Detection of Airborne Inoculum of Hymenoscyphus fraxineus and H. albidus during Seasonal Fluctuations Associated with Absence of Apothecia
Abstract
:1. Introduction
2. Experimental Section
2.1. Sampling Locality
2.2. Air Samplers

2.3. DNA Extraction
2.4. qPCR Conditions
| Nucleotide Designation | Sequence (5′–3′) | Detected Organism | Source |
|---|---|---|---|
| Halb-F | TATATTGTTGCTTTAGCAGGTCGC | H. albidus | Husson et al. [17] |
| Halb-R | ATCCTCTAGCAGGCACGGTC | H. albidus | Husson et al. [17] |
| Halb-P | YY-CCGGGGCGTTGGCCTCG-BHQ2 | H. albidus | Husson et al. [17] |
| Cf-F | CCCTTGTGTATATTATATTGTTGCTTTAGC | H. fraxineus | Chandelier et al. [26] |
| Cf-R | GGGTCCTCTAGCAGGCACAGT | H. fraxineus | Chandelier et al. [26] |
| Cf-S | 6FAM-TCTGGGCGTCGGCCTCGG-BHQ1 | H. fraxineus | Chandelier et al. [26] |
2.5. Absolute Quantification
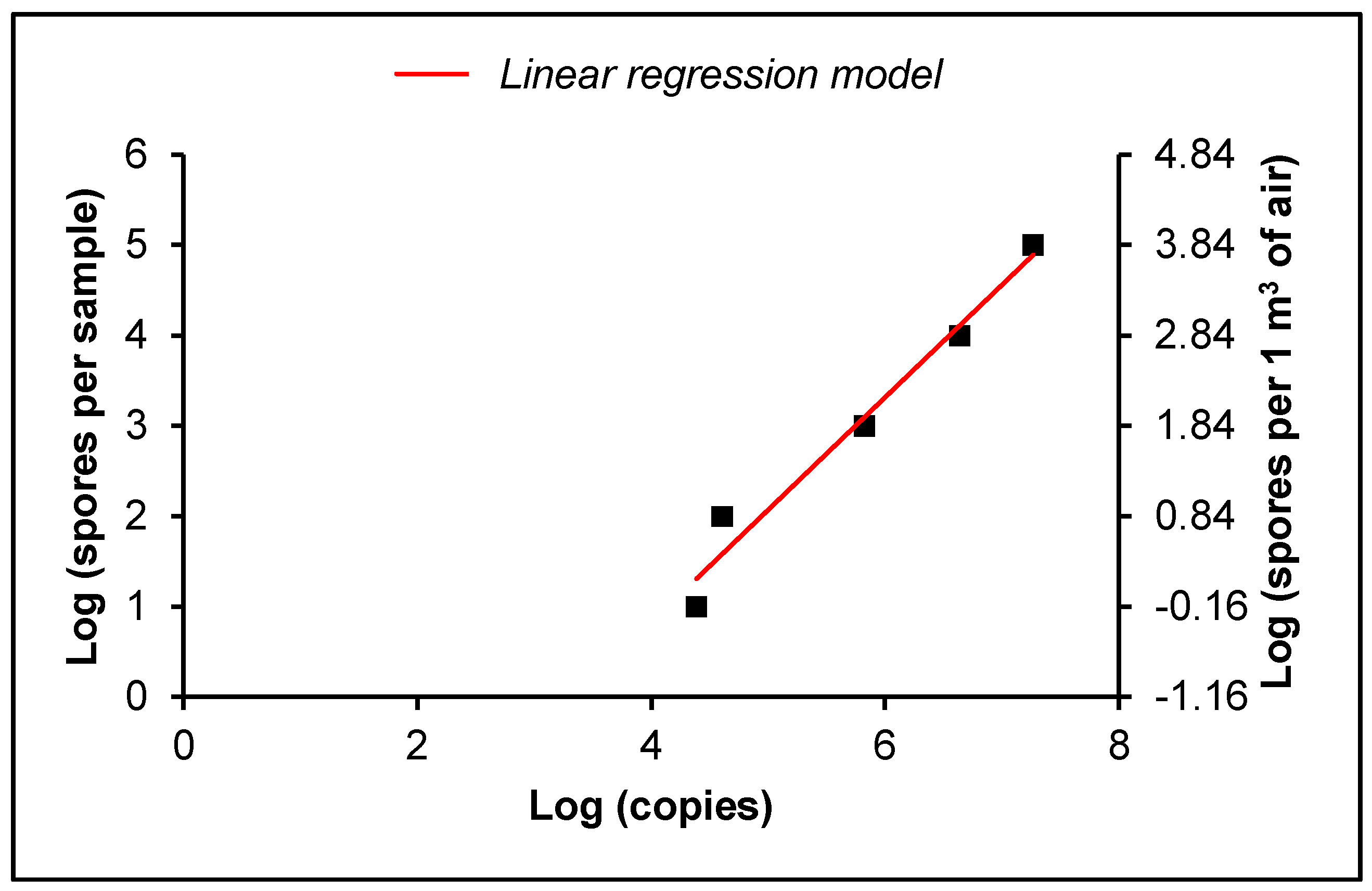
2.6. Statistical Analysis
3. Results
3.1. Detection of H. fraxineus and H. albidus Air Inoculum

3.2. Comparison of Different Air Samplers
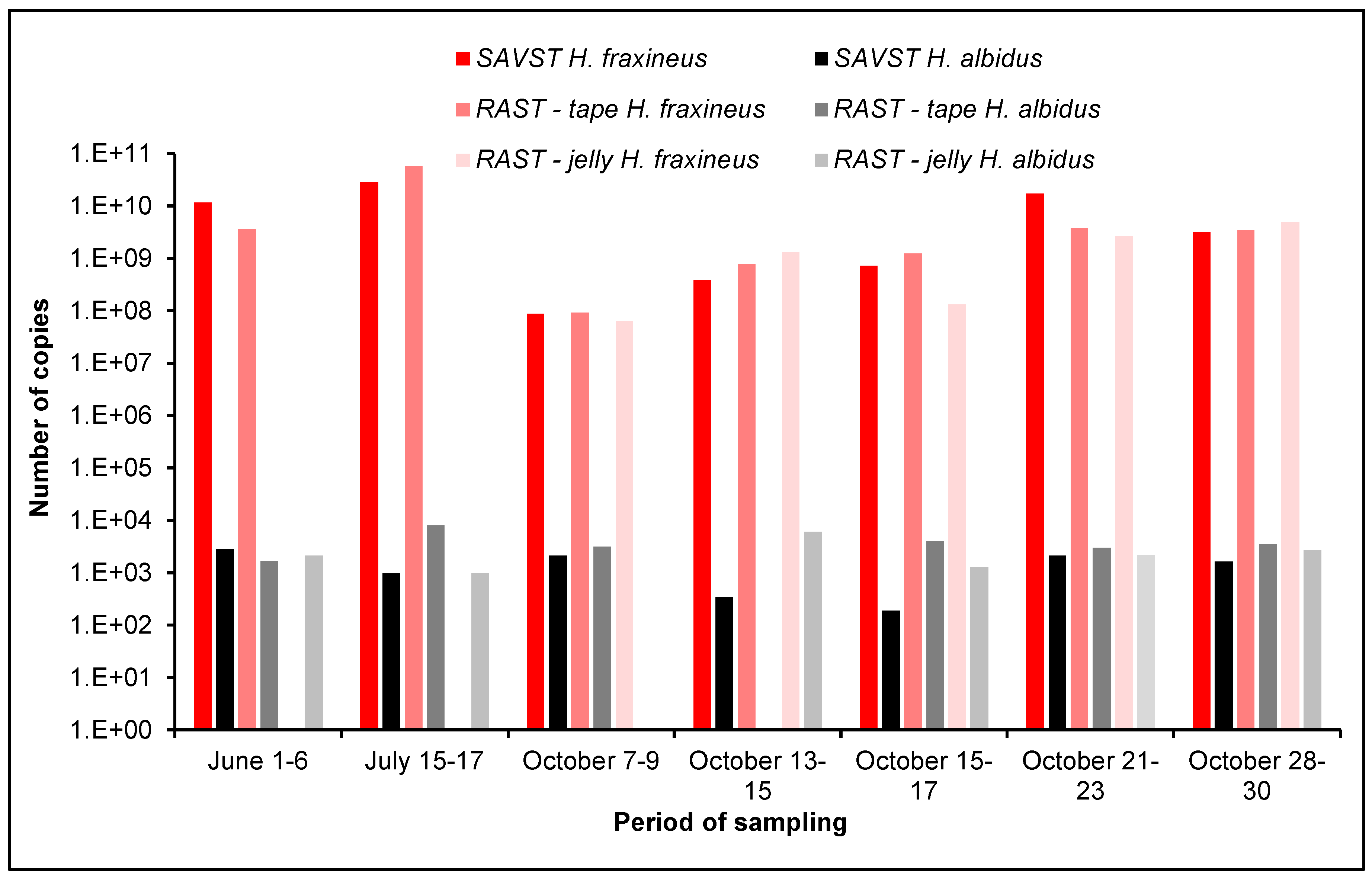
3.3. qPCR Detection of H. fraxineus and H. albidus
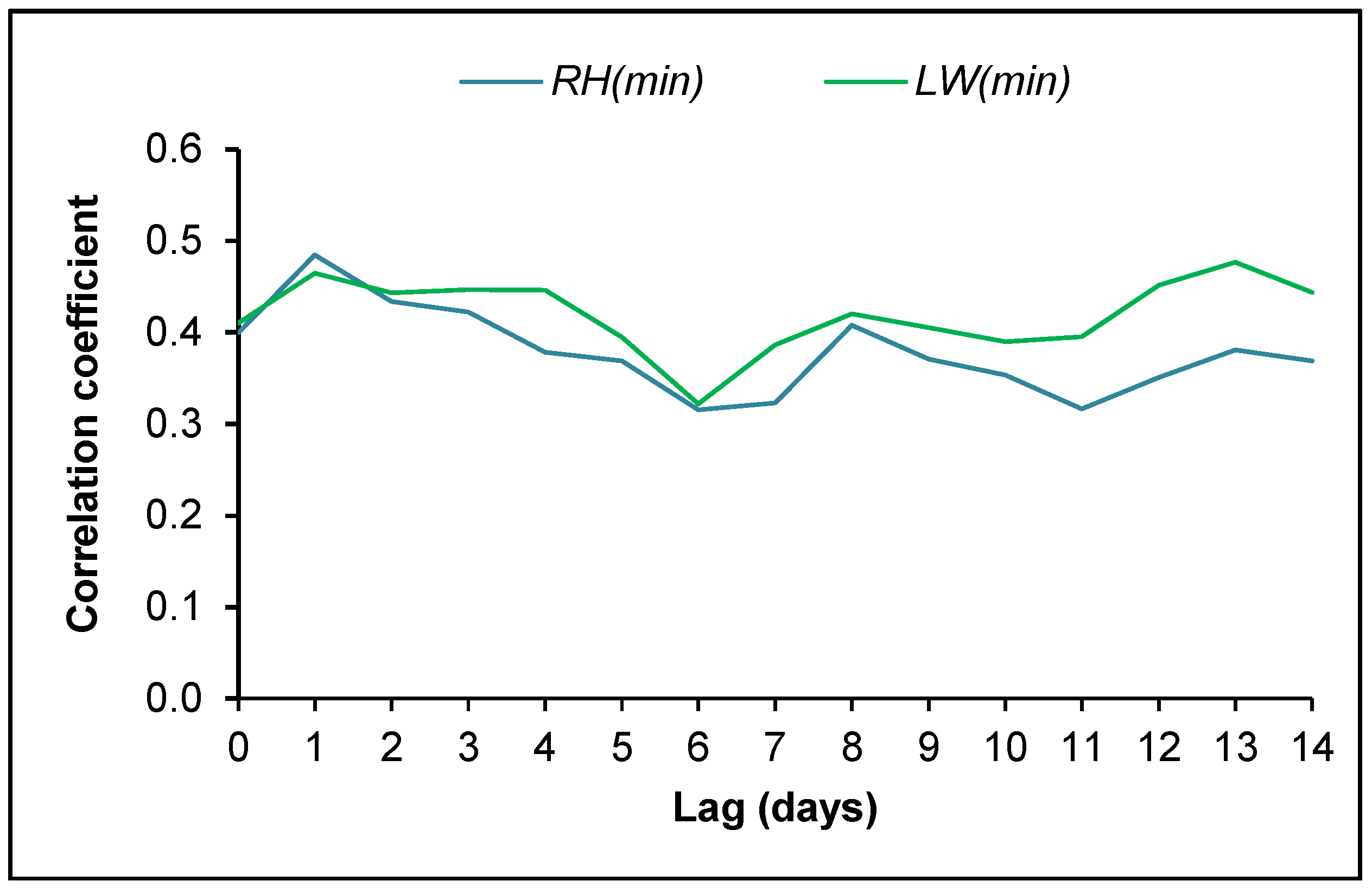
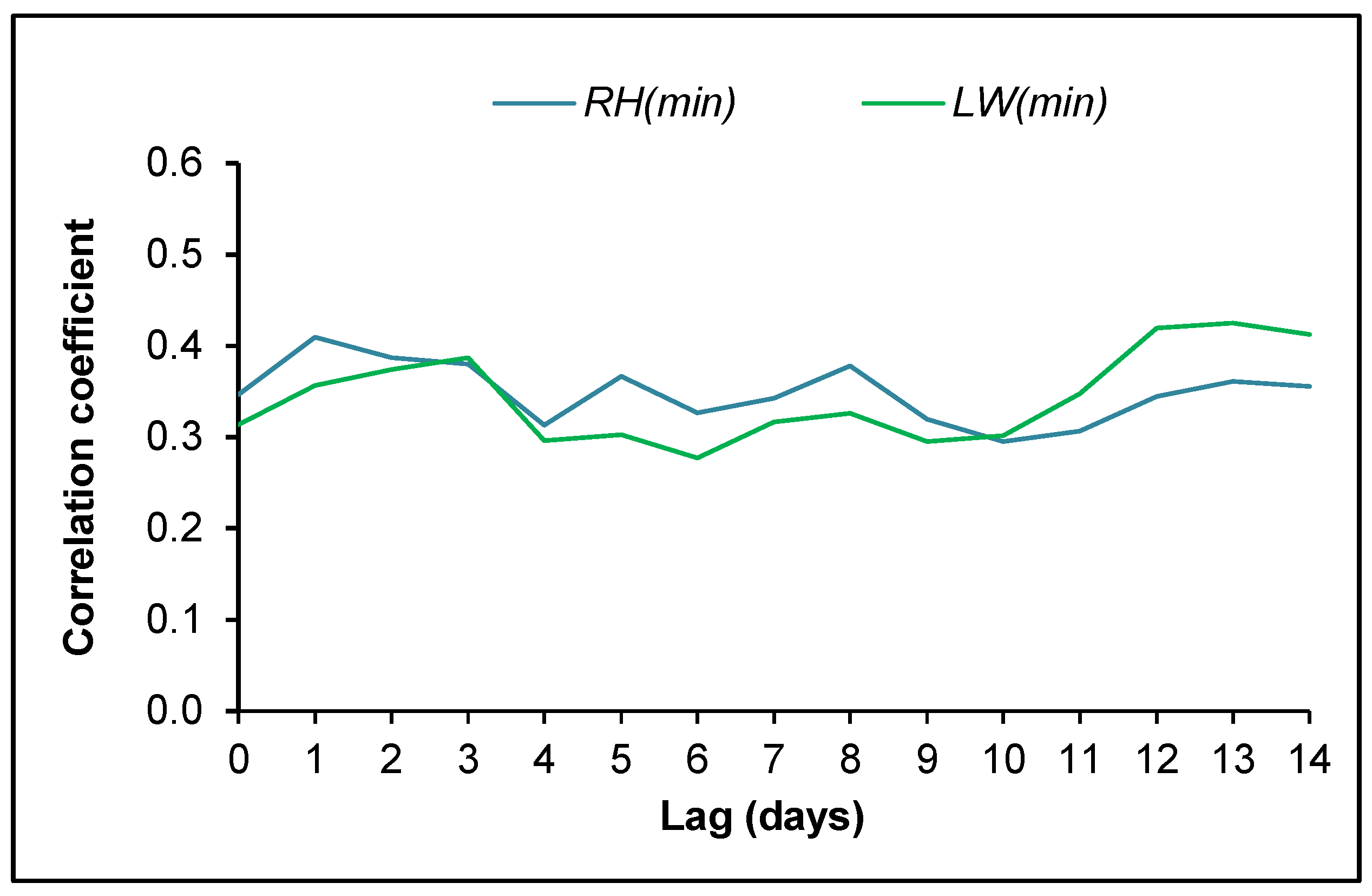
3.4. Ascospore Occurrence vs. Meteorological Data
4. Discussion
4.1. Detection of Air-Inoculum
4.2. Ascospore Production vs. Meteorological Data
4.3. Absolute Quantification
4.4. H. fraxineus vs. H. albidus
4.5. Comparison of Different Air-Samplers
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Queloz, V.; Grünig, C.R.; Berndt, R.; Kowalski, T.; Sieber, T.N.; Holdenrieder, O. Cryptic speciation in Hymenoscyphus albidus. For. Pathol. 2011, 41, 133–142. [Google Scholar] [CrossRef]
- Kowalski, T.; Holdenrieder, O. Pathogenicity of Chalara fraxinea. For. Pathol. 2009, 39, 1–7. [Google Scholar] [CrossRef]
- Zhao, Y.; Hosoya, T.; Baral, H.O.; Hosaka, K.; Kakishima, M. Hymenoscyphus pseudoalbidus, the correct name for Lambertelle albida reported from Japan. Mycotaxon 2012, 122, 25–41. [Google Scholar] [CrossRef]
- Kowalski, T. Chalara fraxinea sp. nov. associated with dieback of ash (Fraxinus excelsior) in Poland. For. Pathol. 2006, 36, 264–270. [Google Scholar] [CrossRef]
- Pautasso, M.; Aas, G.; Queloz, V.; Holdenrieder, O. European ash (Fraxinus excelsior) dieback—A conservation biology challenge. Biol. Conserv. 2013, 158, 37–49. [Google Scholar] [CrossRef]
- Kräutler, K.; Treitler, R.; Kirisits, T. Hymenoscyphus fraxineus can directly infect intact current-year shoots of Fraxinus excelsior and artificially exposed leaf scars. For. Pathol. 2015, 45, 274–280. [Google Scholar] [CrossRef]
- Gross, A.; Zaffarano, P.L.; Duo, A.; Grünig, C.R. Reproductive mode and life cycle of the ash dieback pathogen Hymenoscyphus pseudoalbidus. Fungal Genet. Biol. 2012, 49, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Husson, C.; Caël, O.; Grandjean, J.P.; Nageleisen, L.M.; Marçais, B. Occurrence of Hymenoscyphus pseudoalbidus on infected ash logs. Plant Pathol. 2012, 61, 889–895. [Google Scholar] [CrossRef]
- Kirisits, T.; Schwanda, K. First definite report of natural infection of Fraxinus ornus by Hymenoscyphus fraxineus. For. Pathol. 2015, 45, 430–432. [Google Scholar] [CrossRef]
- Chandelier, A.; Helson, M.; Dvorak, M.; Gischer, F. Detection and quantification of airborne inoculum of Hymenoscyphus pseudoalbidus using real-time PCR assays. Plant Pathol. 2014, 63, 1296–1305. [Google Scholar] [CrossRef]
- Gross, A.; Holdenrieder, O.; Pautasso, M.; Queloz, V.; Sieber, T.N. Hymenoscyphus pseudoalbidus, the causal agent of European ash dieback. Mol. Plant Pathol. 2014, 15, 5–21. [Google Scholar] [CrossRef] [PubMed]
- Timmermann, V.; Borja, I.; Hietala, A.M.; Kirisits, T.; Solheim, H. Ash dieback: Pathogen spread and diurnal patterns of ascospore dispersal, with special emphasis on Norway. EPPO Bull. 2011, 41, 14–20. [Google Scholar] [CrossRef]
- Hietala, A.M.; Timmermann, V.; Borja, I.; Solheim, H. The invasive ash dieback pathogen Hymenoscyphus pseudoalbidus exerts maximal infection pressure prior to the onset of host leaf senescence. Fungal Ecol. 2013, 6, 302–308. [Google Scholar] [CrossRef]
- Kowalski, T.; Bartnik, C. Morphological variation in colonies of Chalara fraxinea isolated from ash (Fraxinus excelsior L.) stems with symptoms of dieback and effects of temperature on colony growth and structure. Acta Agrobot. 2010, 63, 99–106. [Google Scholar] [CrossRef]
- Baral, H.; Bemmann, M. Hymenoscyphus fraxineus vs. Hymenoscyphus albidus—A comparative light microscopic study on the causal agent of European ash dieback and related foliicolous, stroma-forming species. Mycology 2014, 5, 228–290. [Google Scholar] [CrossRef] [PubMed]
- McKinney, L.V.; Thomsen, I.M.; Kjær, E.D.; Bengtsson, S.B.K.; Nielsen, L.R. Rapid invasion by an aggressive pathogenic fungus (Hymenoscyphus pseudoalbidus) replaces a native decomposer (Hymenoscyphus albidus): A case of local cryptic extinction? Fungal Ecol. 2012, 5, 663–669. [Google Scholar] [CrossRef]
- Husson, C.; Scala, B.; Caël, O.; Frey, P.; Feau, N.; Ioos, R. Chalara fraxinea is an invasive pathogen in France. Eur. J. Plant Pathol. 2011, 130, 311–324. [Google Scholar] [CrossRef]
- Hirst, J.M. An automatic volumetric spore-trap. Ann. App. Biol. 1952, 36, 257–265. [Google Scholar] [CrossRef]
- Perkins, W.A. The Rotorod Sampler; 2nd Semiannual Report; Aerosol Laboratory Department of Chemistry and Chemical Engineering, Stanford University; CML: Stanford, CA, USA, 1957; Volume 66, p. 186. [Google Scholar]
- McCartney, H.A.; Fitt, B.D.L.; Schmechel, D. Sampling bioaerosols in plant pathology. J. Aerosol Sci. 1997, 28, 349–364. [Google Scholar] [CrossRef]
- Dhingra, O.; Sinclair, J.B. Basic Plant Pathology Methods; CRC Press, Inc.: Boca Raton, FL, USA, 1985; p. 376. [Google Scholar]
- Jankovsky, L.; Holdenrieder, O. Chalara fraxinea—Ash Dieback in the Czech Republic. Plant Prot. Sci. 2009, 45, 74–78. [Google Scholar]
- Hanackova, Z.; Koukol, O.; Havrdova, L.; Gross, A.; Cleary, M. Local population structure of Hymenoscyphus fraxineus surveyed by an enlarged set of microsatellite markers. For. Pathol. 2015. [Google Scholar] [CrossRef]
- Hospodsky, D.; Yamamoto, N.; Peccia, J. Accuracy, precision, and method detection limits of quantitative PCR for airborne bacteria and fungi. Appl. Environ. Microbiol. 2010, 76, 7004–7012. [Google Scholar] [CrossRef] [PubMed]
- Vainio, E.J.; Korhonen, K.; Hantula, J. Genetic variation in Phlebiopsis gigantea as detected with random amplified microsatellite (RAMS) markers. Mycol. Res. 1998, 102, 187–192. [Google Scholar] [CrossRef]
- Chandelier, A.; André, F.; Laurent, F. Detection of Chalara fraxinea in common ash (Fraxinus excelsior) using real time PCR. For. Pathol. 2010, 40, 87–95. [Google Scholar] [CrossRef]
- Sadys, M.; Skjøth, C.; Kennedy, R. Back-trajectories show export of airborne fungal spores (Ganoderma sp.) from forests to agricultural and urban areas in England. Atmos. Environ. 2014, 84, 88–99. [Google Scholar] [CrossRef]
- Noll, K.E. A rotary inertial impactor for sampling giant particles in the atmosphere. Atmos. Environ. 1970, 4, 9–19. [Google Scholar] [CrossRef]
- Lacey, M.E.; West, J.S. The Air Spora; Springer: Dordrecht, The Netherlands, 2006. [Google Scholar]
- Dvorak, M.; Jankovsky, L.; Drapela, K. Dothistroma septosporum: Spore production and weather conditions. For. Syst. 2012, 21, 323–328. [Google Scholar] [CrossRef]
- Gross, A.; Holdenrieder, O. On the longevity of Hymenoscyphus pseudoalbidus in petioles of Fraxinus excelsior. For. Pathol. 2013, 43, 168–170. [Google Scholar] [CrossRef]
- Brook, P.J. Effects of light, temperature, and moisture on release of ascospores by Venturia inaequalis (Cke.) Wint. N. Z. J. Agric. Res. 1969, 12, 214–227. [Google Scholar] [CrossRef]
- Villalta, O.; Washington, W.S.; Kita, N.; Bardon, D. The use of weather and ascospore data for forecasting apple and pear scab in Victoria, Australia. Australas. Plant Pathol. 2002, 31, 205–215. [Google Scholar] [CrossRef]
- McCartney, H.A.; Lacey, M.E. The production and release of ascospores of Pyrenopeziza brassicae on oilseed rape. Plant Pathol. 2007, 39, 17–32. [Google Scholar] [CrossRef]
- Villalta, O.; Washington, W.S.; Rimmington, G.M.; Taylor, P.A. Effects of temperature and leaf wetness duration on infection of pear leaves by Venturia pirina. Crop Pasture Sci. 1999, 51, 97–106. [Google Scholar] [CrossRef]
- Mathieu, D. Effects of temperature and leaf wetness duration on the infection of celery by Septoria apiicola. Phytopathology 1993, 83, 1036–1040. [Google Scholar] [CrossRef]
- Mooney, H.A.; Cleland, E.E. The evolutionary impact of invasive species. Proc. Natl. Acad. Sci. USA 2001, 98, 5446–5451. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, S.V.; Palumbi, S.R. Testing the utility of internally transcribed spacer sequences in coral phylogenetics. Mol. Ecol. 2004, 13, 2763–2772. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, S.B.K.; Vasaitis, R.; Kirisits, T.; Solheim, H.; Stenlid, J. Population structure of Hymenoscyphus pseudoalbidus and its genetic relationship to Hymenoscyphus albidus. Fungal Ecol. 2012, 5, 147–153. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dvorak, M.; Rotkova, G.; Botella, L. Detection of Airborne Inoculum of Hymenoscyphus fraxineus and H. albidus during Seasonal Fluctuations Associated with Absence of Apothecia. Forests 2016, 7, 1. https://doi.org/10.3390/f7010001
Dvorak M, Rotkova G, Botella L. Detection of Airborne Inoculum of Hymenoscyphus fraxineus and H. albidus during Seasonal Fluctuations Associated with Absence of Apothecia. Forests. 2016; 7(1):1. https://doi.org/10.3390/f7010001
Chicago/Turabian StyleDvorak, Milon, Gabriela Rotkova, and Leticia Botella. 2016. "Detection of Airborne Inoculum of Hymenoscyphus fraxineus and H. albidus during Seasonal Fluctuations Associated with Absence of Apothecia" Forests 7, no. 1: 1. https://doi.org/10.3390/f7010001





