Colonization with Arbuscular Mycorrhizal Fungi Promotes the Growth of Morus alba L. Seedlings under Greenhouse Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Plant Measurements, Nutrient Analysis and Mycorrhizal Colonization
2.3. Chlorophyll Content and Root Activity
2.4. Statistics
3. Results
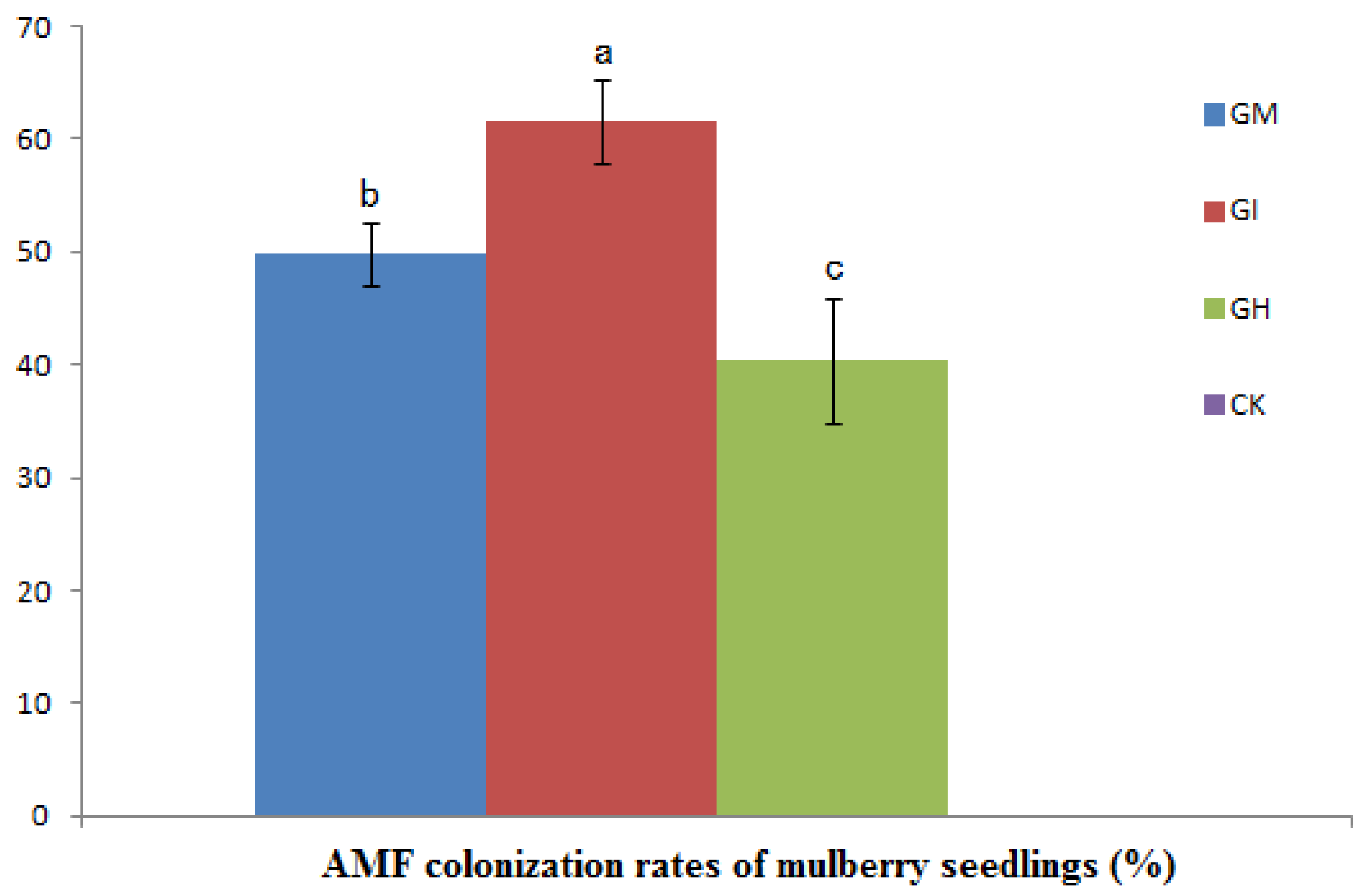
3.1. The AMF Colonization Rate
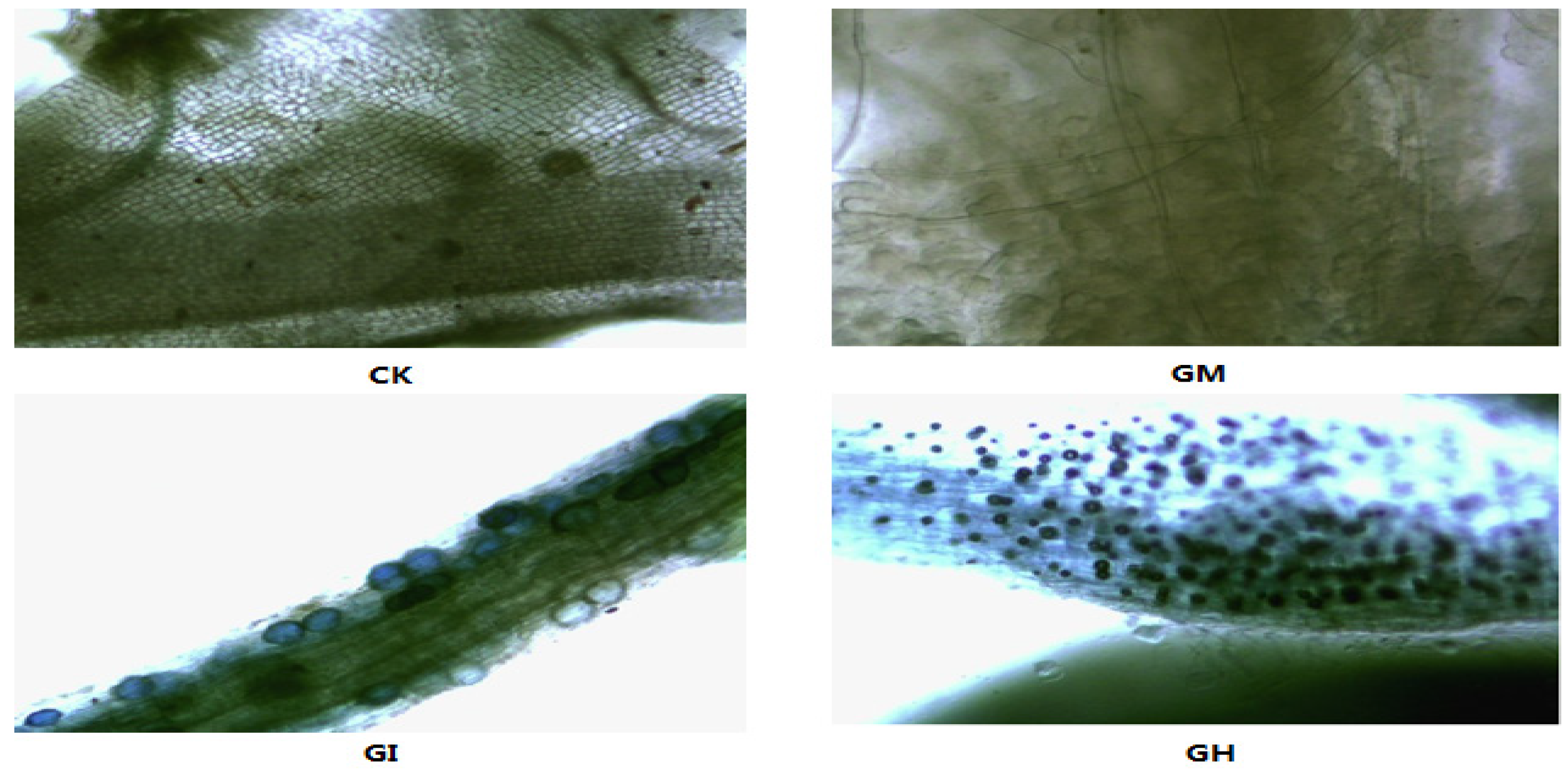
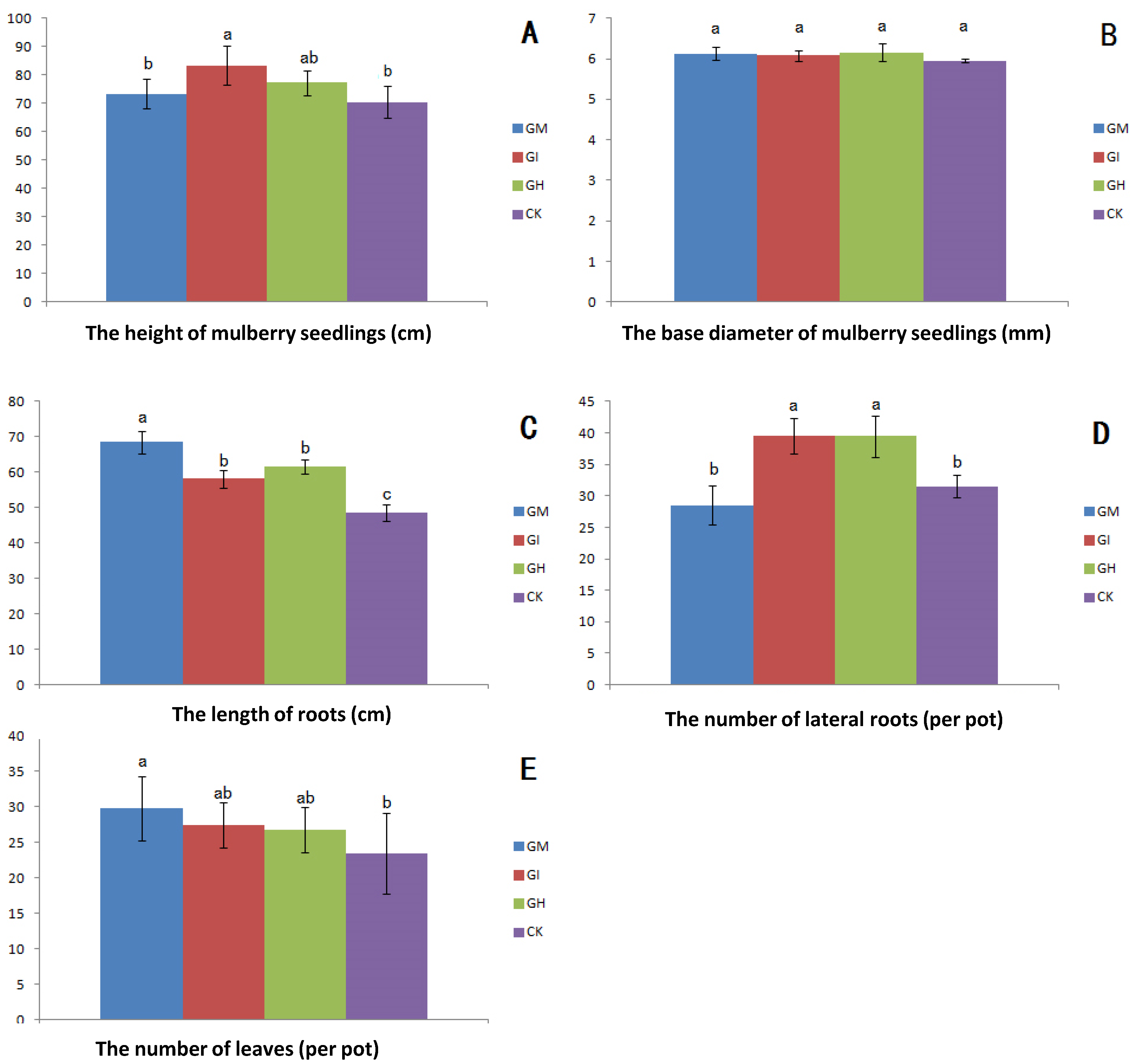
3.2. Plant Growth, Biomass and Mycorrhizal Dependence
| Treatments | Fresh Weight (g/pot) | Total Fresh Weight (g/pot) | Dry Weight (g/pot) | Total Dry Weight (g/pot) | ||
|---|---|---|---|---|---|---|
| Aboveground Plant Parts | Belowground Plant Parts | Aboveground Plant Parts | Belowground Plant Parts | |||
| GM | 76.10 ± 5.33 ab | 69.00 ± 7.33 ab | 145.10 ± 12.66 ab | 38.45 ± 2.76 ab | 34.43 ± 1.95 ab | 72.88 ± 4.71 ab |
| GI | 96.91 ± 8.52 a | 78.42 ± 8.46 a | 175.33 ± 16.98 a | 46.29 ± 4.12 a | 39.39 ± 3.54 a | 85.67 ± 7.66 a |
| GH | 84.61 ± 7.83 ab | 69.88 ± 6.43 ab | 154.49 ± 14.26 ab | 37.99 ± 3.55 ab | 37.39 ± 2.87 a | 75.38 ± 6.42 ab |
| CK | 67.83 ± 5.52 b | 60.28 ± 6.65 b | 128.11 ± 12.17 b | 31.74 ± 3.43 b | 29.43 ± 2.10 b | 61.18 ± 5.53 b |

| Treatment | Dry Biomass (g/pot) | Dry Biomass of Control (g/pot) | MD (%) |
|---|---|---|---|
| GM | 72.88 ± 4.71 b | 61.18 | 119.12 ± 7.70 b |
| GI | 85.67 ± 7.66 a | 140.03 ± 12.52 a | |
| GH | 75.38 ± 6.42 b | 123.21 ± 10.49 b |
3.3. Chlorophyll Content

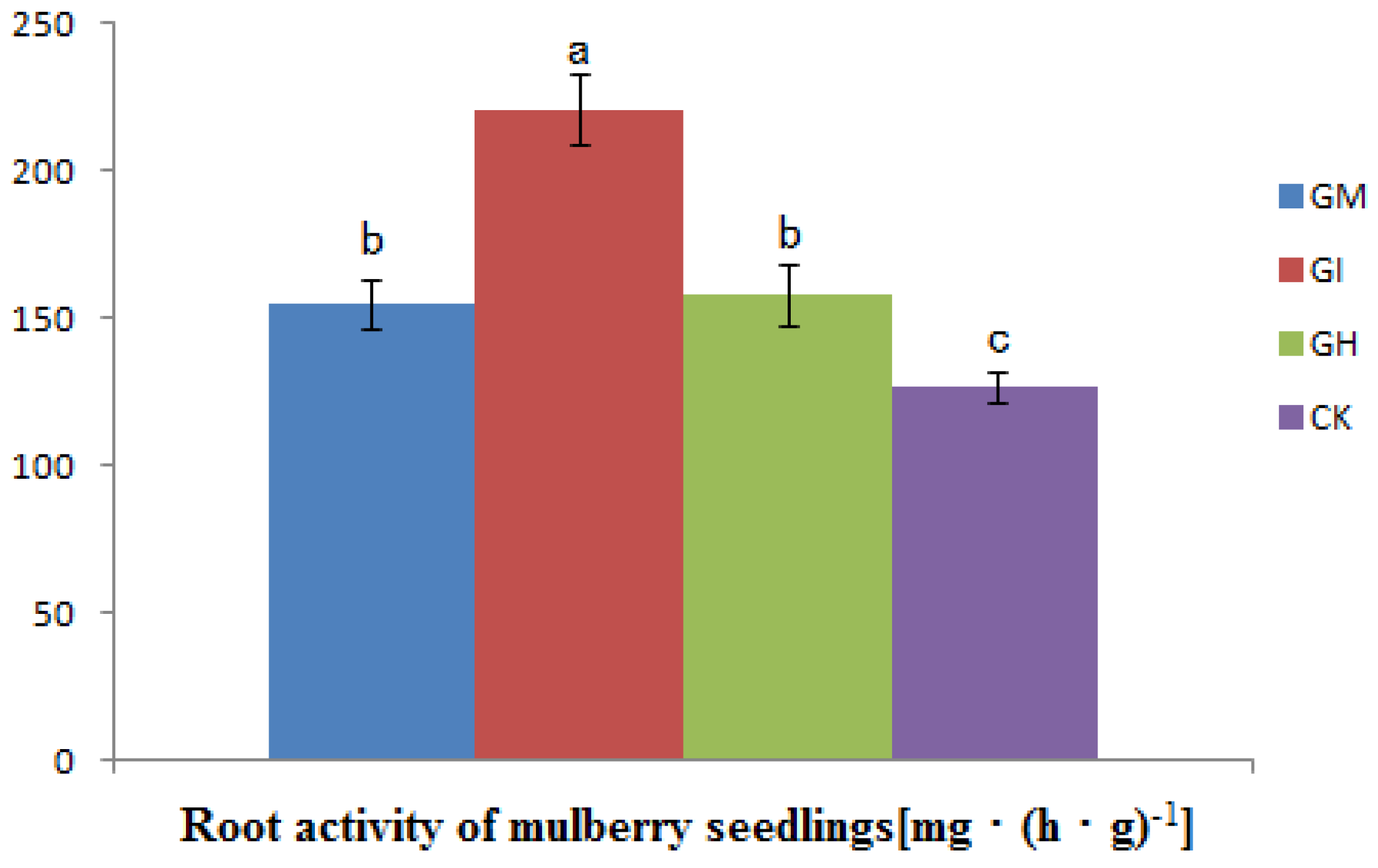
3.4. The Influence on Root Activity
3.5. Nutritional Content
| Treatment | Nitrogen | Phosphorus | Potassium | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Leaf | Stem | Root | Leaf | Stem | Root | Leaf | Stem | Root | |
| GM | 183.1 ± 20.6 b | 218.4 ± 11.9 b | 254.6 ± 1.8 ab | 18.5 ± 2.1 a | 49.9 ± 2.7 ab | 63.8 ± 3.2 ab | 160.9 ± 18.1 b | 248.2 ± 13.5 b | 346.2 ± 5.2 a |
| GI | 223.8 ± 7.4 ab | 328.4 ± 28.3 a | 354.0 ± 91.1 a | 25.1 ± 0.8 a | 59.2 ± 5.1 a | 72.9 ± 9.0 a | 244.9 ± 8.1 a | 312.3 ± 26.9 a | 359.2 ± 19.6 a |
| GH | 275.3 ± 54.9 a | 220.0 ± 20.2 b | 262.0 ± 15.8 ab | 24.5 ± 4.9 a | 47.1 ± 4.3 b | 69.0 ± 6.0 a | 197.6 ± 39.4 ab | 245.8 ± 22.6 b | 366.3 ± 8.8 a |
| CK | 168.4 ± 54.9 b | 178.2 ± 29.4 b | 197.6 ± 6.0 b | 18.9 ± 6.2 a | 41.9 ± 6.9 b | 54.7 ± 1.8 b | 138.3 ± 45.1 b | 203.7 ± 33.6 b | 274.6 ± 27.7 b |
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Academic Press: London, UK, 1996. [Google Scholar]
- Gianinazzi, S.; Gollotte, A.; Binet, M.; van Tuinen, D.; Redecker, D.; Wipf, D. Agroecology: The key role of arbuscular mycorrhizas in ecosystem services. Mycorrhiza 2010, 20, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Smith, F.A. Roles of Arbuscular Mycorrhizas in Plant Nutrition and Growth: New Paradigms from Cellular to Ecosystem Scales. Ann. Rev. Plant Biol. 2011, 62, 227–250. [Google Scholar] [CrossRef]
- Jones, M.D.; Smith, S.E. Exploring functional definitions of mycorrhizas: Are mycorrhizas always mutualisms? Can. J. Bot. 2004, 82, 1089–1110. [Google Scholar] [CrossRef]
- Smith, F.A.; Smith, S.E. Mutualism and parasitism: Diversity in function and structure in the “arbuscular” (VA) mycorrhizal symbiosis. Adv. Bot. Res. 1996, 22, 1–43. [Google Scholar]
- Van der Heijden, M.G.; Klironomos, J.N.; Ursic, M.; Moutoglis, P.; Streitwolf-Engel, R.; Boller, T.; Wiemken, A.; Sanders, I.R. Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature 1998, 396, 69–72. [Google Scholar] [CrossRef]
- Maherali, H.; Klironomos, J.N. Influence of Phylogeny on Fungal Community Assembly and Ecosystem Functioning. Science 2007, 316, 1746–1748. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Huang, K.; Huang, A.; Ho, Y.; Wang, C. Hibiscus anthocyanins-rich extract inhibited LDL oxidation and oxLDL-mediated macrophages apoptosis. Food Chem. Toxicol. 2006, 44, 1015–1023. [Google Scholar] [CrossRef] [PubMed]
- Butt, M.S.; Nazir, A.; Sultan, M.T.; Schroën, K. Morus alba L. Nature’s functional tonic. Trends Food Sci. Technol. 2008, 19, 505–512. [Google Scholar] [CrossRef]
- Katiyar, R.S.; Das, P.K.; Choudhury, P.C.; Ghosh, A.; Singh, G.B.; Datta, R.K. Response of irrigated mulberry (Morus alba L.) to VA-mycorrhizal inoculation under graded doses of phosphorus. Plant Soil. 1995, 170, 331–337. [Google Scholar] [CrossRef]
- Mamatha, G.; Bagyaraj, D.; Jaganath, S. Inoculation of field-established mulberry and papaya with arbuscular mycorrhizal fungi and a mycorrhiza helper bacterium. Mycorrhiza 2002, 12, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Gerdemann, J.W.; Trappe, J.M. Endogonaceae in the Pacific Northwest. Mycologia Mem 1974, 5, 1–76. [Google Scholar]
- Biermann, B.; Linderman, R.G. Quantifying vesicular-Arbuscular mycorrhizae: A proposed method towards standardization. New Phytol. 1981, 87, 63–67. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 118–158. [Google Scholar] [CrossRef]
- Bradstreet, R.B. The Kjeldahl Method for Organic Nitrogen; Academic Press Inc: New York, NY, USA, 1965. [Google Scholar]
- Lu, R.K. Soil Analytical Methods of Agronomic Chemica; China Agricultural Science and Technology Press: Beijing, China, 2000. [Google Scholar]
- Page, A.L. Methods of soil analysis. Part 2. In Chemical and Microbiological Properties; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1982. [Google Scholar]
- Inskeep, W.P.; Bloom, P.R. Extinction coefficients of chlorophyll a and b in N,N-dimethylformamide and 80% acetone. Plant Physiol. 1985, 77, 483–485. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.X.; Ma, Y.Z.; Xiao, J.P.; Zhang, Z.H.; Shi, Z.H. Photosynthesis and root characteristics of rice (Oryza sativa L.) in floating culture. Photosynthetica 2013, 51, 231–237. [Google Scholar] [CrossRef]
- Bennett, A.E.; Bever, J.D. Trade-offs between arbuscular mycorrhizal fungal competitive ability and host growth promotion in Plantago lanceolata. Oecologia 2009, 160, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Pearson, J.N.; Abbott, L.K.; Jasper, D.A. Phosphorus, soluble carbohydrates and the competition between two arbuscular mycorrhizal fungi colonizing subterranean clover. New Phytol. 1994, 127, 101–106. [Google Scholar] [CrossRef]
- Hepper, C.M.; Azcon-Aguilar, C.; Rosendahl, S.; Sen, R. Competition Between Three Species of Glomus Used as Spatially Separated Introduced and Indigenous Mycorrhizal Inocula for Leek (Allium porrum L.). New Phytol. 1988, 110, 207–215. [Google Scholar] [CrossRef]
- Pearson, J.N.; Abbott, L.K.; Jasper, D.A. Mediation of Competition between Two Colonizing VA Mycorrhizal Fungi by the Host Plant. New Phytol. 1993, 123, 93–98. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.H.; Chaoxing, H. Effect of arbuscular mycorrhizal fungi on growth, mineral nutrition, antioxidant enzymes activity and fruit yield of tomato grown under salinity stress. Sci. Hortic. 2011, 127, 228–233. [Google Scholar] [CrossRef]
- Wu, Q.S.; Xia, R.X. Effects of arbuscular mycorrhizal fungi on plant growth and osmotic adjustment matter content of trifoliate orange seedling under water stress. J. Plant Physiol. Mol. Biol. 2004, 30, 583–588. [Google Scholar]
- Smith, G.S.; Roncadori, R.W. Responses of three vesicular-arbuscular mycorrhizal fungi at four soil temperatures and their effects on cotton growth. New Phytol. 1986, 104, 89–95. [Google Scholar] [CrossRef]
- Shrestha, Y.H.; Ishii, T.; Kadoya, K. Effect of vesicular-arbuscular mycorrhizal fungi on the growth, photosynthesis, transpiration and the distribution of photosynthates of bearing satsuma mandarin (Citrus reticulata) trees. J. Jpn. Soc. Hortic. Sci. 1995, 64, 517–525. [Google Scholar] [CrossRef]
- Artursson, V.; Finlay, R.D.; Jansson, J.K. Interactions between arbuscular mycorrhizal fungi and bacteria and their potential for stimulating plant growth. Environ. Microbiol. 2006, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sheng, M.; Tang, M.; Chen, H.; Yang, B.; Zhang, F.; Huang, Y. Influence of arbuscular mycorrhizae on photosynthesis and water status of maize plants under salt stress. Mycorrhiza 2008, 18, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Al-Karaki, G.N. Growth of mycorrhizal tomato and mineral acquisition under salt stress. Mycorrhiza 2000, 10, 51–54. [Google Scholar] [CrossRef]
- Jia, Y.; Gray, V.M.; Straker, C.J. The influence of Rhizobium and arbuscular mycorrhizal fungi on nitrogen and phosphorus accumulation by Vicia faba. Ann. Bot. 2004, 94, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Menéndez, M.J.; Herrera-Silveira, J.; Comín, F.A. Effect of nitrogen and phosphorus supply on growth, chlorophyll content and tissue composition of the macroalga Chaetomorpha linum (O.F. Müll.) Kütz in a Mediterranean coastal lagoon. Sci. Mar. 2002, 66, 355–364. [Google Scholar] [CrossRef]
- Zhao, D.; Oosterhuis, D.M.; Bednarz, C.W. Influence of potassium deficiency on photosynthesis, chlorophyll content, and chloroplast ultrastructure of cotton plants. Photosynthetica 2001, 39, 103–109. [Google Scholar] [CrossRef]
- Buttery, B.R.; Buzzell, R.I. The relationship between chlorophyll content and rate of photosynthesis in soybeans. Can. J. Plant Sci. 1977, 57, 1–5. [Google Scholar] [CrossRef]
- Murchie, E.H.; Horton, P. Acclimation of photosynthesis to irradiance and spectral quality in British plant species: Chlorophyll content, photosynthetic capacity and habitat preference. Plant Cell Environ. 1997, 20, 438–448. [Google Scholar] [CrossRef]
- Reynolds, H.L.; Hartley, A.E.; Vogelsang, K.M.; Bever, J.D.; Schultz, P.A. Arbuscular mycorrhizal fungi do not enhance nitrogen acquisition and growth of old-field perennials under low nitrogen supply in glasshouse culture. New Phytol. 2005, 167, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Eom, A.H.; Hartnett, D.C.; Wilson, G.W.T. Host plant species effects on arbuscular mycorrhizal fungal communities in tallgrass prairie. Oecologia 2000, 122, 435–444. [Google Scholar] [CrossRef]
- Estaun, V.; Camprubi, A.; Calvet, C.; Pinochet, J. Nursery and Field Response of Olive Trees Inoculated with Two Arbuscular Mycorrhizal Fungi, Glomus intraradices and Glomus mosseae. J. Am. Soc. Hortic. Sci. 2003, 128, 767. [Google Scholar]
- Wu, S.C.; Cao, Z.H.; Li, Z.G.; Cheung, K.C.; Wong, M.H. Effects of biofertilizer containing N-fixer, P and K solubilizers and AM fungi on maize growth: A greenhouse trial. Geoderma 2005, 125, 155–166. [Google Scholar] [CrossRef]
- Berta, G.; Gianinazzi-Pearson, V.; Gianinazzi, S. Arbuscular mycorrhizal induced changes to plant growth and root system morphology in Prunus cerasifera. Tree Physiol. 1995, 15, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Jansa, J.; Smith, F.A.; Smith, S.E. Are there benefits of simultaneous root colonization by different arbuscular mycorrhizal fungi? New Phytol. 2008, 177, 779–789. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, N.; Zhou, X.; Cui, M.; Yu, M.; Zhou, J.; Qin, Y.; Li, Y. Colonization with Arbuscular Mycorrhizal Fungi Promotes the Growth of Morus alba L. Seedlings under Greenhouse Conditions. Forests 2015, 6, 734-747. https://doi.org/10.3390/f6030734
Lu N, Zhou X, Cui M, Yu M, Zhou J, Qin Y, Li Y. Colonization with Arbuscular Mycorrhizal Fungi Promotes the Growth of Morus alba L. Seedlings under Greenhouse Conditions. Forests. 2015; 6(3):734-747. https://doi.org/10.3390/f6030734
Chicago/Turabian StyleLu, Nan, Xia Zhou, Ming Cui, Meng Yu, Jinxing Zhou, Yongsheng Qin, and Yun Li. 2015. "Colonization with Arbuscular Mycorrhizal Fungi Promotes the Growth of Morus alba L. Seedlings under Greenhouse Conditions" Forests 6, no. 3: 734-747. https://doi.org/10.3390/f6030734
APA StyleLu, N., Zhou, X., Cui, M., Yu, M., Zhou, J., Qin, Y., & Li, Y. (2015). Colonization with Arbuscular Mycorrhizal Fungi Promotes the Growth of Morus alba L. Seedlings under Greenhouse Conditions. Forests, 6(3), 734-747. https://doi.org/10.3390/f6030734






