Nitrogen Nutrition of Trees in Temperate Forests—The Significance of Nitrogen Availability in the Pedosphere and Atmosphere
Abstract
:1. Introduction
2. Distribution and Fluxes of Nitrogen in Marginal Soil
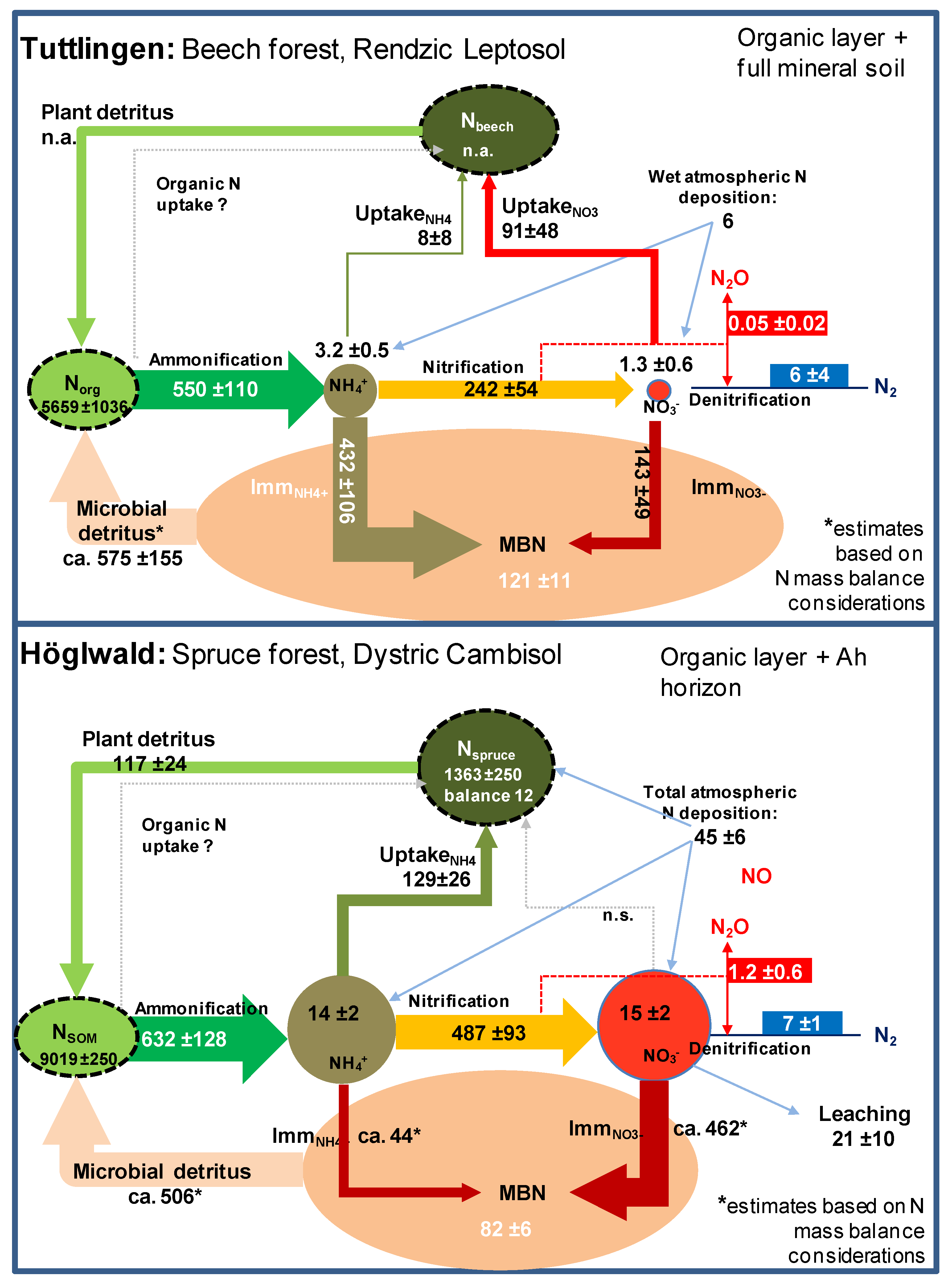
3. Distribution and Fluxes of Nitrogen in Forests Exposed to High Nitrogen Loads
4. Regulation of N Acquisition and Distribution in Trees
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rosegrant, M.W.; Ringler, C.; Zhu, T. Water for agriculture: Maintaining food security under growing scarcity. Annu. Rev. Environ. Resour. 2009, 34, 205–222. [Google Scholar] [CrossRef]
- Strzepek, K.; Boehlert, B. Competition for water for the food system. Philos. Trans. R. Soc. B 2010, 365, 2927–2940. [Google Scholar] [CrossRef] [PubMed]
- Mekonnen, M.M.; Hoekstra, A.Y. Water footprint benchmarks for crop production: A first global assessment. Ecol. Indic. 2014, 46, 214–223. [Google Scholar] [CrossRef]
- De Vries, W.; Kros, J.; Kroeze, C.; Seitzinger, S.P. Assessing planetary and regional nitrogen boundaries related to food security and adverse environmental impacts. Curr. Opin. Environ. Sustain. 2013, 5, 392–402. [Google Scholar] [CrossRef]
- Wang, Y.P.; Houlton, B.Z. Nitrogen constraints on terrestrial carbon uptake: Implications for the global carbon-climate feedback. Geophys. Res. Lett. 2009, 36, L24403. [Google Scholar] [CrossRef]
- Zaehle, S. Terrestrial nitrogen-carbon cycle interactions at the global scale. Philos. Trans. R. Soc. B 2013, 368, 20130125. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Martinez, M.; Vicca, S.; Janssens, I.A.; Sardans, J.; Luyssaert, S.; Cmpioli, M.; Chapin, F.S., III; Ciais, P.; Malhi, Y.; Obersteiner, M.; et al. Nutrient availability as the key regulator of global forest carbon balance. Nat. Clim. Chang. 2014, 4, 471–476. [Google Scholar] [CrossRef]
- Batjes, N.H. Total carbon and nitrogen in the soils of the world. Eur. J. Soil Sci. 1996, 47, 151–163. [Google Scholar] [CrossRef]
- IPCC. Climate change 2007: The physical science basis. In Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M.M.H.L., Eds.; Cambridge University Press: Cambridge, United Kingdom, 2007. [Google Scholar]
- IPCC. Climate Change 2013: The Physical Science Basis. In Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, United Kingdom and New York, NY, USA, 2013; p. 1535. [Google Scholar]
- Rennenberg, H.; Seiler, W.; Matyssek, R.; Gessler, A.; Kreuzwieser, J. Die Buche (Fagus sylvatica L.)—Ein Waldbaum ohne Zukunft im südlichen Mitteleuropa? Allg. Forst- und Jagdzeitschrift 2004, 175, 210–224. [Google Scholar]
- Rennenberg, H.; Dannenmann, M.; Gessler, A.; Kreuzwieser, J.; Simon, J.; Papen, H. Nitrogen balance in forests: Nutritional limitation of plants under climate change stresses. Plant Biol. 2009, 11, 4–23. [Google Scholar] [CrossRef] [PubMed]
- Gessler, A.; Keitel, C.; Kreuzwieser, L.; Matyssek, R.; Seiler, W.; Rennenberg, H. Potential risks for European beech (Fagus sylvatica L.) in a changing climate. Trees 2007, 21, 1–11. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Howarth, R.W. Nitrogen limitation on landand in the sea: How can it occur? Biogeochemistry 1991, 13, 87–115. [Google Scholar] [CrossRef]
- Oren, R.; Ellsworth, D.S.; Johnsen, K.H.; Philips, N.; Ewers, B.E.; Maier, C.; Schäfer, K.V.; McCarthy, H.; Hendrey, G.; McNulty, S.G.; et al. Soil fertility limits carbon sequestration by forest ecosystems in a CO2-enriched atmosphere. Nature 2001, 411, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Heffer, P.; Prud’homme, M. Fertilizer Outlook 2013–2017; International Fertilizer Industry Association (IFA): Paris, France, 2013; Available online: http://www.fertilizer.org (accessed on 1 May 2015.).
- Galloway, J.N.; Aber, J.D.; Erisman, J.W.; Seitzinger, S.P.; Howarth, R.H.; Cowling, E.B.; Cosby, B.J. The nitrogen cascade. Bioscience 2003, 53, 341–356. [Google Scholar] [CrossRef]
- Dise, N.B.; Rothwell, J.J.; Gauci, V.; van der Salm, C.; de Vries, W. Predicting dissolved inorganic nitrogen leaching in European forests using two independent databases. Sci. Total Environ. 2009, 407, 1798–1808. [Google Scholar] [CrossRef] [PubMed]
- Maynard, D.G.; Pare, D.; Thiffault, E.; Lafleur, B.; Hogg, K.E.; Kishchuk, B. How do natural disturbances and human activities affect soils and tree nutrition and growth in the Canadian boreal forest? Environ. Rev. 2014, 22, 161–178. [Google Scholar] [CrossRef]
- Raven, J.A.; Andrews, M. Evolution of tree nutrition. Tree Physiol. 2010, 30, 1050–1071. [Google Scholar] [CrossRef] [PubMed]
- Rennenberg, H.; Schmidt, S. Perennial lifestyle—An adaptation to nutrient limitation? Tree Physiol. 2010, 30, 1047–1049. [Google Scholar] [CrossRef] [PubMed]
- Ollivier, J.; Töwe, S.; Bannert, A.; Hai, B.; Kastl, E.-M.; Meyer, A.; Su, M.X.; Kleineidam, K.; Schloter, M. Nitrogen turnover in soil and global change. FEMS Microbiol. Ecol. 2011, 78, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Schimel, J.P.; Bennett, J. Nitrogen mineralization: Challenges of a changing paradigm. Ecology 2004, 85, 591–602. [Google Scholar] [CrossRef]
- Dungait, J.A.J.; Hopkins, D.W.; Gregory, A.S.; Whitmore, A.P. Soil organic matter turnover is governed by accessibility not recalcitrance. Glob. Chang. Biol. 2012, 18, 1781–1796. [Google Scholar] [CrossRef]
- Schmidt, M.W.I.; Torn, M.S.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.A.; Kleber, M.; Kögel-Knabner, I.; Lehmann, J.; Manning, D.A.C.; et al. Persistence of soil organic matter as an ecosystem property. Nature 2011, 478, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Kreutzer, K.; Butterbach-Bahl, K.; Rennenberg, H.; Papen, H. The complete nitrogen cycle in a N-saturated spruce forest ecosystem. Plant Biol. 2009, 11, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Butterbach-Bahl, K.; Gundersen, P.; Ambus, P.; Augustin, J.; Beier, C.; Boeckx, P.; Dannenmann, M.; Gimeno, B.S.; Kiese, R.; Kitzler, B.; et al. Nitrogen turnover processes and effects in terrestrial ecosystems. In The European Nitrogen Assessment, 1st ed.; Sutton, M.A., Howard, C.M., Erisman, J.W., et al., Eds.; Cambridge University Press: London, UK, 2011. [Google Scholar]
- Kreutzweiser, D.P.; Hazlett, P.W.; Gunn, J.M. Logging impacts on the biogeochemistry of boreal forest soils and nutrient export to aquatic systems: A review. Environ. Rev. 2008, 16, 157–179. [Google Scholar] [CrossRef]
- Rennenberg, H.; Kreutzer, K.; Papen, H.; Weber, P. Consequences of high loads of nitrogen for spruce (Picea abies L.) and beech (Fagus sylvatica L.) forests. New Phytol. 1998, 139, 71–86. [Google Scholar] [CrossRef]
- Cleveland, C.C.; Townsend, A.R.; Schimel, D.S.; Fisherm, H.; Howarth, R.W.; Hedin, L.O.; Perakis, S.S.; Latty, E.F.; von Fischer, J.C.; Elseroad, A.; et al. Global patterns of terrestrial biological nitrogen (N2) fixation in natural ecosystems. Glob. Biogeochem. Cycles 1999, 13, 623–645. [Google Scholar] [CrossRef]
- Cole, D.W. Soil nutrient supply in natural and managed forests. Plant Soil 1995, 168, 43–53. [Google Scholar] [CrossRef]
- Morford, S.L.; Houlton, B.J.; Dahlgren, R.A. Increased forest ecosystem carbon and nitrogen storage from nitrogen rich bedrock. Nature 2011, 477, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, C.C.; Houlton, B.Z.; Kolby Smith, W.; Marklein, A.R.; Reed, S.C; Parton, W.; del Grosso, S.J.; Running, S.W. Patterns of new versus recycled primary production in the terrestrial biosphere. Proc. Natl. Acad. Sci. U.S.A. 2013, 110, 12733–12737. [Google Scholar] [CrossRef] [PubMed]
- Hackl, E.; Bachmann, G.; Zechmeister-Boltenstern, S. Microbial nitrogen turnover in soils under different types of natural forest. For. Ecol. Manag. 2004, 188, 101–112. [Google Scholar] [CrossRef]
- Dannenmann, M.; Gasche, R.; Ledebuhr, A.; Papen, H. Effects of forest management on soil N cycling in beech forests stocking on calcareous soils. Plant Soil 2006, 287, 279–300. [Google Scholar] [CrossRef]
- West, J.B.; Espeleta, J.F.; Donovan, L.A. Fine root production and turnover across a complex edaphic gradient of a Pinus palustris-Aristida stricta savanna ecosystem. For. Ecol. Manag. 2004, 189, 397–406. [Google Scholar] [CrossRef]
- Guo, C.J.; Simon, J.; Gasche, R.; Naumann, P.S.; Bimüller, C.; Pena, R.; Polle, A.; Kögel-Knabner, I.; Zeller, B.; Rennenberg, H.; et al. Minor contribution of leaf litter to N nutrition of beech (Fagus sylvatica) seedlings in a mountainous beech forest of Southern Germany. Plant Soil 2013, 369, 657–668. [Google Scholar] [CrossRef]
- Guo, C.J; Dannenmann, M.; Gasche, R.; Zeller, B.; Papen, H.; Polle, A.; Rennenberg, H.; Simon, J. Preferential use of root litter compared to leaf litter by beech seedlings and soil microorganisms. Plant Soil 2013, 368, 519–534. [Google Scholar] [CrossRef]
- Dannenmann, M.; Butterbach-Bahl, K.; Gasche, R.; Willibald, S.; Papen, H. Dinitrogen emissions 40 and the N2:N2O emission ratio of a Rendzic Leptosol as influenced by pH and forest thinning. Soil Biol. Biochem. 2008, 40, 2317–2323. [Google Scholar] [CrossRef]
- Dannenmann, M.; Gasche, R.; Ledebuhr, A.; Holst, T.; Mayer, H.; Papen, H. The effect of forest management on trace gas exchange at the pedosphere-atmosphere interface in beech (Fagus sylvatica L.) forests stocking on calcareous soils. Eur. J. For. Res. 2007, 126, 331–346. [Google Scholar] [CrossRef]
- Dannenmann, M.; Gasche, R.; Papen, H. Nitrogen turnover and N2O production in the forest floor of beech stands as influenced by forest management. J. Plant Nutr. Soil Sci. 2007, 170, 134–144. [Google Scholar] [CrossRef]
- Dannenmann, M.; Simon, J.; Gasche, R.; Holst, J.; Pena, R.; Naumann, P.S.; Kögel-Knabner, I.; Knicker, H.; Mayer, H.; Schloter, M.; et al. Tree girdling provides insight on the role of labile carbon in nitrogen partitioning between soil microorganisms and adult European beech. Soil Biol. Biochem. 2009, 41, 1622–1631. [Google Scholar] [CrossRef]
- Simon, J.; Dannenmann, M.; Gasche, R.; Holst, J.; Mayer, H.; Papen, H.; Rennenberg, H. Competition for nitrogen between adult European beech and its offspring is reduced by avoidance strategy. For. Ecol. Manag. 2011, 262, 105–114. [Google Scholar] [CrossRef]
- Rosenkranz, P.; Dannenmann, M.; Brüggemann, N.; Papen, H.; Berger, U.; Zumbusch, E.; Butterbach-Bahl, K. Gross rates of ammonification and nitrification at a nitrogen-saturated spruce (Picea abies (L.) Karst.) stand in Southern Germany. Eur. J. Soil Sci. 2010, 61, 745–758. [Google Scholar] [CrossRef]
- Dannenmann, M.; Bimüller, C.; Gschwendtner, S.; Leberecht, M.; Tejedor, J.; Bilela, S.; Gasche, R.; Hanewinkel, M.; Baltensweiler, A.; Kögel-Knabner, I.; et al. Climate change impairs nitrogen cycling in European beech forests. PLoS ONE 2015. Submitted for publication. [Google Scholar]
- Näsholm, T.; Kielland, K.; Ganeteg, U. Uptake of organic nitrogen by plants. New Phytol. 2009, 182, 31–48. [Google Scholar] [CrossRef] [PubMed]
- Stoelken, G.; Simon, L.; Ehlting, B.; Rennenberg, H. The presence of amino acids affects inorganic N uptake in non-mycorrhizal seedlings of European beech (Fagus sylvatica L.). Tree Physiol. 2010, 30, 1118–1128. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, C.; Fuchslueger, L.; Koranda, M.; Gorfer, M.; Stange, C.F.; Kitzler, B.; Rasche, F.; Strauss, J.; Sessitsch, A.; Zechmeister-Boltenstern, S.; et al. Plants control the seasonal dynamics of microbial N cycling in a beech forest soil by belowground C allocation. Ecology 2011, 92, 1036–1051. [Google Scholar] [CrossRef] [PubMed]
- Pena, R.; Offermann, C.; Simon, J.; Naumann, P.; Gessler, A.; Holst, J.; Dannenmann, M.; Mayer, H.; Kögel-Knabner, I.; Rennenberg, H.; et al. Girdling affects ectomycorrhizal diversity and reveals functional differences of EM community composition in a beech forest. Appl. Environ. Microbiol. 2010, 76, 1831–1841. [Google Scholar] [CrossRef] [PubMed]
- Rennenberg, H.; Gessler, A. Consequences of N deposition to forest ecosystems—Recent results and future research needs. Water Air Soil Pollut. 1999, 116, 47–64. [Google Scholar] [CrossRef]
- Gessler, A.; Rienks, M.; Rennenberg, H. NH3 and NO2 fluxes between beech trees and the atmosphere—Correlation with climatic and physiological parameters. New Phytol. 2000, 147, 539–560. [Google Scholar] [CrossRef]
- Gessler, A.; Rienks, M.; Rennenberg, H. NH3 and NOx exchange between spruce (Picea abies) trees and the atmosphere. New Phytol. 2002, 156, 179–194. [Google Scholar] [CrossRef]
- Grulke, N.E.; Dobrowolski, W.; Mingus, P.; Fenn, M.E. California black oak response to nitrogen amendment at a high nitrogen-saturated site. Environ. Pollut. 2005, 137, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Herschbach, C.; Gessler, A.; Rennenberg, H. Long-distance transport and plant internal cycling of N- and S-compounds. Prog. Bot. 2012, 73, 161–188. [Google Scholar]
- Ding, W.J.; Wang, R.Q.; Yuan, Y.F.; Liang, X.Q.; Liu, J. Effects of nitrogen deposition on growth and relationship of Robinia pseudoacacia and Quercus acutissima seedlings. Dendrobiology 2012, 67, 3–13. [Google Scholar]
- Ochoa-Huesco, R.; Manrique, E. Impacts of altered precipitation, nitrogen deposition and plant competition on a Mediterranian seed bank. J. Veg. Sci. 2014, 25, 1289–1298. [Google Scholar] [CrossRef]
- Muller, B.; Touraine, B.; Rennenberg, H. Interaction between atmospheric and pedospheric nitrogen nutrition in spruce seedlings. Plant Cell Environ. 1996, 19, 345–355. [Google Scholar] [CrossRef]
- Rennenberg, H.; Herschbach, C.; Polle, A. Consequences of air pollution on shoot-root interactions. J. Plant Physiol. 1996, 148, 296–301. [Google Scholar] [CrossRef]
- Gessler, A.; Schneider, S.; Weber, P.; Hanemann, U.; Rennenberg, H. Soluble N compounds in trees exposed to high loads of N: A comparison between the roots of Norway spruce (Picea abies (L.) Karst) and beech (Fagus sylvatica) trees grown under field conditions. New Phytol. 1998, 138, 385–399. [Google Scholar] [CrossRef]
- Aber, J.D.; Goodale, C.L.; Ollinger, S.V.; Smith, M.L.; Magill, A.H.; Martin, M.E.; Hallett, R.A.; Stoddard, J.L. Is nitrogen deposition altering the nitrogen status of northeastern forests? BioScience 2003, 53, 375–389. [Google Scholar] [CrossRef]
- Aber, J.D.; McDowell, W.; Nadelhoffer, K.; Magill, A.; Berntson, G.; Kamakea, M.; McNulty, S.; Currie, W.; Rustad, L.; Fernandez, I. Nitrogen saturation in temperate forest ecosystems: Hypothesis revisited. BioScience 1998, 48, 921–934. [Google Scholar] [CrossRef]
- Aber, J.D.; Nadelhoffer, K.; Steudler, P.; Melillo, J.M. Nitrogen saturation in northern forest ecosystems. BioScience 1989, 39, 378–386. [Google Scholar] [CrossRef]
- Agren, G.I.; Bosatta, E. Nitrogen saturation of terrestrial ecosystems. Environ. Pollut. 1988, 54, 185–198. [Google Scholar] [CrossRef]
- Gao, W.; Yang, H.; Kou, L.; Li, S. Effects of nitrogen deposition and fertilization on N transformations in forest soils: A review. J. Soils Sediments 2015, 15, 863–879. [Google Scholar] [CrossRef]
- Corre, M.D.; Brumme, R.; Veldkamp, E.; Beese, F.O. Changes in nitrogen cycling and retention processes in soils under spruce forests along a nitrogen enrichment gradient in Germany. Glob. Chang. Biol. 2007, 13, 1509–1527. [Google Scholar] [CrossRef]
- Butterbach-Bahl, K.; Dannenmann, M. Soil carbon and nitrogen interactions and biosphere-atmosphere exchange of methane and nitrous oxide. In Recarbonization of the Biosphere–Ecosystems and the Global Carbon Cycle, 1st ed.; Lal, R., Lorenz, K., Hüttl, R.F., Schneider, B.U., von Braun, J., Eds.; Springer: Dordrecht, the Netherlands; Heidelberg, Germany; NewYork, USA; London, UK, 2012; pp. 429–443. [Google Scholar]
- Frey, S.D.; Ollinger, S.; Nadelhoffer, K.; Bowden, R.; Brzostek, E.; Burton, A.; Caldwell, B.A.; Crow, S.; Goodale, C.L.; Grandy, A.S.; et al. Chronic nitrogen additions suppress decomposition and sequester soil carbon in temperate forests. Biogeochemistry 2014, 121, 305–316. [Google Scholar] [CrossRef]
- Gessler, A.; Weber, P.; Schneider, S.; Rennenberg, H. Bidirectional exchange of amino compounds between phloem and xylem during long distance transport in Norway spruce trees (Picea abies [L.] Karst). J. Exp. Bot. 2003, 54, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Rennenberg, H.; Wildhagen, H.; Ehlting, B. Nitrogen nutrition of poplar trees. Plant Biol. 2010, 12, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Wildhagen, H.; Bilela, S.; Rennenberg, H. Low temperatures counteract short-day induced nitrogen storage, but not accumulation of bark storage protein transcripts in bark of Gray poplar (Populus × canescens). Plant Biol. 2013, 15 (Suppl. 1), 44–56. [Google Scholar] [CrossRef] [PubMed]
- Wildhagen, H.; Dürr, J.; Ehlting, B.; Rennenberg, H. Seasonal nitrogen cycling in the bark is correlated with gene expression and meteorological factors in grey poplar plants. Tree Physiol. 2010, 30, 1096–1110. [Google Scholar] [CrossRef] [PubMed]
- Gessler, A.; Schulte, M.; Schrempp, S.; Rennenberg, H. Interaction of phloem-translocated amino compounds with nitrate net uptake by the roots of beech (Fagus sylvatica) seedlings. J. Exp. Bot. 1998, 4, 1529–1537. [Google Scholar] [CrossRef]
- Collier, M.; Fotelli, M.; Nahm, M.; Kopriva, S.; Rennenberg, H.; Hanke, D.; Gessler, A. Regulation of nitrogen uptake by Fagus sylvatica on a whole plant level—Interactions between cytokinins and soluble N compounds. Plant Cell Environ. 2003, 26, 1549–1560. [Google Scholar] [CrossRef]
- Dluzniewska, P.; Gessler, A.; Kopriva, S.; Strnad, M.; Novak, O.; Dietrich, H.; Rennenberg, H. Exogenous supply of glutamine and active cytokinin to the roots reduces NO3—Uptake rates in poplar. Plant Cell Environ. 2006, 29, 1284–1297. [Google Scholar] [CrossRef] [PubMed]
- Ruffel, S.; Gojon, A.; Lejay, L. Signal interactions in the regulation of root nitrate uptake. J. Exp. Bot. 2014, 65, 5509–5517. [Google Scholar] [CrossRef] [PubMed]
- Ruffel, S.; Krouk, G.; Ristova, D.; Shasha, D.; Birnbaum, K.D.; Coruzzi, G.M. Nitrogen economics of root foraging: Transitive closure of the nitrate-cytokinin relay and distinct systemic signalling for N supply vs. demand. Proc. Natl. Acad. Sci. USA 2011, 108, 18524–18529. [Google Scholar] [CrossRef] [PubMed]
- Forde, B.G. Local and long-range signaling pathways regulating plant responses to nitrate. Annu. Rev. Plant Biol. 2002, 53, 203–224. [Google Scholar] [CrossRef] [PubMed]
- Chellamuthu, V.-R.; Ermilova, E.; Lapina, T.; Lüddecke, J.; Minaeva, E.; Herrmann, C.; Hartmann, M.-D.; Forchhammer, K. A widespread glutamine sensing mechanism in the plant kingdom. Cell 2014, 159, 1188–1199. [Google Scholar] [CrossRef] [PubMed]
- Rubin, G.; Tohge, T.; Matsuda, F.; Saito, K.; Scheible, W.R. Members of the LBD family of transcription factors repress anthocyanin synthesis and affect additional nitrogen responses in Arabidopsis. Plant Cell 2009, 21, 3567–3584. [Google Scholar] [CrossRef] [PubMed]
- Widiez, T.; El Kafafi, E.S.; Girin, T.; Berr, A.; Ruffel, S.; Krouk, G.; Vayssieres, A.; Shen, W.-H.; Coruzzi, G.M.; Gojon, A. High nitrogen insensitive 9(NHI9)-mediated systemic repression of root NO3- uptake is associated with changes in histone methylation. Proc. Natl. Acad. Sci. USA 2011, 108, 13329–13334. [Google Scholar] [CrossRef] [PubMed]
- Gessler, A.; Kopriva, S.; Rennenberg, H. Regulation of nitrate uptake of trees at the whole plant level: Interaction between nitrogen compounds, cytokinins and carbon metabolism. Tree Physiol. 2004, 24, 1313–1321. [Google Scholar] [PubMed]
- Camanes, G.; Cerezo, M.; Primo-Millo, E.; Gojon, A.; Garcia-Agustin, P. Ammonium transport and CitAMT1 expression are regulated by N in Citrus plants. Planta 2009, 229, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Rütting, T.; Boeckx, P.; Müller, C.; Klemedtsson, L. Assessment of the importance of dissimilatory nitrate reduction to ammonium for the terrestrial nitrogen cycle. Biogeosciences 2011, 8, 1779–1791. [Google Scholar] [CrossRef]
- Simon, J.; Waldhecker, P.; Brüggemann, N.; Rennenberg, H. Competition for nitrogen sources between European beech (Fagus sylvatica) and sycamore maple (Acer pseudoplatanus) seedlings. Plant Biol. 2010, 12, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.; Dong, F.; Buegger, H.; Rennenberg, H. Rhizospheric NO affects N uptake and metabolism in Scots pine (Pinus sylvestris L.) seedlings depending on soil N availability and N sources. Plant Cell Environ. 2013, 36, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- Medinets, S.; Skiba, U.; Rennenberg, H.; Butterbach-Bahl, K. A review of soil NO transformation: Associated processes and possible physiological significance on organisms. Soil Biol. Biochem. 2015, 80, 92–117. [Google Scholar] [CrossRef]
- Simon, J.; Stoelken, G.; Rienks, M.; Rennenberg. Rhizospheric NO interacts with the acquisition of reduced nitrogen sources by the roots of European beech (Fagus sylvatica). FEBS Lett. 2009, 583, 2907–2910. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Simon, J.; Rienks, M.; Rennenberg, H. Effects of rhizospheric nitric oxide (NO) on N uptake depend on soil CO2 concentration, soil N availability, and N source. Tree Physiol. 2015, in press. [Google Scholar] [CrossRef] [PubMed]
- Leitner, M.; Vandelle, E.; Gaupells, F.; Bellin, D.; Delledonne, M. NO signals in the haze: Nitric oxide signaling in plant defense. Curr. Opin. Plant Biol. 2009, 12, 451–458. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rennenberg, H.; Dannenmann, M. Nitrogen Nutrition of Trees in Temperate Forests—The Significance of Nitrogen Availability in the Pedosphere and Atmosphere. Forests 2015, 6, 2820-2835. https://doi.org/10.3390/f6082820
Rennenberg H, Dannenmann M. Nitrogen Nutrition of Trees in Temperate Forests—The Significance of Nitrogen Availability in the Pedosphere and Atmosphere. Forests. 2015; 6(8):2820-2835. https://doi.org/10.3390/f6082820
Chicago/Turabian StyleRennenberg, Heinz, and Michael Dannenmann. 2015. "Nitrogen Nutrition of Trees in Temperate Forests—The Significance of Nitrogen Availability in the Pedosphere and Atmosphere" Forests 6, no. 8: 2820-2835. https://doi.org/10.3390/f6082820
APA StyleRennenberg, H., & Dannenmann, M. (2015). Nitrogen Nutrition of Trees in Temperate Forests—The Significance of Nitrogen Availability in the Pedosphere and Atmosphere. Forests, 6(8), 2820-2835. https://doi.org/10.3390/f6082820





