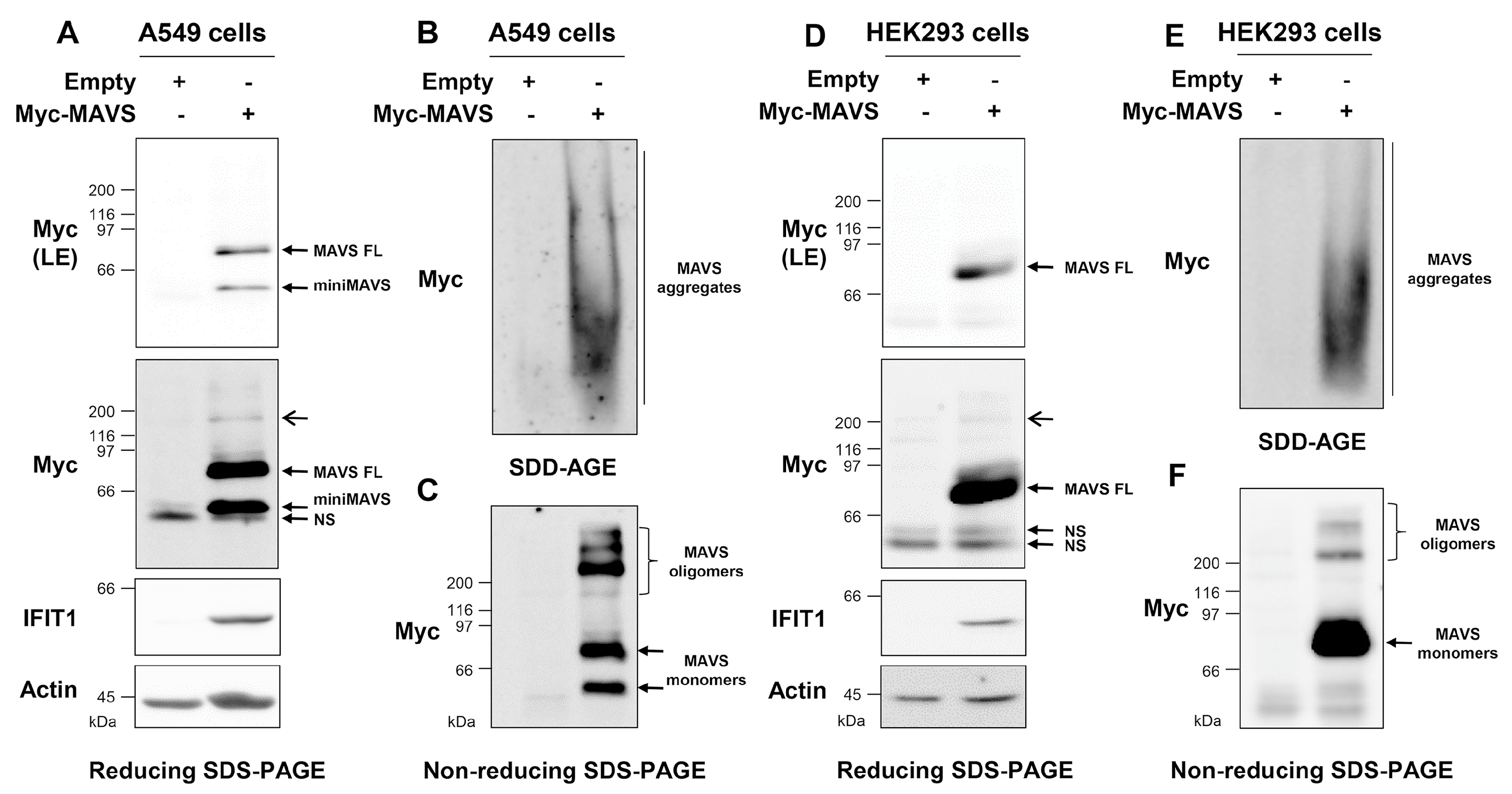
3.3. Ectopically Expressed MAVS Is Detected as Oligomeric and Aggregated States
3.4. MAVS Anchoring to Intracellular Membranes Is Essential for Functional Polymer Formation
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BME | β-mercaptoethanol |
| SEC | size-exclusion chromatography |
| CARD | caspase recruitment domain |
| FRET | fluorescence resonance energy transfer |
| HCV | hepatitis C virus |
| HypF | [NiFe]-hydrogenase maturation factor |
| IFIT1 | interferon-induced protein with tetratricopeptide repeats 1 |
| IFN | interferon |
| IRF | interferon regulatory factors |
| ISG | IFN-stimulated gene |
| MAM | mitochondrial-associated endoplasmic reticulum membrane |
| MAVS | mitochondrial antiviral signaling protein |
| MDA5 | melanoma differentiation-associated protein 5 |
| MW | molecular weight |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| P53 | cellular tumor antigen p53 |
| PRR | pathogen recognition receptor |
| RIG-I | retinoic acid-inducible gene I protein |
| SDD-AGE | semi-denaturing detergent agarose gel electrophoresis |
| SDS-PAGE | sodium dodecyl sulfate-polyacrylamide gel electrophoresis |
| SOD1 | superoxide dismutase [Cu-Zn] |
| SeV | Sendai virus |
| TRAF3 | TNF receptor-associated factor 3 |
| TRAF6 | TNF receptor-associated factor 6 |
| WCE | whole cell extract |
References
- Yoneyama, M.; Kikuchi, M.; Natsukawa, T.; Shinobu, N.; Imaizumi, T.; Miyagishi, M.; Taira, K.; Akira, S.; Fujita, T. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 2004, 5, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, M.; Kikuchi, M.; Matsumoto, K.; Imaizumi, T.; Miyagishi, M.; Taira, K.; Foy, E.; Loo, Y.M.; Gale, M., Jr.; Akira, S.; et al. Shared and unique functions of the DExD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity. J. Immunol. 2005, 175, 2851–2858. [Google Scholar] [CrossRef] [PubMed]
- Odendall, C.; Kagan, J.C. Activation and pathogenic manipulation of the sensors of the innate immune system. Microbes Infect. 2017, 19, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Dixit, E.; Kagan, J.C. Intracellular pathogen detection by RIG-I-like receptors. Adv. Immunol. 2013, 117, 99–125. [Google Scholar] [PubMed]
- Seth, R.B.; Sun, L.; Ea, C.-K.; Chen, Z.J. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-κB and IRF 3. Cell 2005, 122, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Takahashi, K.; Sato, S.; Coban, C.; Kumar, H.; Kato, H.; Ishii, K.J.; Takeuchi, O.; Akira, S. IPS-1, an adaptor triggering RIG-I- and MDA5-mediated type I interferon induction. Nat. Immunol. 2005, 6, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.-G.; Wang, Y.-Y.; Han, K.-J.; Li, L.-Y.; Zhai, Z.; Shu, H.-B. Visa is an adapter protein required for virus-triggered IFN-β signaling. Mol. Cell 2005, 19, 727–740. [Google Scholar] [CrossRef] [PubMed]
- Meylan, E.; Curran, J.; Hofmann, K.; Moradpour, D.; Binder, M.; Bartenschlager, R.; Tschopp, J. Cardif is an adaptor protein in the Rig-I antiviral pathway and is targeted by hepatitis C virus. Nature 2005, 437, 1167–1172. [Google Scholar] [CrossRef] [PubMed]
- Dixit, E.; Boulant, S.; Zhang, Y.; Lee, A.S.; Odendall, C.; Shum, B.; Hacohen, N.; Chen, Z.J.; Whelan, S.P.; Fransen, M.; et al. Peroxisomes are signaling platforms for antiviral innate immunity. Cell 2010, 141, 668–681. [Google Scholar] [CrossRef] [PubMed]
- Horner, S.M.; Liu, H.M.; Park, H.S.; Briley, J.; Gale, M. Mitochondrial-associated endoplasmic reticulum membranes (MAM) form innate immune synapses and are targeted by hepatitis C virus. Proc. Natl. Acad. Sci. USA 2011, 108, 14590–14595. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.L.; Coyne, C.B. Mechanisms of MAVS regulation at the mitochondrial membrane. J. Mol. Biol. 2013, 425, 5009–5019. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Morosky, S.A.; Delorme-Axford, E.; Dybdahl-Sissoko, N.; Oberste, M.S.; Wang, T.; Coyne, C.B. The coxsackievirus B 3C protease cleaves Mavs and TRIF to attenuate host type I interferon and apoptotic signaling. PLoS Pathog. 2011, 7, e1001311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Li, S.; Dorf, M.E. Nemo binds ubiquitinated tank-binding kinase 1 (TBK1) to regulate innate immune responses to RNA viruses. PLoS ONE 2012, 7, e43756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belgnaoui, S.M.; Paz, S.; Hiscott, J. Orchestrating the interferon antiviral response through the mitochondrial antiviral signaling (MAVS) adapter. Curr. Opin. Immunol. 2011, 23, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, C.; Horner, S.M. Mavs coordination of antiviral innate immunity. J. Virol. 2015, 89, 6974–6977. [Google Scholar] [CrossRef] [PubMed]
- Oshiumi, H.; Kouwaki, T.; Seya, T. Accessory factors of cytoplasmic viral RNA sensors required for antiviral innate immune response. Front. Immunol. 2016, 7, 200. [Google Scholar] [CrossRef] [PubMed]
- Odendall, C.; Dixit, E.; Stavru, F.; Bierne, H.; Franz, K.M.; Durbin, A.F.; Boulant, S.; Gehrke, L.; Cossart, P.; Kagan, J.C. Diverse intracellular pathogens activate type III interferon expression from peroxisomes. Nat. Immunol. 2014, 15, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Bender, S.; Reuter, A.; Eberle, F.; Einhorn, E.; Binder, M.; Bartenschlager, R. Activation of type I and III interferon response by mitochondrial and peroxisomal mavs and inhibition by hepatitis c virus. PLoS Pathog. 2015, 11, e1005264. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Su, C. Herpes simplex virus 1 infection dampens the immediate early antiviral innate immunity signaling from peroxisomes by tegument protein VP16. Virol. J. 2017, 14, 35. [Google Scholar] [CrossRef] [PubMed]
- Peisley, A.; Wu, B.; Yao, H.; Walz, T.; Hur, S. Rig-I forms signaling-competent filaments in an ATP-dependent, ubiquitin-independent manner. Mol. Cell 2013, 51, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Peisley, A.; Tetrault, D.; Li, Z.; Egelman, E.H.; Magor, K.E.; Walz, T.; Penczek, P.A.; Hur, S. Molecular imprinting as a signal-activation mechanism of the viral RNA sensor RIG-I. Mol. Cell 2014, 55, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Hou, F.; Sun, L.; Zheng, H.; Skaug, B.; Jiang, Q.X.; Chen, Z.J. Mavs forms functional prion-like aggregates to activate and propagate antiviral innate immune response. Cell 2011, 146, 448–461. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; He, X.; Zheng, H.; Huang, L.J.; Hou, F.; Yu, Z.; de la Cruz, M.J.; Borkowski, B.; Zhang, X.; Chen, Z.J.; et al. Structural basis for the prion-like mavs filaments in antiviral innate immunity. eLife 2014, 3, e01489. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Lührs, T.; Ritter, C. Solid-state NMR resonance assignments of the filment-forming card domain of the innate immunity signaling protein mavs. Biomol. NMR Assign. 2015, 9, 223–227. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Bardiaux, B.; Ahmed, M.; Spehr, J.; Konig, R.; Lunsdorf, H.; Rand, U.; Luhrs, T.; Ritter, C. Structure determination of helical filaments by solid-state NMR spectroscopy. Proc. Nat. Acad. Sci. USA 2016, 113, E272–E281. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Huoh, Y.-S.; Hur, S. Measuring Monomer-to-Filament Transition of Mavs as an in Vitro Activity Assay for Rig-I-like Receptors. In Toll-Like Receptors: Practice and Methods; McCoy, C.E., Ed.; Springer: New York, NY, USA, 2016; pp. 131–142. [Google Scholar]
- Baril, M.; Racine, M.-E.; Penin, F.; Lamarre, D. Mavs dimer is a crucial signaling component of innate immunity and the target of hepatitis C virus ns3/4a protease. J. Virol. 2009, 83, 1299–1311. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Sun, X.; Nie, X.; Sun, L.; Tang, T.-S.; Chen, D.; Sun, Q. COX5B regulates MAVS-mediated antiviral signaling through interaction with ATG5 and repressing ROS production. PLoS Pathog. 2012, 8, e1003086. [Google Scholar] [CrossRef] [PubMed]
- Buskiewicz, I.A.; Montgomery, T.; Yasewicz, E.C.; Huber, S.A.; Murphy, M.P.; Hartley, R.C.; Kelly, R.; Crow, M.K.; Perl, A.; Budd, R.C.; et al. Reactive oxygen species induce virus-independent MAVS oligomerization in systemic lupus erythematosus. Sci. Signal. 2016, 9, ra115. [Google Scholar] [CrossRef] [PubMed]
- Westermark, G.T.; Fändrich, M.; Westermark, P. AA amyloidosis: Pathogenesis and targeted therapy. Annu. Rev. Pathol. Mech. Dis. 2015, 10, 321–344. [Google Scholar] [CrossRef] [PubMed]
- Michaels, T.C.T.; Lazell, H.W.; Arosio, P.; Knowles, T.P.J. Dynamics of protein aggregation and oligomer formation governed by secondary nucleation. J. Chem. Phys. 2015, 143, 054901. [Google Scholar] [CrossRef] [PubMed]
- Giacomelli, C.; Daniele, S.; Martini, C. Potential biomarkers and novel pharmacological targets in protein aggregation-related neurodegenerative diseases. Biochem. Pharmacol. 2017, 131, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Fändrich, M. Oligomeric intermediates in amyloid formation: Structure determination and mechanisms of toxicity. J. Mol. Biol. 2012, 421, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Breiman, A.; Grandvaux, N.; Lin, R.; Ottone, C.; Akira, S.; Yoneyama, M.; Fujita, T.; Hiscott, J.; Meurs, E.F. Inhibition of RIG-I-dependent signaling to the interferon pathway during hepatitis C virus expression and restoration of signaling by ikkepsilon. J. Virol. 2005, 79, 3969–3978. [Google Scholar] [CrossRef] [PubMed]
- Halfmann, R.; Lindquist, S. Screening for amyloid aggregation by semi-denaturing detergent-agarose gel electrophoresis. J. Vis. Exp. 2008, 838. [Google Scholar] [CrossRef] [PubMed]
- Seiberlich, V.; Goldbaum, O.; Zhukareva, V.; Richter-Landsberg, C. The small molecule inhibitor PR-619 of deubiquitinating enzymes affects the microtubule network and causes protein aggregate formation in neural cells: Implications for neurodegenerative diseases. Biochim. Biophys. Acta Mol. Cell Res. 2012, 1823, 2057–2068. [Google Scholar] [CrossRef] [PubMed]
- Robitaille, A.C.; Mariani, M.K.; Fortin, A.; Grandvaux, N. A high resolution method to monitor phosphorylation-dependent activation of IRF3. J. Vis. Exp. 2016, e53723. [Google Scholar] [CrossRef] [PubMed]
- Orte, A.; Birkett, N.R.; Clarke, R.W.; Devlin, G.L.; Dobson, C.M.; Klenerman, D. Direct characterization of amyloidogenic oligomers by single-molecule fluorescence. Proc. Natl. Acad. Sci. USA 2008, 105, 14424–14429. [Google Scholar] [CrossRef] [PubMed]
- Brubaker, S.W.; Gauthier, A.E.; Mills, E.W.; Ingolia, N.T.; Kagan, J.C. A bicistronic mavs transcript highlights a class of truncated variants in antiviral immunity. Cell 2014, 156, 800–811. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Aparicio, M.T.; Ayllón, J.; Leo-Macias, A.; Wolff, T.; García-Sastre, A. Subcellular localizations of RIG-I, TRIM25, and MAVS complexes. J. Virol. 2017, 91, e01155-16. [Google Scholar] [CrossRef] [PubMed]
- Koshiba, T.; Yasukawa, K.; Yanagi, Y.; Kawabata, S.-I. Mitochondrial membrane potential is required for mavs-mediated antiviral signaling. Sci. Signal. 2011, 4, ra7. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Yuan, B.; Zhu, W.; Zhang, R.; Li, L.; Hao, X.; Chen, S.; Hou, F. Ube2d3 and ube2n are essential for rig-I-mediated mavs aggregation in antiviral innate immunity. Nat. Commun. 2017, 8, 15138. [Google Scholar] [CrossRef] [PubMed]
- Hames, D.R.; David Hames, B. Gel Electrophoresis of Proteins: A Practical Approach, 3rd ed.; Oxford University Press Inc.: New York, NY, USA, 2002; pp. 98–218. [Google Scholar]
- Gallagher, S.R. SDS-polyacrylamide gel electrophoresis (SDS-PAGE). Curr. Protoc. Essent. Lab. Tech. 2012. [Google Scholar] [CrossRef]
- Rath, A.; Glibowicka, M.; Nadeau, V.G.; Chen, G.; Deber, C.M. Detergent binding explains anomalous SDS-PAGE migration of membrane proteins. Proc. Natl. Acad. Sci. USA 2009, 106, 1760–1765. [Google Scholar] [CrossRef] [PubMed]
- Campioni, S.; Mannini, B.; Zampagni, M.; Pensalfini, A.; Parrini, C.; Evangelisti, E.; Relini, A.; Stefani, M.; Dobson, C.M.; Cecchi, C.; et al. A causative link between the structure of aberrant protein oligomers and their toxicity. Nat. Chem. Biol. 2010, 6, 140. [Google Scholar] [CrossRef] [PubMed]
- Baftizadeh, F.; Biarnes, X.; Pietrucci, F.; Affinito, F.; Laio, A. Multidimensional view of amyloid fibril nucleation in atomistic detail. J. Am. Chem. Soc. 2012, 134, 3886–3894. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-D.; Sun, L.; Seth, R.B.; Pineda, G.; Chen, Z.J. Hepatitis c virus protease ns3/4a cleaves mitochondrial antiviral signaling protein off the mitochondria to evade innate immunity. Proc. Natl. Acad. Sci. USA 2005, 102, 17717–17722. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Fersht, A.R. First-order rate-determining aggregation mechanism of p53 and its implications. Proc. Natl. Acad. Sci. USA 2012, 109, 13590–13595. [Google Scholar] [CrossRef] [PubMed]
- Leal, S.S.; Cristovao, J.S.; Biesemeier, A.; Cardoso, I.; Gomes, C.M. Aberrant zinc binding to immature conformers of metal-free copper-zinc superoxide dismutase triggers amorphous aggregation. Metallomics 2015, 7, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Invernizzi, G.; Papaleo, E.; Sabate, R.; Ventura, S. Protein aggregation: Mechanisms and functional consequences. Int. J. Biochem. Cell Biol. 2012, 44, 1541–1554. [Google Scholar] [CrossRef] [PubMed]
- Schoborg, J.A.; Hershewe, J.; Stark, J.C.; Kightlinger, W.; Kath, J.E.; Jaroentomeechai, T.; Natarajan, A.; DeLisa, M.P.; Jewett, M.C. A cell-free platform for rapid synthesis and testing of active oligosaccharyltransferases. Biotechnol. Bioeng. 2017, 115, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Guan, Y.; Wang, S.; Xu, H.; Tao, N. Study of small-molecule–membrane protein binding kinetics with nanodisc and charge-sensitive optical detection. Anal. Chem. 2016, 88, 2375–2379. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zamorano Cuervo, N.; Osseman, Q.; Grandvaux, N. Virus Infection Triggers MAVS Polymers of Distinct Molecular Weight. Viruses 2018, 10, 56. https://doi.org/10.3390/v10020056
Zamorano Cuervo N, Osseman Q, Grandvaux N. Virus Infection Triggers MAVS Polymers of Distinct Molecular Weight. Viruses. 2018; 10(2):56. https://doi.org/10.3390/v10020056
Chicago/Turabian StyleZamorano Cuervo, Natalia, Quentin Osseman, and Nathalie Grandvaux. 2018. "Virus Infection Triggers MAVS Polymers of Distinct Molecular Weight" Viruses 10, no. 2: 56. https://doi.org/10.3390/v10020056