Changes in Whole-Tree Water Use Following Live-Crown Pruning in Young Plantation-Grown Eucalyptus pilularis and Eucalyptus cloeziana
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Treatment Design
2.2. Sap Flow Measurements
2.3. Radial Variation in Sap Velocity
2.4. Sapwood Area, Volume Fractions of Wood and Water and Tree Growth
2.5. Microclimatic Conditions
2.6. Analysis
3. Results
3.1. Tree Characteristics
| E. pilularis | E. cloeziana | |||
|---|---|---|---|---|
| Unpruned | Pruned | Unpruned | Pruned | |
| Before pruning | ||||
| DBH (cm) | 11.0 (0.247) | 10.9 (0.247) | 12.5 (0.190) | 11.8 (0.190) |
| Basal area at 1.3 m (cm2) | 95.0 (4.29) | 92.8 (4.29) | 123.8 (3.70) | 110.3 (3.70) |
| Stem area at 0.12 m (cm2) | 143.7 (6.38) | 147.7 (6.38) | 194.4 (4.39) | 179.0 (4.39) |
| Tree height (m) | 10.2 (0.129) | 10.1 (0.129) | 11.1 (0.337) | 10.8 (0.337) |
| Crown area (m2) | 10.7 (0.463) | 11.7 (0.463) | 14.9 (1.033) | 15.2 (1.033) |
| Live-crown length (m) | 6.7 (0.224) | 6.4 (0.224) | 10.1 (0.351) | 10.1 (0.351) |
| 88 days after pruning | ||||
| DBH at 1.3 m (cm) | 11.6 (0.247) | 11.4 (0.247) | 13.4 (0.220) | 12.6 (0.220) |
| Basal area at 1.3 m (cm2) | 105.7 (4.49) | 102.7 (4.49) | 141.0 (4.55) | 125.4 (4.55) |
| Basal area growth since pruning (cm2) | 10.6 (0.99) | 9.9 (1.07) | 17.1 (1.06) | 15.0 (0.94) |
| Stem area at 0.12 m (cm2) | 153.9 (6.27) | 157.3 (6.27) | 203.8 (5.14) | 184.1 (5.14) |
| Sapwood area 0.12 m (cm2) | 79.6 (3.93) | 78.9 (3.93) | 102.9 (4.17) | 88.0 (4.17) |
| Tree height (m) | 11.0 (0.127) | 11.0 (0.127) | 11.9 (0.434) | 11.7 (0.434) |
| Vw | 0.30 (0.02) | 0.33 (0.02) | 0.38 (0.02) | 0.37 (0.02) |
| Vh | 0.64 (0.02) | 0.61 (0.02) | 0.56 (0.02) | 0.53 (0.02) |
3.2. Pre-Pruning Transpiration
3.3. Radial Variation in Sap Velocity
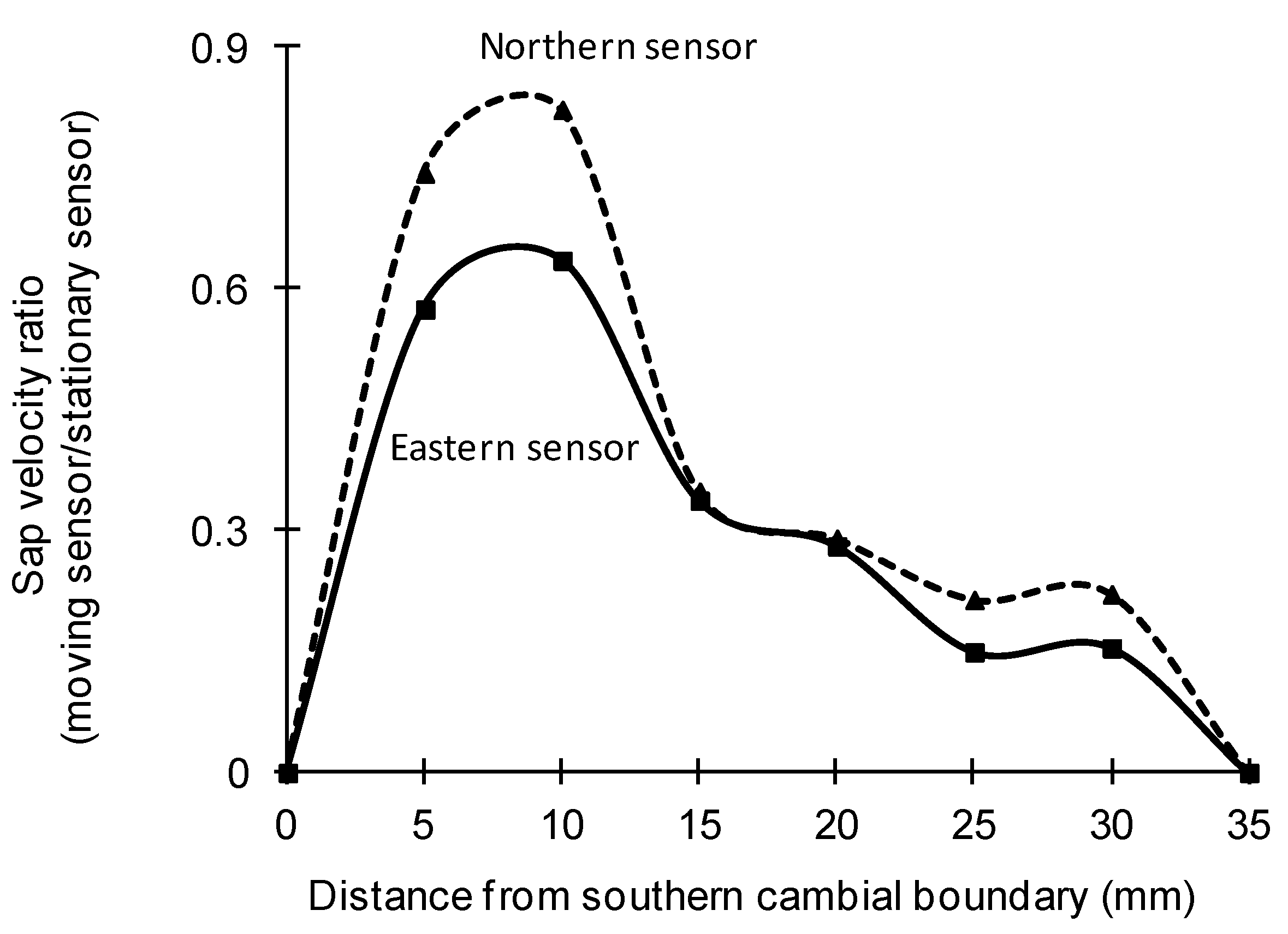
| Species | Treatment | Mean Correction Coefficient | |
| Northern Aspect | Eastern Aspect | ||
| E. pilularis | Unpruned | 0.51 (0.08) | 0.52 (0.09) |
| Pruned | 0.62 (0.08) | 0.65 (0.09) | |
| E. cloeziana | Unpruned | 0.65 (0.25) | 0.48 (0.11) |
| Pruned | 0.57 (0.25) | 0.54 (0.11) | |
3.4. Water Use Patterns of Reference Trees
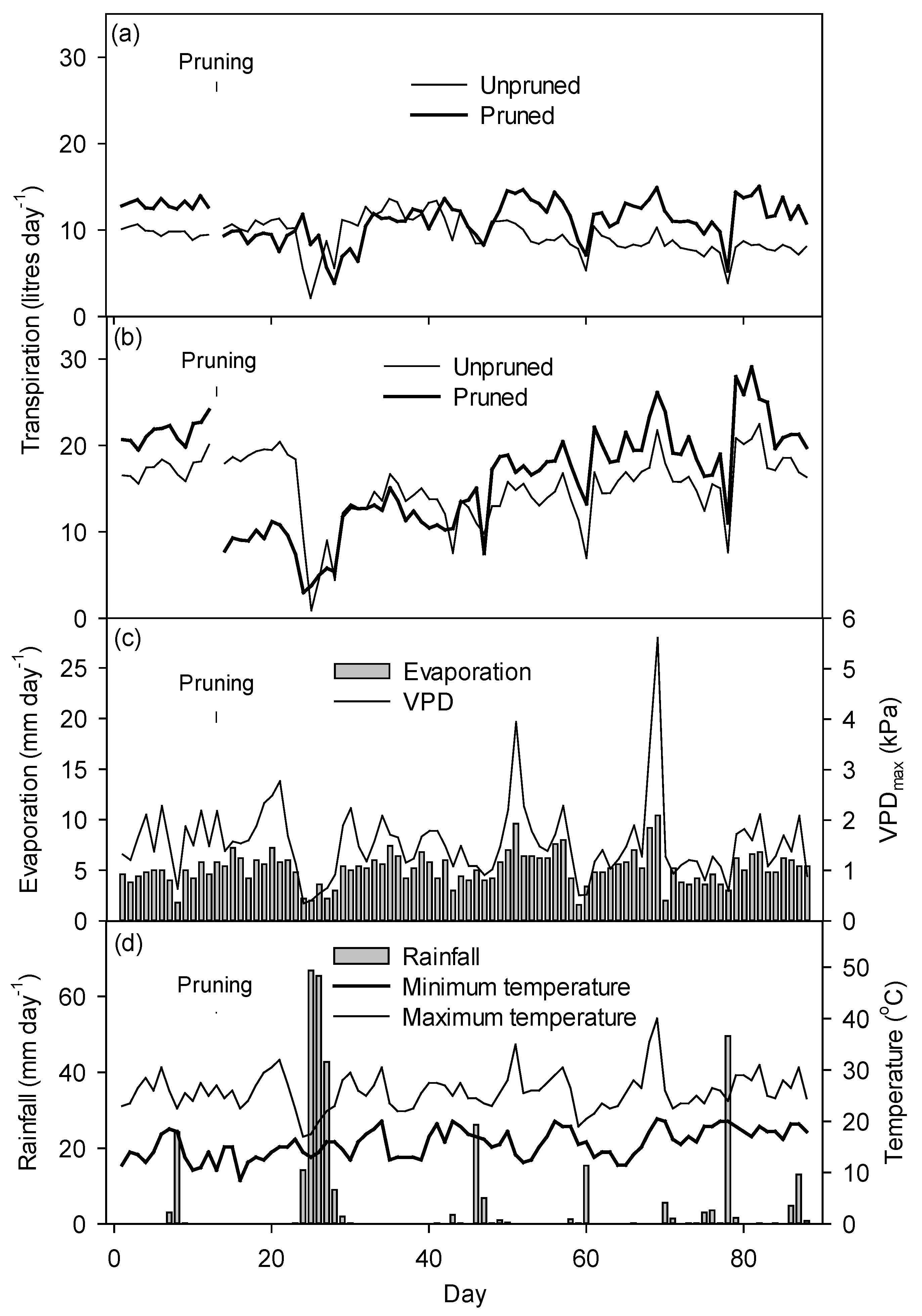
3.5. Mean Daily Transpiration

| E. pilularis | E. cloeziana | |||||
|---|---|---|---|---|---|---|
| F | df | P | F | df | P | |
| Pruning | 1.50 | 1 | 0.275 | 4.27 | 1 | 0.131 |
| Time | 3.44 | 5 | 0.052 | 4.28 | 5 | 0.062 |
| Pruning × Time | 7.56 | 5 | 0.004 | 6.40 | 5 | 0.027 |
3.6. Weather
4. Discussion
5. Conclusions
Acknowledgments
Conflict of Interest
References
- Sosebee, R.E.; Wiebe, H.H. Effect of water stress and clipping on photosynthate translocation in two grasses. Agron. J. 1971, 63, 14–17. [Google Scholar] [CrossRef]
- Ovaska, J.; Walls, M.; Mutikainen, P. Changes in leaf gas exchange properties of cloned Betula pendula saplings after partial defoliation. J. Exp. Bot. 1992, 43, 1301–1307. [Google Scholar] [CrossRef]
- McJannet, D.; Vertessy, R. Effects of thinning on wood production, leaf area index, transpiration and canopy interception of a plantation subject to drought. Tree Physiol. 2001, 21, 1001–1008. [Google Scholar] [CrossRef]
- Zeppel, M.J.B.; Murray, B.R.; Barton, C.; Eamus, D. Seasonal responses of xylem sap velocity to VPD and solar radiation during drought in a stand of native trees in temperate Australia. Funct. Plant Biol. 2004, 31, 461–470. [Google Scholar]
- Whitehead, D.; Jarvis, P.G.; Waring, R.H. Stomatal conductance, transpiration, and resistance to water uptake in a Pinus sylvestris spacing experiment. Can. J. For. Res. 1984, 14, 692–700. [Google Scholar] [CrossRef]
- Jarvis, P.G.; McNaughton, K.G. Stomatal control of transpiration: Scaling up from leaf to region. Adv. Ecol. Res. 1986, 15, 1–49. [Google Scholar]
- Wullschleger, S.D.; Meinzer, F.C.; Vertessy, R.A. A review of whole-plant water use studies in trees. Tree Physiol. 1998, 18, 499–512. [Google Scholar]
- Pepin, S.; Livingston, N.J.; Whitehead, D. Responses of transpiration and photosynthesis to reversible changes in photosynthetic foliage area in western red cedar (Thuja plicata) seedlings. Tree Physiol. 2002, 22, 363–371. [Google Scholar] [CrossRef]
- Whitehead, D.; Livingston, N.J.; Kelliher, F.M.; Hogan, K.P.; Pepin, S.; McSeveny, T.M.; Byers, J.N. Response of transpiration and photosynthesis to a transient change in illuminated foliage area for a Pinus radiata D. Don tree. Plant Cell Environ. 1996, 19, 949–957. [Google Scholar] [CrossRef]
- Quentin, A.G.; O’Grady, A.P.; Beadle, C.L.; Pinkard, E.L.; Worldege, D. Resonses of transpiration and canopy conductance to partial defoliation of Eucalyptus globulus trees. Agric. For. Meteorol. 2011, 151, 356–364. [Google Scholar] [CrossRef]
- Quentin, A.G.; O’Grady, A.P.; Beadle, C.L.; Mohammed, C.; Pinkard, E.A. Interactive effects of water supply and defoliation on photosynthesis, plant water status and growth of Eucalyptus globulus Labill. Tree Physiol. 2012, 32, 958–967. [Google Scholar] [CrossRef]
- Forrester, D.I.; Collopy, J.J.; Beadle, C.L.; Warren, C.R.; Baker, T.G. Effect of thinning, pruning and nitrogen fertiliser application on transpiration, photosynthesis and water-use efficiency in a young Eucalyptus nitens plantation. For. Ecol. Manag. 2012, 266, 286–300. [Google Scholar] [CrossRef]
- Pinkard, E.A.; Beadle, C.L.; Davidson, N.J.; Battaglia, M. Photosynthetic responses of Eucalyptus nitens (Deane and Maiden) Maiden to green pruning. Trees-Struct. Funct. 1998, 12, 119–129. [Google Scholar]
- Pinkard, E.A. Physiological and growth responses related to pattern and severity of green pruning in young Eucalyptus globulus. For. Ecol. Manag. 2003, 182, 231–245. [Google Scholar] [CrossRef]
- Heichel, G.H.; Turner, N.C. Co2 assimilation of primary and regrowth foliage of red maple (Acer rubrum L.) and red oak (Quercus rubra L.): Response to defoliation. Oecologia 1983, 57, 14–19. [Google Scholar] [CrossRef]
- Wallace, L.L.; McNaughton, S.J.; Coughenour, M.B. Compensatory photosynthetic responses of three African graminoids to different fertilization, watering and clipping regimes. Bot. Gazette 1984, 145, 151–156. [Google Scholar]
- Whitehead, D.; Kelliher, F.M. A canopy water balance model for a Pinus radiata stand before and after thinning. Agric. For. Meteorol. 1991, 55, 109–126. [Google Scholar] [CrossRef]
- Singh, K.A.; Thompson, F.B. Effect of lopping on water potential, transpiration, regrowth, 14C-photosynthate distribution and biomass production in Alnus glutinosa. Tree Physiol. 1995, 15, 197–202. [Google Scholar] [CrossRef]
- Alcorn, P.J.; Forrester, D.I.; Smith, R.G.B.; Thomas, D.S.; James, R.N.; Nicotra, A.B.; Bauhus, J. Crown structure and vertical foliage distribution in 4-year-old plantation-grown Eucalyptus pilularis and Eucalyptus cloeziana. Trees 2012, in press.. [Google Scholar]
- Milford, H.B. Soil Landscapes of the Coffs Harbour 1:100,000 Sheet Map; Department of Land and Water Conservation: Sydney, NSW, Australia, 1999. [Google Scholar]
- Gilligan, L.B.; Brownlow, J.W.; Cameron, R.G.; Henley, H.F. Dorrigo-Coffs Harbour 1:250,000 Metallogenic Map sh/56–10, sh/56–11: Metallogenic Study and Mineral Deposit Data Sheet; Department of Mineral Resources: Sydney, NSW, Australia, 1992. [Google Scholar]
- Smith, D.M.; Larson, B.C.; Kelty, M.J.; Ashton, P.M.S. The Practice of Silviculture: Applied Forest Ecology, 9th ed; John Wiley and Sons Inc.: New York, NY, USA, 1997. [Google Scholar]
- Soares, P.; Tomé, M. A tree crown ratio prediction equation for eucalypt plantations. Ann. For. Sci. 2001, 58, 193–202. [Google Scholar]
- Vertessy, R.A.; Benyon, R.G.; O’Sullivan, S.K.; Gribben, P.R. Relationships between stem diameter, sapwood area, leaf area and transpiration in a young mountain ash forest. Tree Physiol. 1995, 15, 559–567. [Google Scholar] [CrossRef]
- Hunt, M.A.; Beadle, C.L. Whole-tree transpiration and water-use partitioning between Eucalyptus nitens and Acacia dealbata weeds in a short-rotation plantation in northeastern Tasmania. Tree Physiol. 1998, 18, 557–563. [Google Scholar] [CrossRef]
- Medhurst, J.L.; Battaglia, M.; Beadle, C.L. Measured and predicted changes in tree and stand water use following high-intensity thinning of an 8-year-old Eucalyptus nitens plantation. Tree Physiol. 2002, 22, 775–784. [Google Scholar] [CrossRef]
- Zang, D.; Beadle, C.L.; White, D.A. Variation of sapflow velocity in Eucalyptus globulus with position in sapwood and use of a correction coefficient. Tree Physiol. 1996, 16, 697–703. [Google Scholar] [CrossRef]
- NRM Natural Resource Management. Queensland Government Web site, 2005. Available online: www.nrm.qld.gov.au/silo/datadrill (accessed on 1 January 2005).
- Buck, A.L. New equations for computing vapor pressure deficit and enhancement factor. J. Appl. Meteorol. 1981, 20, 1527–1532. [Google Scholar]
- Alcorn, P.J.; Bauhus, J.; Smith, R.G.B.; Thomas, D.; James, R.; Nicotra, A. Growth response following green crown pruning in plantation-grown Eucalyptus pilularis and E. Cloeziana. Can. J. For. Res. 2008, 38, 770–781. [Google Scholar] [CrossRef]
- Forrester, D.I.; Collopy, J.J.; Beadle, C.L.; Baker, T.G. Interactive effects of simultaneously applied thinning, pruning and fertiliser application treatments on growth, biomass production and crown architecture in a young Eucalyptus nitens plantation. For. Ecol. Manag. 2012, 267, 104–116. [Google Scholar] [CrossRef]
- Forrester, D.I.; Collopy, J.J.; Beadle, C.L.; Baker, T.G. Effect of thinning, pruning and nitrogen fertiliser application on light interception and light-use efficiency in a young Eucalyptus nitens plantation. For. Ecol. Manag. 2013, 288, 21–30. [Google Scholar] [CrossRef]
- Forrester, D.I.; Medhurst, J.L.; Wood, M.; Beadle, C.L.; Valencia, J.C. Growth and physiological responses to silviculture for producing solid-wood products from Eucalyptus plantations: An australian perspective. For. Ecol. Manag. 2010, 259, 1819–1835. [Google Scholar] [CrossRef]
- Reich, P.B.; Walters, M.B.; Krause, S.C.; Vanderklein, D.W.; Raffa, K.F.; Tabone, T. Growth, nutrition and gas exchange of Pinus resinosa following artificial defoliation. Trees Struct. Funct. 1993, 7, 67–77. [Google Scholar]
- Tschaplinski, T.; Blake, T.J. Growth and carbohydrate status of coppice shoots of hybrid poplar following shoot pruning. Tree Physiol. 1995, 15, 333–338. [Google Scholar]
- Pataki, D.E.; Oren, R.; Katul, G.; Sigmon, J. Canopy conductance of Pinus taeda, Liquidambar styraciflua and Quercus phellos under varying atmospheric and soil water conditions. Tree Physiol. 1998, 18, 307–315. [Google Scholar] [CrossRef]
- Florence, R.G. Ecology and Silviculture of Eucalypt Forests; CSIRO: Collingwood, Australia, 1996; p. 413. [Google Scholar]
- Whitehead, D.; Beadle, C.L. Physiological regulation of productivity and water use in Eucalyptus: A review. For. Ecol. Manag. 2004, 193, 113–140. [Google Scholar]
- Hatton, T.; Reece, P.; Taylor, P.; McEwan, K. Does leaf water efficiency vary among eucalypts in water-limited environments? Tree Physiol. 1998, 18, 529–536. [Google Scholar] [CrossRef]
- Benyon, R.G.; Theiveyanathan, S.; Doody, T.M. Impacts of tree plantations on groundwater in south-eastern Australia. Aust. J. Bot. 2006, 54, 181–192. [Google Scholar]
- Forrester, D.I.; Collopy, J.J.; Morris, J.D. Transpiration along an age series of Eucalyptus globulus plantations in southeastern australia. For. Ecol. Manag. 2010, 259, 1754–1760. [Google Scholar] [CrossRef]
- Cohen, Y.; Kelliher, F.M.; Black, T.A. Determination of sap flow in douglas-fir trees using the heat pulse technique. Can. J. For. Res. 1985, 15, 422–428. [Google Scholar]
- Dye, P.J.; Olbrich, B.W.; Poulter, A.G. The influence of growth rings in pinus patula on heat pulse velocity and sap flow measurement. J. Exp. Bot. 1991, 42, 867–870. [Google Scholar] [CrossRef]
- Jiménez, M.S.; Nadezhdina, N.; Cermak, J.; Morales, D. Radial variation in sap flow in five laurel forest tree species in tenerife, canary islands. Tree Physiol. 2000, 20, 1149–1156. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Alcorn, P.J.; Forrester, D.I.; Thomas, D.S.; James, R.; Smith, R.G.B.; Nicotra, A.B.; Bauhus, J. Changes in Whole-Tree Water Use Following Live-Crown Pruning in Young Plantation-Grown Eucalyptus pilularis and Eucalyptus cloeziana. Forests 2013, 4, 106-121. https://doi.org/10.3390/f4010106
Alcorn PJ, Forrester DI, Thomas DS, James R, Smith RGB, Nicotra AB, Bauhus J. Changes in Whole-Tree Water Use Following Live-Crown Pruning in Young Plantation-Grown Eucalyptus pilularis and Eucalyptus cloeziana. Forests. 2013; 4(1):106-121. https://doi.org/10.3390/f4010106
Chicago/Turabian StyleAlcorn, Philip J., David I. Forrester, Dane S. Thomas, Ryde James, R. Geoff B. Smith, Adrienne B. Nicotra, and Jürgen Bauhus. 2013. "Changes in Whole-Tree Water Use Following Live-Crown Pruning in Young Plantation-Grown Eucalyptus pilularis and Eucalyptus cloeziana" Forests 4, no. 1: 106-121. https://doi.org/10.3390/f4010106
APA StyleAlcorn, P. J., Forrester, D. I., Thomas, D. S., James, R., Smith, R. G. B., Nicotra, A. B., & Bauhus, J. (2013). Changes in Whole-Tree Water Use Following Live-Crown Pruning in Young Plantation-Grown Eucalyptus pilularis and Eucalyptus cloeziana. Forests, 4(1), 106-121. https://doi.org/10.3390/f4010106





