The Importance of Microtopography and Nurse Canopy for Successful Restoration Planting of the Slow-Growing Conifer Pilgerodendron uviferum
Abstract
:1. Introduction
2. Experimental Section
2.1. Study Area
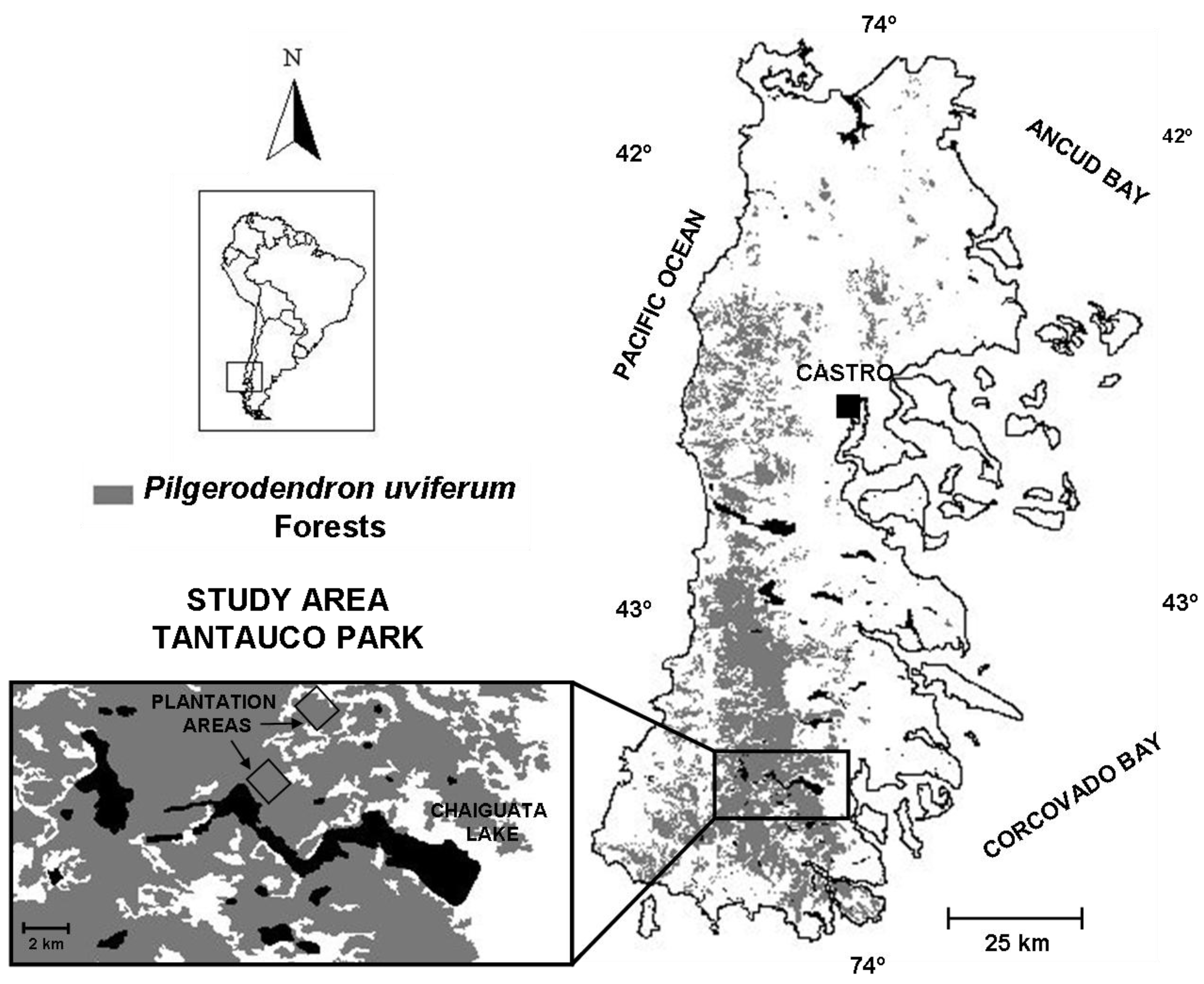
2.2. Planting Design and Implementation

2.3. Data Collection
2.4. Net Photosynthesis and Chlorophyll Fluorescence Measurements
2.5. Data Analysis
3. Results
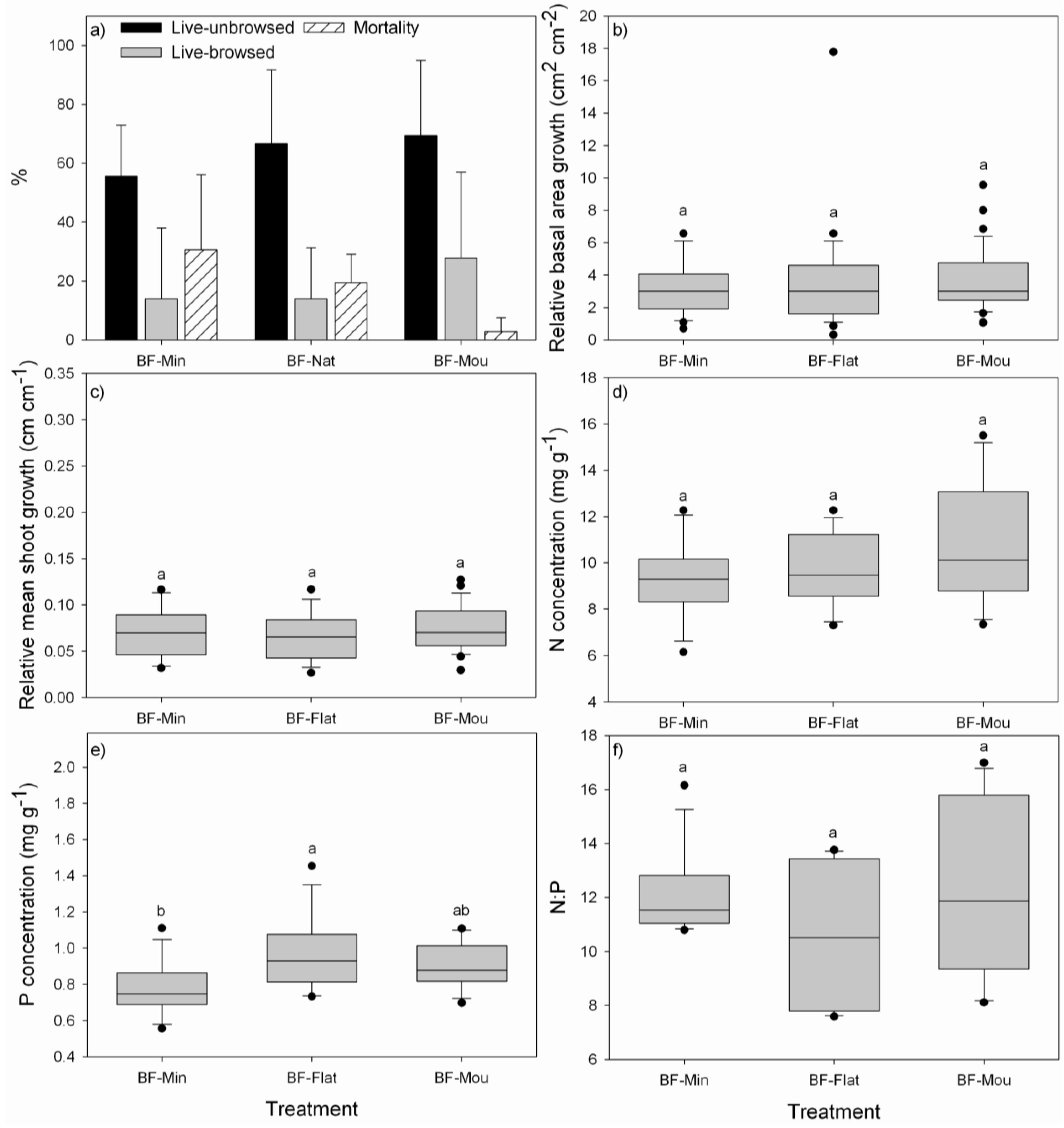
3.1. Performance of P. uviferum Seedlings in Bogs
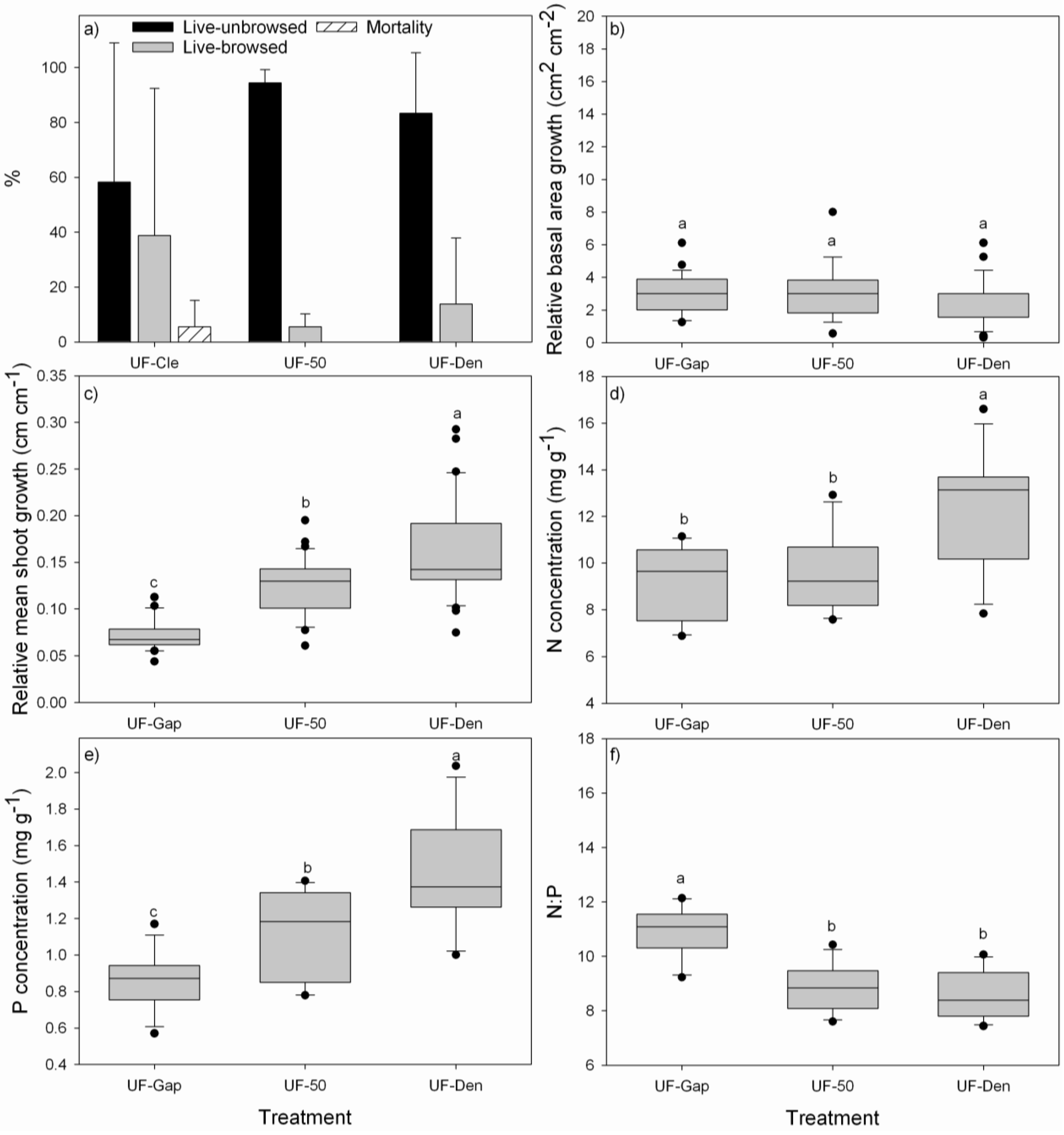
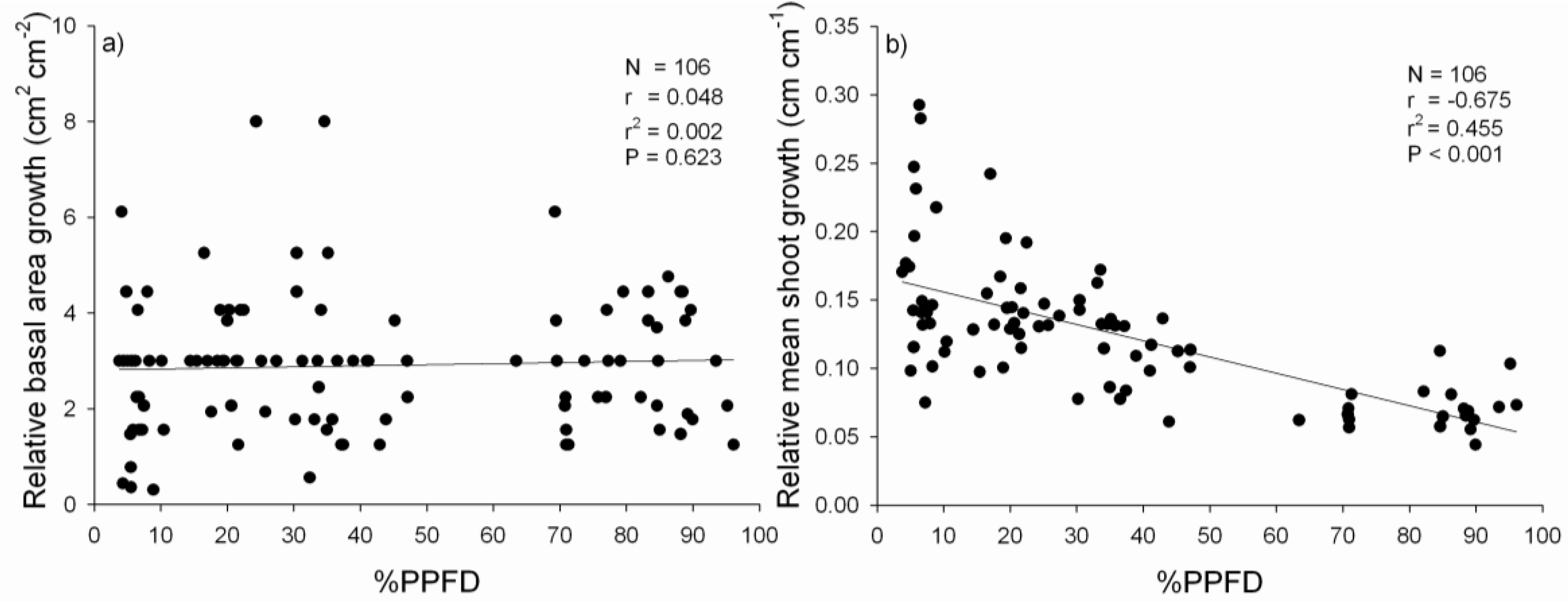
3.2. Performance of P. uviferum Seedlings in Upland Areas
| Treatment | LSP (µmol m−2·s−1) | LCP (µmol m−2·s−1) | AQE | Amax (µmol m−2·s−1) | Rd (µmol m−2·s−1) | Fv/Fm | LMA (g·m−2) |
|---|---|---|---|---|---|---|---|
| UF-Gap | 1624 ± 117 | 17.6 ± 2.0 | 0.099 ± 0.012 | 20.0 ± 2.0 | −1.51 ± 0.12 | 0.78 ± 0.01 | 669.4 ± 58.4 |
| UF-50 | 834 ± 39 | 7.6 ± 1.2 | 0.101 ± 0.007 | 9.5 ± 0.7 | −0.32 ± 0.04 | 0.81 ± 0.00 | 338.7 ± 21.3 |
| UF-Den | 746 ± 110 | 5.0 ± 0.7 | 0.111 ± 0.016 | 9.5 ± 0.9 | −0.21 ± 0.02 | 0.83 ± 0.00 | 421.7 ± 10.9 |
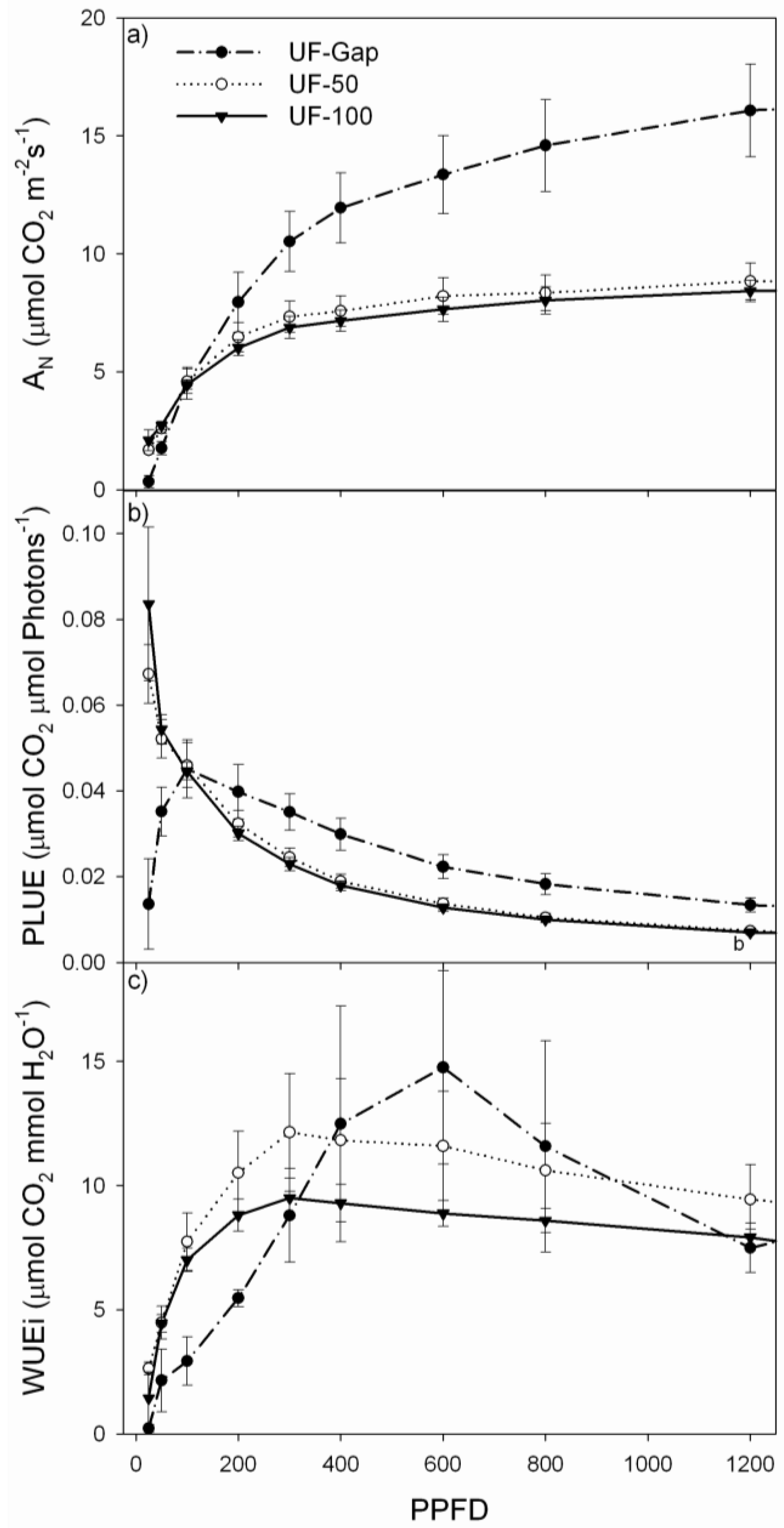
4. Discussion
4.1. Performance of P. uviferum Seedlings in Bogs
4.2. Performance of P. uviferum Seedlings in Upland Areas
5. Implications for Restoration Plantings
- The high effort associated with mounding the soil is not justified since survival of seedlings established on mounds was only slightly improved and there were no effects on growth. Therefore, seedlings should be planted in the grown substrate of flat areas or on natural mounds and small elevated areas in the landscape to avoid waterlogging conditions for seedlings.
- Existing vegetation, e.g., of shrubs, such as Bacharis sp., Gaultheria sp., and ferns, such as Gleichenia sp., should not be removed or only partially removed to offer more suitable environments to seedlings [12].
- The creation of gaps or strips is not effective and may be detrimental to seedling establishment and growth.
- Planting seedlings beneath existing canopies of other trees, like D. winteri, W. trichosperma, N. nitida or T. stipularis, is a good option to promote better nutrition and faster growth of P. uviferum seedlings. Under these shady conditions, P. uviferum can persist over long periods of time [6].
Acknowledgments
Conflict of Interest
References
- Hobbs, R.J.; Norton, D.A. Towards a conceptual framework for restoration ecology. Restor. Ecol. 1996, 4, 93–110. [Google Scholar] [CrossRef]
- Benayas, J.M.R.; Bullock, J.M.; Newton, A.C. Creating woodland islets to reconcile ecological restoration, conservation and agricultural land use. Front. Ecol. Environ. 2008, 6, 329–336. [Google Scholar] [CrossRef] [Green Version]
- Morrison, E.B.; Lindell, C.A. Active or passive forest restoration? Assessing restoration alternatives with avian foraging behavior. Restor. Ecol. 2011, 19, 170–177. [Google Scholar] [CrossRef]
- Holl, K.D.; Aide, T.M. When and where to actively restore ecosystems? For. Ecol. Manag. 2011, 261, 1558–1563. [Google Scholar] [CrossRef]
- Lara, A.; Donoso, C.; Escobar, B.; Rovere, A.; Premoli, A.; Soto, D.P.; Bannister, J.R. Pilgerodendron uviferum (D. Don) Florin. In Las Especies Arbóreas de los Bosques Templados de Chile y Argentina, Autoecología; Donoso, C., Ed.; Marisa Cuneo: Valdivia, Chile, 2006; pp. 82–91. [Google Scholar]
- Bannister, J.R.; Donoso, P.J.; Bauhus, J. Persistence of the slow growing conifer Pilgerodendron uviferum in old-growth and fire-disturbed southern bog forests. Ecosystems 2012, 15, 1158–1172. [Google Scholar] [CrossRef]
- Bannister, J.R.; Lara, A.; Le Quesne, C. Estructura y dinámica de bosques de Pilgerodendron uviferum afectados por incendios en la Cordillera de la Costa de la Isla Grande de Chiloé. Bosque 2008, 29, 33–43. [Google Scholar]
- Walter, K.S.; Gillet, H.J. 1997 IUCN Red List of Threatened Plants; IUCN: Cambridge, UK, 1998. [Google Scholar]
- Hobbs, R.J.; Norton, D.A. Ecological Filters, Thresholds and Gradients in Resistance to Ecosystem Reassembly. In Assembly Rules and Restoration Ecology: Bridging the Gap between Theory and Practice; Temperton, V.M., Hobbs, R.J., Nuttle, T., Halle, S., Eds.; Island Press: Washington, DC, USA, 2004; pp. 72–95. [Google Scholar]
- Bannister, J.R.; Wagner, S.; Donoso, P.J.; Bauhus, J. The importance of seed trees for the passive restoration of disturbed Pilgerodendron uviferum bog forests in Northern Patagonia. 2013; Unpublished work. [Google Scholar]
- Armesto, J.J.; Bustamante-Sánchez, M.A.; Díaz, M.F.; González, M.E.; Holz, A.; Nuñez-Avila, M.; Smith-Ramirez, C. Fire Disturbance Regimes, Ecosystem Recovery and Restoration Strategies in Mediterranean and Temperate Regions of Chile. In Fire Effects on Soils and Restoration Strategies; Cerda, A., Robichaud, P.R., Eds.; Science Publishers: Enfield, NH, USA, 2009; pp. 537–564. [Google Scholar]
- Carmona, M.R.; Aravena, J.C.; Bustamante-Sanchez, M.A.; Celis-Diez, J.L.; Charrier, A.; Díaz, I.A.; Díaz-Forestier, J.; Díaz, M.F.; Gaxiola, A.; Gutiérrez, A.G.; et al. Estación biológica Senda Darwin: Investigación ecológica de largo plazo en la interfase ciencia-sociedad. Rev. Chil. Hist. Nat. 2010, 83, 113–142. [Google Scholar]
- Lara, A.; Echeverría, C.; Thiers, O.; Huss, E.; Escobar, B.; Tripp, K.; Zamorano, C.; Altamirano, A. Restauración ecológica de coníferas longevas: el caso del alerce (Fitzroya cupressoides) en el sur de Chile. In Restauración de Bosques en América Latina; González-Espinosa, M., Rey-Benayas, J.M., Ramírez-Marcial, N., Eds.; Mundi-Prensa México: México City, México, 2008; pp. 40–55. [Google Scholar]
- Hörnberg, G.; Ohlson, M.; Zackrisson, O. Influence of bryophytes and microrelief conditions on Picea abies seed regeneration patterns in boreal old-growth swamp forests. Can. J. For. Res. 1997, 27, 1015–1023. [Google Scholar] [CrossRef]
- Ohlson, M. Growth and nutrient characteristics in bog and fen populations of Scots pine (Pinus sylvestris). Plant Soil 1995, 172, 235–245. [Google Scholar] [CrossRef]
- Díaz, M.; Armesto, J.J. Limitantes físicos y bióticos de la regeneración arbórea en matorrales sucesionales de la Isla Grande de Chiloé, Chile. Rev. Chil. Hist. Nat. 2007, 80, 13–26. [Google Scholar]
- Rydin, H.; Jeglum, J.K. The Biology of Peatlands; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Cruz, G.; Lara, A. Tipificación, cambio de estructura y normas de manejo para Ciprés de las Guaitecas (Pilgerodendron uviferum D. Don Florin) en la isla Grande de Chiloé. Forest Engineer Thesis, Universidad de Chile, Santiago, Chile, 1981. [Google Scholar]
- Baker, N.R.; Rosenqvist, E. Applications of chlorophyll fluorescence can improve crop production strategies: An examination of future possibilities. J. Exp. Bot. 2004, 55, 1607–1621. [Google Scholar] [CrossRef]
- Walters, R.G. Towards an understanding of photosynthetic acclimation. J. Exp. Bot. 2005, 56, 435–447. [Google Scholar] [CrossRef]
- Givnish, T. Adaptation to sun and shade: A whole-plant perspective. Funct. Plant Biol. 1988, 15, 63–92. [Google Scholar]
- Holz, A.; Veblen, T.T. The amplifying effects of humans on fire regimes in temperate rainforests in western Patagonia. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2011, 311, 82–92. [Google Scholar] [CrossRef]
- Di Castri, F.; Hajek, E. Bioclimatología de Chile; Vicerrectoría Académica de la Universidad Católica de Chile: Santiago, Chile, 1976. [Google Scholar]
- Bannister, J.R.; Vidal, O.J.; Teneb, E.; Sandoval, V. Latitudinal patterns and regionalization of plant diversity along a 4270-km gradient in continental Chile. Austral Ecol. 2012, 37, 500–509. [Google Scholar] [CrossRef]
- Pérez, C.A.; Armesto, J.J.; Torrealba, C.; Carmona, M.R. Litterfall dynamics and nitrogen use efficiency in two evergreen temperate rainforests of southern Chile. Austral Ecol. 2008, 28, 591–600. [Google Scholar] [CrossRef]
- Villagrán, C. Late quaternary vegetation of southern Isla Grande de Chiloé, Chile. Quat. Res. 1988, 29, 294–306. [Google Scholar]
- Donoso, C.; Sandoval, V.; Grez, R.; Rodríguez, J. Dynamics of Fitzroya cupressoides forests in southern Chile. J. Veg. Sci. 1993, 4, 303–312. [Google Scholar] [CrossRef]
- Parent, S.; Messier, C. A simple and efficient method to estimate microsite light availability under a forest canopy. Can. J. For. Res. 1996, 26, 151–154. [Google Scholar] [CrossRef]
- König, N. Handbuch Forstliche Analytik: Gessellschaft für Analytik; Bundesministerium für Verbraucherschutz, Ernährung und Landwirtschaft: Bonn, Germany, 2005. [Google Scholar]
- Genty, B.; Briantais, J.M. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Hurlbert, S.H. Pseudoreplication and the design of ecological field experiments. Ecol. Monogr. 1984, 54, 187–211. [Google Scholar]
- SPSS Inc. SPSS Statistics for Windows, version 17, SPSS Inc.: Chicago, IL, USA, 2008.
- R Development Core Team, R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria, 2012.
- Koerselman, W.; Meuleman, A.F.M. The vegetation N:P ratio: A new tool to detect the nature of nutrient limitation. J. Appl. Ecol. 1996, 33, 1441–1450. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Simard, M.; Lecomte, N.; Bergeron, Y.; Bernier, P.Y.; Paré, D. Forest productivity decline caused by successional paludification of Boreal soils. Ecol. Appl. 2007, 17, 1619–1637. [Google Scholar] [CrossRef]
- Jones, M.D.; Durall, D.M.; Cairney, J.W.G. Ectomycorrhizal fungal communities in young forest stands regenerating after clearcut logging. New Phytol. 2003, 157, 399–422. [Google Scholar] [CrossRef]
- Simard, S.W. The foundational role of mycorrhizal networks in self-organization of interior Douglas-fir forests. Forest Ecol. Manag. 2009, 258, S95–S107. [Google Scholar]
- Simard, S.W.; Beiler, K.J.; Bingham, M.A.; Deslippe, J.R.; Philip, L.J.; Teste, F.P. Mycorrhizal networks: Mechanisms, ecology and modelling. Fungal Biol. Rev. 2012, 26, 39–60. [Google Scholar] [CrossRef]
- Coopman, R.E.; Reyes-Díaz, M.; Briceño, V.F.; Corcuera, L.J.; Cabrera, H.M.; Bravo, L.A. Changes during early development in photosynthetic light acclimation capacity explain the shade to sun transition in Nothofagus nitida. Tree Physiol. 2008, 28, 1561–1571. [Google Scholar] [CrossRef]
- Dirección Meteorológica de Chile (DMC), Estadística Climatológica: Tomo III; Dirección Meteorológica de Chile: Santiago, Chile, 2001.
- Roderick, M.; Farquhar, G.; Berry, S.; Noble, I. On the direct effect of clouds and atmospheric particles on the productivity and structure of vegetation. Oecologia 2001, 129, 21–30. [Google Scholar] [CrossRef]
- Alton, P.B.; North, P.R.; Los, S.O. The impact of diffuse sunlight on canopy light-use efficiency, gross photosynthetic product and net ecosystem exchange in three forest biomes. Glob. Chang. Biol. 2007, 13, 776–787. [Google Scholar] [CrossRef]
- Reich, P.; Oleksyn, J.; Wright, I. Leaf phosphorus influences the photosynthesis-nitrogen relation: A cross-biome analysis of 314 species. Oecologia 2009, 160, 207–212. [Google Scholar]
- Mercado, L.M.; Patiño, S.; Domingues, T.F.; Fyllas, N.M.; Weedon, G.P.; Sitch, S.; Quesada, C.A.; Phillips, O.L.; Aragão, L.E.; Malhi, Y.; et al. Variations in Amazon forest productivity correlated with foliar nutrients and modeled rates of photosynthetic carbon supply. Phil. Trans. R. Soc. B 2011, 366, 3316–3329. [Google Scholar] [CrossRef]
- Silva-Rodriguez, E.A.; Verdugo, C.; Alejandro Aleuy, O.; Sanderson, J.G.; Ortega-Solis, G.R.; Osorio-Zuniga, F.; Gonzalez-Acuna, D. Evaluating mortality sources for the vulnerable Pudu Pudu puda in Chile: Implications for the conservation of a threatened deer. Oryx 2010, 44, 97–103. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bannister, J.R.; Coopman, R.E.; Donoso, P.J.; Bauhus, J. The Importance of Microtopography and Nurse Canopy for Successful Restoration Planting of the Slow-Growing Conifer Pilgerodendron uviferum. Forests 2013, 4, 85-103. https://doi.org/10.3390/f4010085
Bannister JR, Coopman RE, Donoso PJ, Bauhus J. The Importance of Microtopography and Nurse Canopy for Successful Restoration Planting of the Slow-Growing Conifer Pilgerodendron uviferum. Forests. 2013; 4(1):85-103. https://doi.org/10.3390/f4010085
Chicago/Turabian StyleBannister, Jan R., Rafael E. Coopman, Pablo J. Donoso, and Jürgen Bauhus. 2013. "The Importance of Microtopography and Nurse Canopy for Successful Restoration Planting of the Slow-Growing Conifer Pilgerodendron uviferum" Forests 4, no. 1: 85-103. https://doi.org/10.3390/f4010085





