Oribatid Mite Community Decline Two Years after Low-Intensity Burning in the Southern Cascade Range of California, USA
Abstract
:1. Introduction
2. Experimental Section
2.1. Site Description
2.2. Treatments
2.3. Soil Microarthropod Sampling
2.4. Data Analyses
3. Results and Discussion
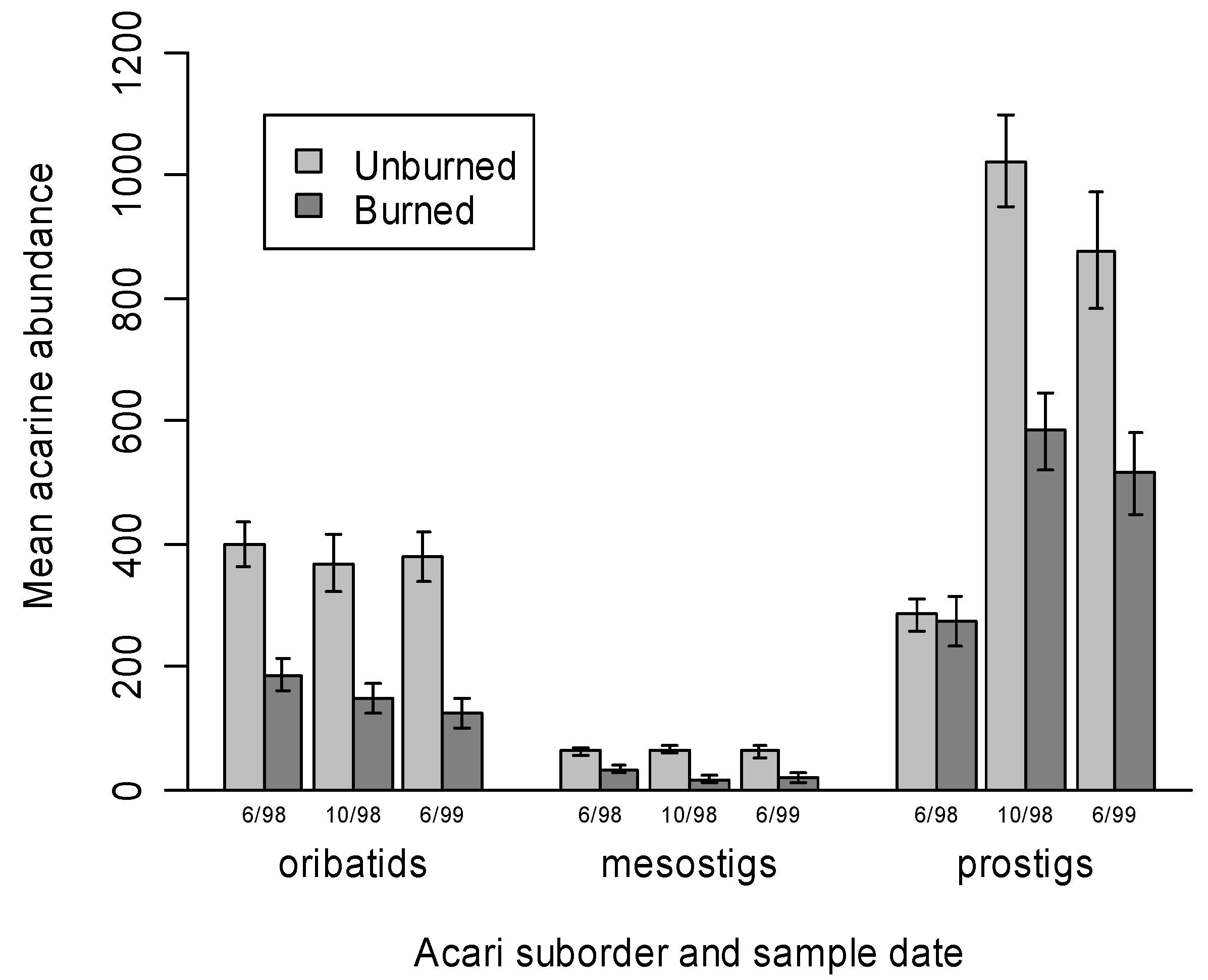
3.1. Mite Suborder Responses
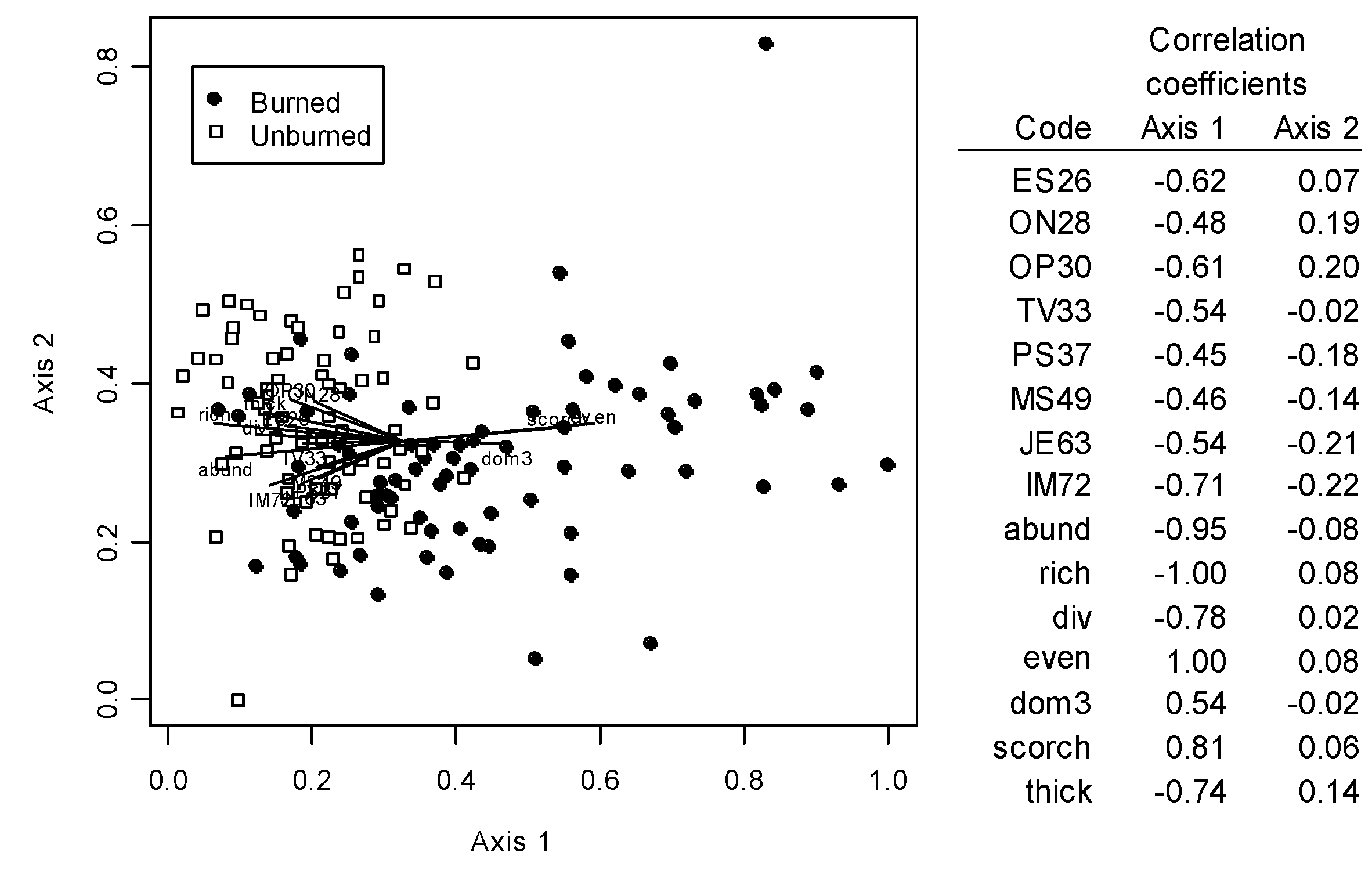
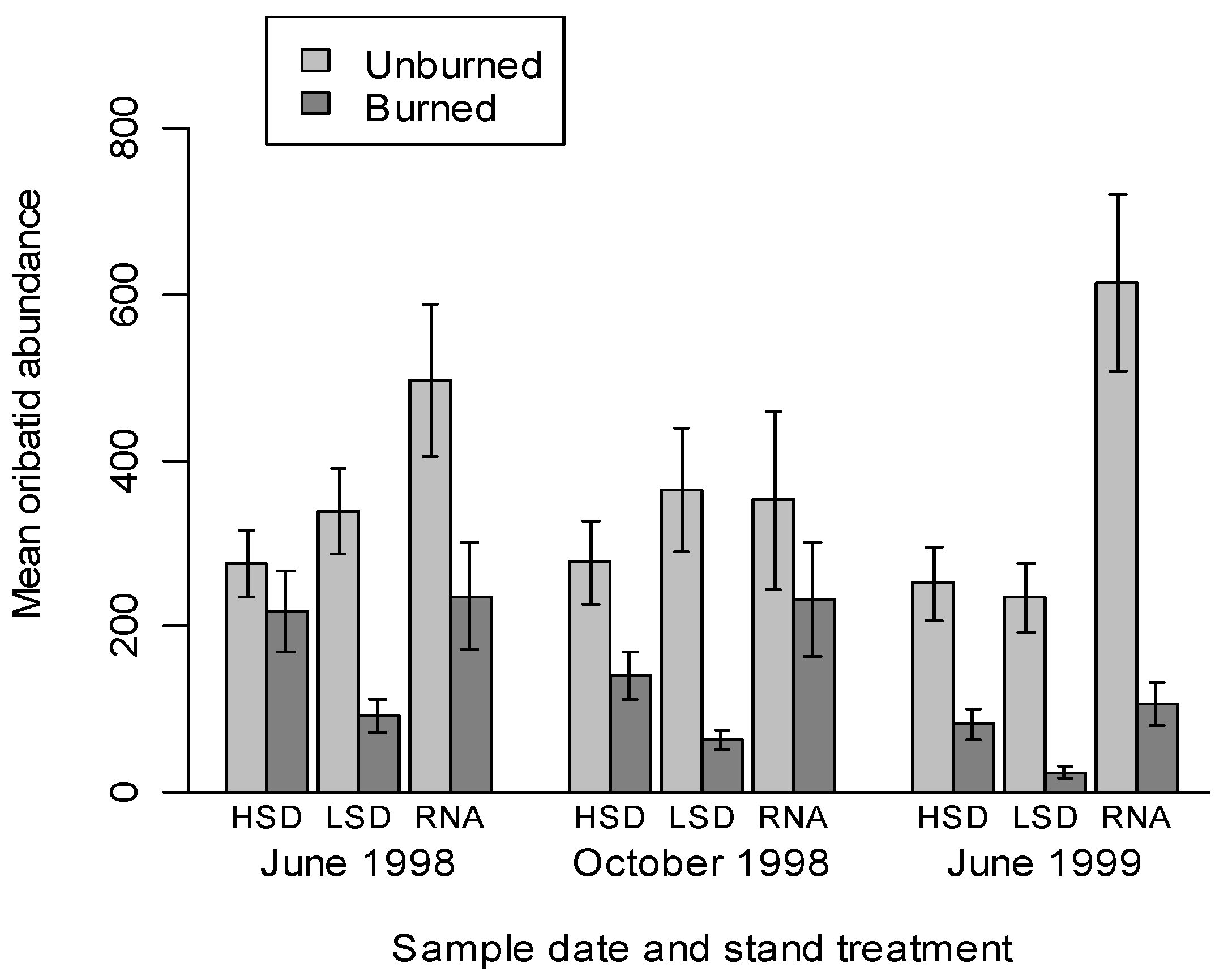
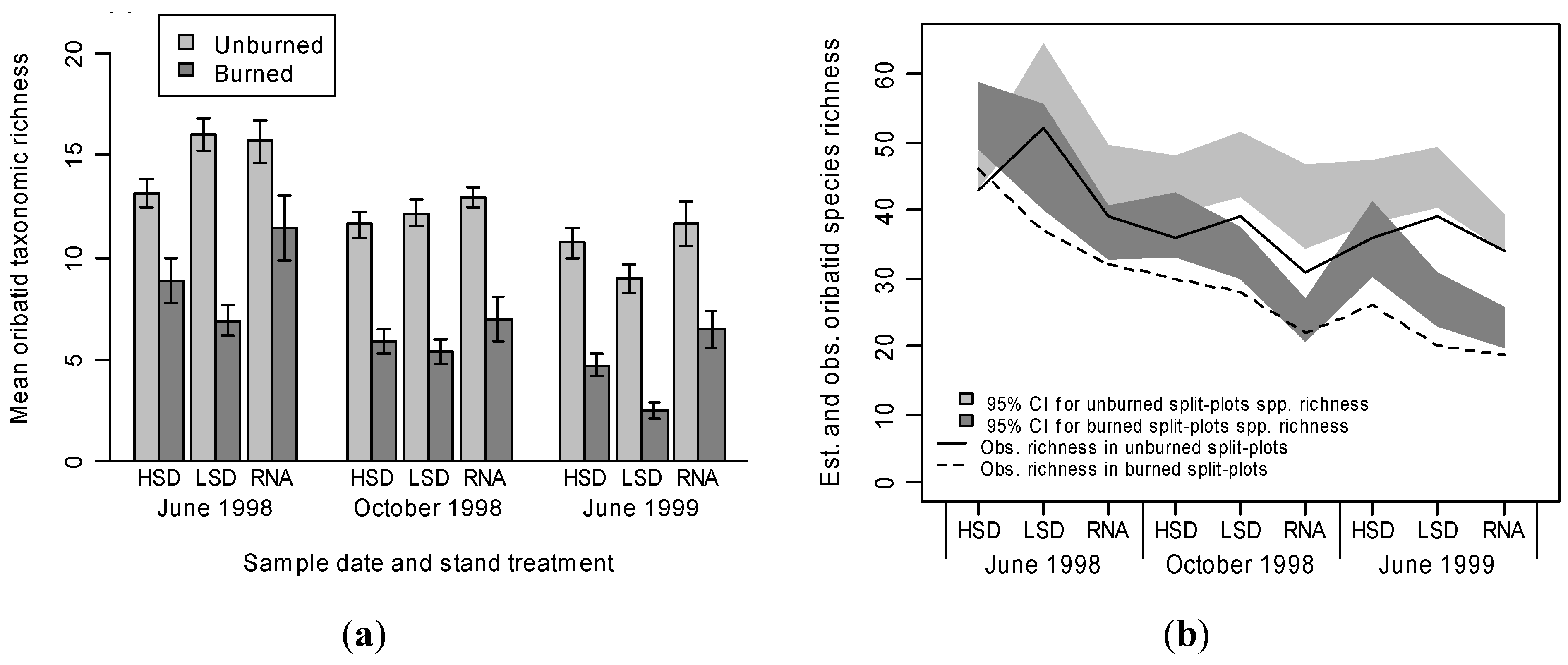
3.2. Oribatid Assemblage Responses
| Taxon | Code | N | Treatment | IV1 | Fire status | IV2 |
|---|---|---|---|---|---|---|
| immature oribatids | IM72 | 26,472 | LSD | 25.7 ** | unburned | 65.4 *** |
| Oppia parviaures | OP30 | 23,976 | RNA | 40.6 *** | unburned | 58.5 *** |
| Aphelacarus acarinus | AA2 | 15,777 | - | ns | - | ns |
| Oppiella nova | ON28 | 15,725 | RNA | 42.8 *** | unburned | 49.7 *** |
| Propelops sp. a | PS37 | 9,885 | all | ns | unburned | ns |
| Jacotella enoplura | JE63 | 7,035 | RNA | 27.8 *** | unburned | 58.0 *** |
| Tectocepheus velatus | TV33 | 6,227 | RNA | 27.9 *** | unburned | 49.4 *** |
| Zachvatkinibates sp. | ZS40 | 5,888 | HSD | 24.5 *** | unburned | 35.2 *** |
| Suctobelbella sp. a | SS67 | 3,941 | HSD | 21.5 *** | unburned | 38.2 *** |
| Maculobates sp. a | MS49 | 2,701 | LSD | 15.6 * | unburned | 43.4 *** |
| Epidamaeus sp. a | ES26 | 2,175 | RNA | 30.2 *** | unburned | 64.7 *** |
| Eueremaeus alvordensis | EA22 | 1,826 | RNA | 16.9 ** | burned | 26.9 * |
| Quadroppia sp. a | QS27 | 1,816 | RNA | 55.1 *** | unburned | 41.7 *** |
| Joshuella sp. nr striata | JS25 | 1,617 | RNA | 17.0 ** | burned | 25.3 ** |
| Nortonella gildersleeveae | NG24 | 1,318 | - | ns | - | ns |
| Pilogalumna sp. | PS43 | 1,027 | HSD | 13.1 * | unburned | 36.1 *** |
| Eobrachychthonius latior | EL11 | 978 | LSD | 18.6 *** | unburned | 18.3 *** |
| Ametroproctus sp. | AS55 | 813 | LSD | 14.0 ** | unburned | ns |
| Eueremaeus stiktos | ES54 | 718 | LSD | 30.2 *** | unburned | 27.7 *** |
| Oribatella dentaticuspis | OD59 | 601 | LSD | 27.1 *** | unburned | 23.4 *** |
| Oribatula tibialis | OT62 | 432 | RNA | 14.7 *** | burned | 7.0 * |
| Microppia minus | MM64 | 400 | RNA | 7.3 * | unburned | 7.3 * |
| Ceratozetes cuspidatus | CC41 | 385 | LSD | 6.9 * | unburned | 11.2 ** |
| Scheloribates sp. b | SS51 | 290 | - | ns | - | ns |
| Liochthonius brevis | LB20 | 273 | LSD | 11.8 ** | unburned | 7.5 ** |
| Galumna sp. | GS66 | 256 | HSD | 4.8 * | - | ns |
| Cultoribula vtouri | CV39 | 253 | HSD | 9.8 ** | unburned | 19.1 *** |
| Scheloribates sp. a | SS38 | 226 | RNA | 12.3 *** | - | ns |
| Brachychthonius sp. a | BS8 | 201 | - | ns | - | 6.3 * |
| Gymnodamaeus sp. | GS50 | 182 | HSD | 14.4 *** | unburned | 18.4 *** |
| Ramusella manifera | RM36 | 179 | LSD | 5.7 ** | unburned | 6.7 ** |
| Verachthonius sp. a | VS5 | 174 | LSD | 8.2 ** | unburned | 7.3 ** |
| Maculobates sp. b | MS69 | 167 | LSD | 10.3 ** | unburned | 8.6 ** |
| Cosmochthonius lanatus | CL77 | 163 | HSD | 9.8 ** | - | ns |
| Oribatella sp. a | OS58 | 123 | RNA | 5.4 * | unburned | 5.6 ** |
| Scapheremaeus sp. | SS73 | 120 | LSD | 5.7 ** | burned | 3.0 ** |
| Brachychthonius bimaculatus | BB7 | 118 | RNA | 12.9 *** | unburned | 6.3 * |
| Eremaeus sp. | ES68 | 82 | LSD | 8.7 *** | unburned | 6.7 ** |
| Scheloribates sp. d | SS75 | 75 | - | ns | - | ns |
| Scheloribates sp. c | SS70 | 61 | - | ns | - | ns |
| Quadroppia sp. c | QS46 | 59 | - | ns | - | ns |
| Verachthonius sp. b | VS6 | 57 | RNA | 4.5 * | unburned | 3.7 ** |
| Camisia horrida | CH61 | 48 | - | ns | - | ns |
| Sellnickochthonius rostratus | SR9 | 34 | - | ns | unburned | 5.1 ** |
| Passalozetes striatus | PS60 | 33 | LSD | 4.2 * | - | ns |
| Carabodes sp. | CS84 | 31 | LSD | 2.7 * | - | ns |
| Zygoribatula sp. a | ZS71 | 29 | - | ns | - | ns |
| Paraleius sp. | PS45 | 29 | - | ns | unburned | 4.4 ** |
| Fosseremus quadripertitus | FQ23 | 29 | HSD | 2.5 * | - | ns |
| Microppia sp. a | MS31 | 18 | - | ns | - | ns |
| Quadroppia sp. b | QS52 | 15 | - | ns | - | ns |
| Charassobatidae sp. | C57 | 11 | LSD | 3.2 * | - | ns |
| Autogneta longilamellata | AL44 | 11 | - | ns | - | ns |
| Xylobates robustior | XR53 | 8 | - | ns | - | ns |
| Sellnickochthonius immaculatus | SS48 | 8 | HSD | 3.4 ** | - | ns |
| Senoribula sp. | SS87 | 6 | RNA | 3.1 * | - | ns |
| Tectocepheus sp. b | T81 | 6 | HSD | 3.5 ** | - | ns |
| Licnodamaeus sp. | L70 | 5 | - | ns | - | ns |
| Poecilochthonius italicus | PI12 | 4 | - | ns | - | ns |
| Oppiod sp. b | OS88 | 3 | RNA | 3.4 ** | - | ns |
| Ramusella clavipectinata | RC82 | 3 | - | ns | - | ns |
| Allosuctobelba sp. | A65 | 3 | - | ns | - | ns |
| Kalyptrazetes sp. | KS56 | 3 | - | ns | - | ns |
| Epidamaeus sp. b | ES86 | 2 | - | ns | - | ns |
| Banksinoma sp. | BS85 | 2 | - | ns | - | ns |
| Sellnickochthonius sp. b | SS18 | 2 | - | ns | - | ns |
| Beklemisheria galeodula | BG1 | 2 | - | ns | - | ns |
| Metrioppia sp. | MS83 | 1 | - | ns | - | ns |
| Multioppia sp. | M79 | 1 | - | ns | - | ns |
| Pirnodus sp. | PS78 | 1 | - | ns | - | ns |
| Parisuctobelba sp. | PS74 | 1 | - | ns | - | ns |
| Nanhermannia dorsalis | ND21 | 1 | - | ns | - | ns |
| Mesotritia sp. | ME10 | 1 | - | ns | - | ns |
| Treatment | Species | Unburned split-plot abundance | Burned split-plot abundance | % change | ||
|---|---|---|---|---|---|---|
| Mean | Proportion | Mean | Proportion | |||
| HSD | Suctobelbella sp. a | 16.6 ± 4.3 | 0.068 | 1.4 ± 0.7 | 0.011 | −91.3 |
| O. nova | 30.6 ± 5.5 | 0.124 | 3.8 ± 1.4 | 0.029 | −87.3 | |
| Zachvatkinibates sp. | 20.9 ± 3.5 | 0.085 | 4.7 ± 3.4 | 0.037 | −77.2 | |
| T. velatus | 18.8 ± 4.9 | 0.076 | 5.2 ± 1.8 | 0.040 | −72.2 | |
| O. parviaures | 53.1 ± 8.8 | 0.216 | 17.5 ± 7.8 | 0.136 | −66.7 | |
| J. enoplura | 16.5 ± 2.8 | 0.067 | 8.9 ± 1.6 | 0.069 | −45.6 | |
| LSD | O. nova | 29.1 ± 8.9 | 0.119 | 1.1 ± 0.6 | 0.024 | −96.0 |
| O. parviaures | 48.5 ± 9.2 | 0.199 | 4.1 ± 2.5 | 0.085 | −91.5 | |
| Immature oribatids | 69.9 ± 7.3 | 0.286 | 15.1 ± 2.5 | 0.316 | −78.3 | |
| A. acarinus | 51.7 ± 23.8 | 0.213 | 11.8 ± 2.9 | 0.248 | −76.9 | |
| T. velatus | 10.4 ± 2.1 | 0.043 | 2.4 ± 0.8 | 0.051 | −76.7 | |
| J. enoplura | 14.0 ± 2.4 | 0.057 | 3.4 ± 1.0 | 0.072 | −75.3 | |
| Propelops sp. a | 20.7 ± 3.5 | 0.085 | 9.7 ± 1.6 | 0.201 | −52.8 | |
| RNA | Suctobelbella sp. a | 15.7 ± 5.7 | 0.031 | 0.5 ± 0.3 | 0.003 | −96.9 |
| Quadroppia sp. a | 18.1 ± 4.9 | 0.039 | 1.9 ± 1.1 | 0.012 | −89.1 | |
| O. parviaures | 136.6 ± 29.0 | 0.295 | 14.2 ± 10.3 | 0.148 | −81.7 | |
| O. nova | 117.2 ± 38.2 | 0.253 | 21.1 ± 12.2 | 0.129 | −81.4 | |
| T. velatus | 25.3 ± 6.7 | 0.055 | 6.7 ± 1.5 | 0.041 | −72.3 | |
| J. enoplura | 25.6 ± 4.8 | 0.055 | 7.8 ± 2.2 | 0.047 | −68.6 | |
| Propelops sp. a | 20.2 ± 3.4 | 0.044 | 8.2 ± 2.1 | 0.050 | −58.2 | |
| Immature oribatids | 53.5 ± 9.6 | 0.116 | 29.9 ± 4.6 | 0.184 | −42.1 | |
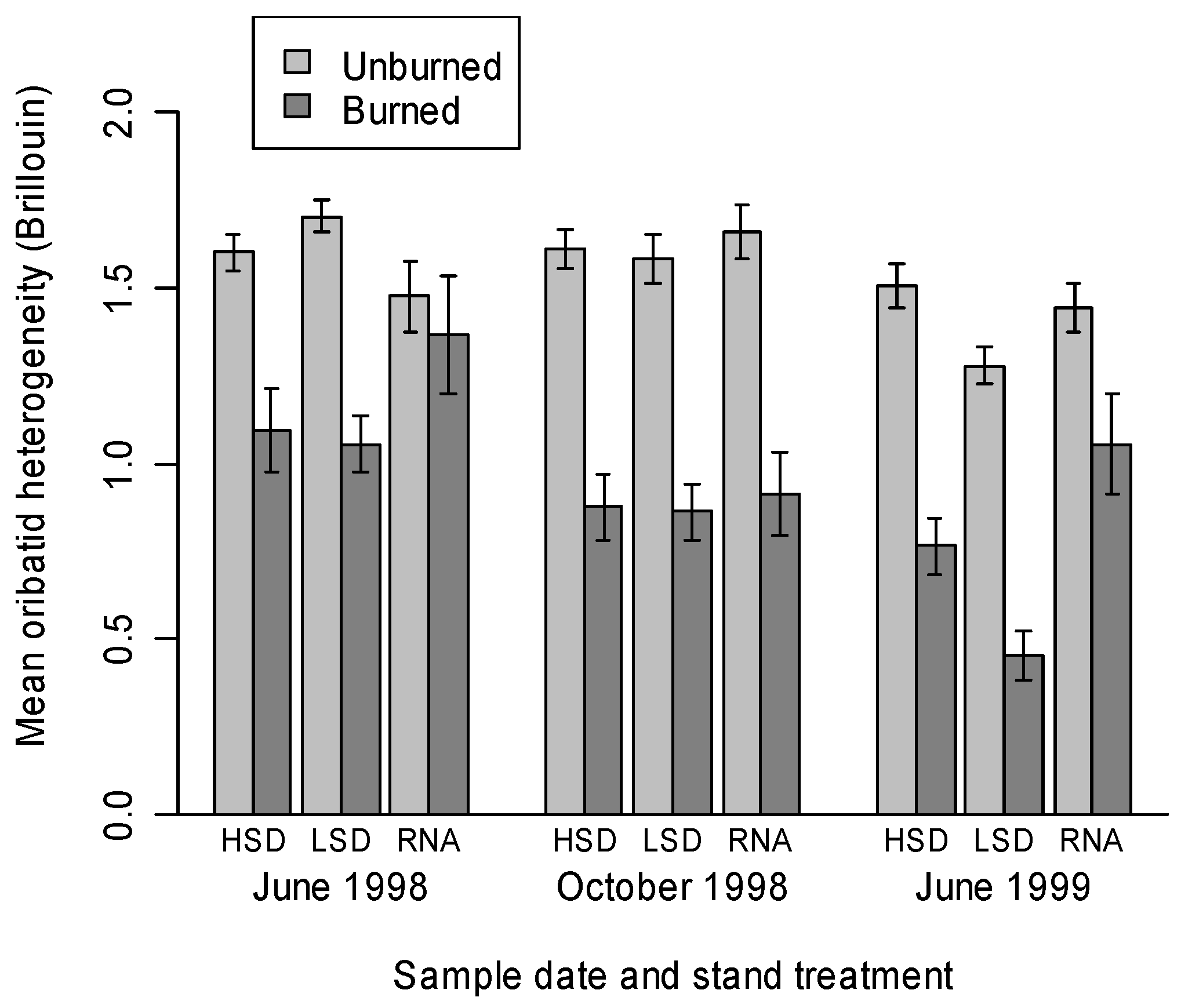
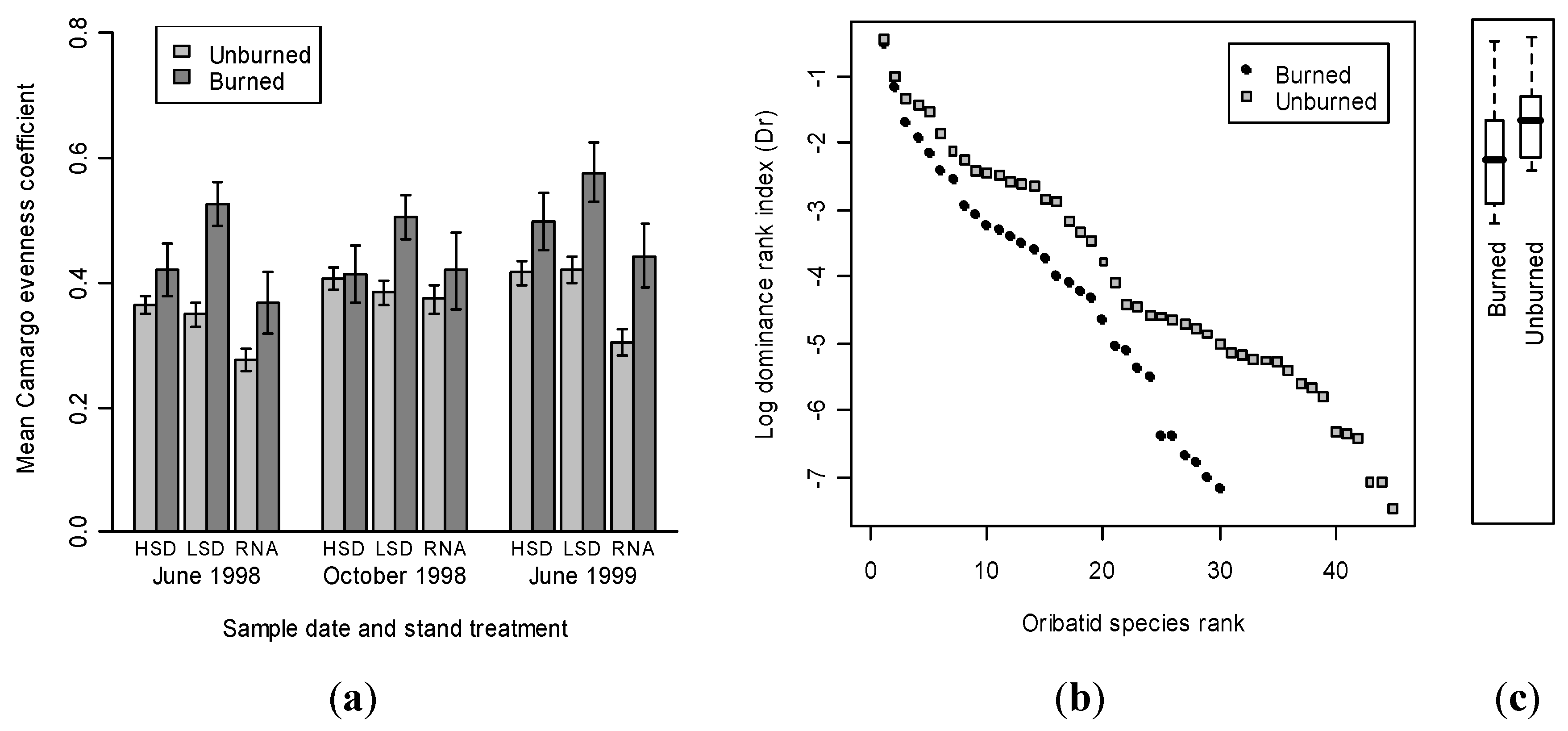

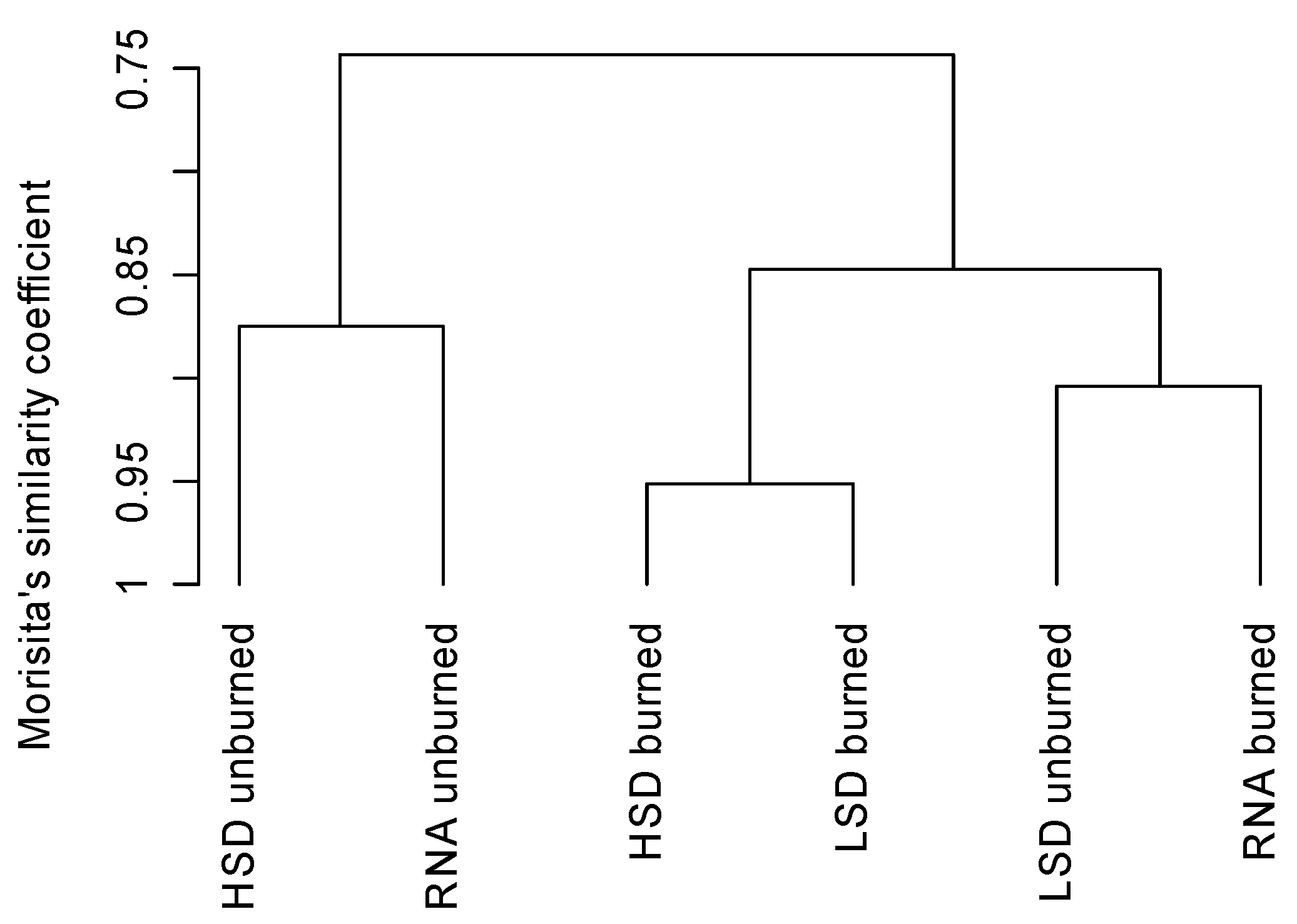
4. Conclusions.
Appendix
Appendix A: Expanded description of Methods
A1. Study Site
A2. Treatments
| Block | Plot | Structure | Harvest year | Fire year |
|---|---|---|---|---|
| 1 | 38 | HSD | 1996 | 1997 |
| 39 | LSD | 1996 | 1997 | |
| 41 | HSD | 1996 | 1997 | |
| 43 | LSD | 1996 | 1997 | |
| 2 | 42 | HSD | 1997 | 1999 |
| 44 | LSD | 1997 | 1999 | |
| 47 | HSD | 1997 | 1999 | |
| 45 | LSD | 1997 | 1999 | |
| 3 | 48 | HSD | 1998 | 2000 |
| 40 | LSD | 1998 | 2000 | |
| 49 | HSD | 1998 | 2000 | |
| 46 | LSD | 1998 | 2000 | |
| NA | RNA A | Old growth | Not harvested | Not burned |
| RNA B | Old growth | Not harvested | 1999 | |
| RNA C | Old growth | Not harvested | 1997 | |
| RNA D | Old growth | Not harvested | Not burned |
A3. Soil Arthropod Sampling
A4. Data Analysis
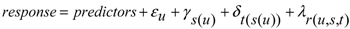
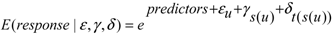
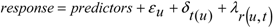
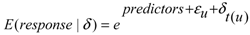
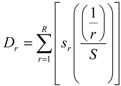
Acknowledgments
Conflict of Interest
References
- Anderson, J.M. The Organization of soil animal communities. Ecol. Bull. 1977, 25, 15–23. [Google Scholar]
- Behan-Pelletier, V.M.; Bissett, B. Oribatida of Canadian peatlands. Mem. Entomol. Soc. Can. 1994, 169, 73–88. [Google Scholar] [CrossRef]
- Hansen, R.A.; Coleman, D.C. Litter complexity and composition are determinants of the diversity and species composition of oribatid mites (Acari: Oribatida) in litterbags. Appl. Soil Ecol. 1998, 9, 17–23. [Google Scholar] [CrossRef]
- Lamoncha, K.L.; Crossley, D.A., Jr. Oribatid mite diversity along an elevation gradient in a southeastern Appalachian forest. Pedobiologia 1998, 42, 43–55. [Google Scholar]
- Crossley, D.A., Jr.; Hansen, R.A.; Lamoncha, K.L. Response of forest floor microarthropods to a forest regeneration burn at Wine Spring watershed (Southern Appalachians). In Proceedings of First Biennial North American Forest Ecology Workshop, Raleigh, NC, USA; 1999. [Google Scholar]
- Camann, M.A.; Gillette, N.E.; Lamoncha, K.L.; Mori, S.R. Response of forest soil Acari to prescribed fire following stand structure manipulation in the Southern Cascade range. Can. J. For. Res. 2008, 38, 956–968. [Google Scholar] [CrossRef]
- Crossley, D.A., Jr. Oribatid mites and nutrient cycling. In Proceedings of Symposium on the Oribatei—Eastern Branch Meeting of the Entomological Society of America, Philadelphia, PA, USA, 2 October 1975; Syracuse State University: New York, NY, USA, 1977; pp. 71–85. [Google Scholar]
- Mitchell, M.J. Energetics of oribatid mites (Acari: Cryptostigmata) in an aspen woodland soil. Pedobiologia 1979, 19, 89–98. [Google Scholar]
- Swift, M.J.; Heal, O.W.; Anderson, J.M. Decomposition in Terrestrial Ecosystems; University of California Press: Berkeley, CA, USA, 1979. [Google Scholar]
- Seastedt, T.R. The role of microarthropods in decomposition and mineralization processes. Annu. Rev. Entomol. 1984, 29, 25–46. [Google Scholar] [CrossRef]
- Harding, D.J.L. Faunal participation in the breakdown of cellophane inserts in the forest floor. In Progress in Soil Biology; North-Holland Publisher Co.: Amsterdam, The Netherlands, 1967; pp. 162–170. [Google Scholar]
- Wall, D.H.; Bradford, M.A.; St. John, M.G.; Trofymow, J.A.; Behan-Pelletier, V.; Bignell, D.E.; Dangerfield, J.M.; Parton, W.J.; Josef Rusek, J.; Voigt, W.; et al. Global decomposition experiment shows soil animal impacts on decomposition are climate-dependent. Glob. Chang. Biol. 2008, 14, 2661–2677. [Google Scholar]
- Knoepp, J.D.; Swank, W.T. Site preparation burning to improve southern Appalachian pine-hardwood stands: Nitrogen responses in soil, soil water, and streams. Can. J. For. Res. 1993, 23, 2263–2270. [Google Scholar] [CrossRef]
- Dress, W.J.; Boerner, R.E.J. Patterns of microarthropod abundance in oak-hickory forest ecosystems in relation to prescribed fire and landscape position. Pedobiologia 2004, 48, 1–8. [Google Scholar] [CrossRef]
- Knapp, E.E.; Keeley, J.E.; Ballenger, E.A.; Brennan, T.J. Fuel reduction and coarse woody debris dynamics with early season and late season prescribed fire in a Sierra Nevada mixed conifer forest. For. Ecol. Manag. 2005, 208, 383–397. [Google Scholar] [CrossRef]
- Abbott, D.T.; Seastedt, T.R.; Crossley, D.A., Jr. Abundance, distribution, and effects of clearcutting on Cryptostigmata in thesouthern Appalachians. Environ. Entomol. 1980, 9, 618–623. [Google Scholar]
- Seastedt, T.R.; Crossley, D.A., Jr. Microarthropod response following cable logging and clear-cutting in the southern Appalachians. Ecology 1981, 62, 126–135. [Google Scholar] [CrossRef]
- Blair, J.M.; Crossley, D.A., Jr. Litter decomposition, nitrogen dynamics, and litter microarthropods in a southern Appalachian hardwood forest 8 years following clearcutting. J. Appl. Ecol. 1988, 25, 683–698. [Google Scholar] [CrossRef]
- Sgardelis, S.P.; Margaris, N.S. Effects of fire on soil microarthropods of a phryganic ecosystem. Pedobiologia 1993, 37, 83–94. [Google Scholar]
- Paquin, P.; Coderre, D. Deforestation and fire impact on edaphic insect larvae and other macroarthropods. Environ. Entomol. 1997, 26, 21–30. [Google Scholar]
- Agee, J.K. Fire Ecology of Pacific Northwestern Forests; Island Press: Washington, DC, USA, 1993. [Google Scholar]
- Arno, S.F.; Fiedler, C.E. Mimicking Nature’s Fire: Restoring Fire-Prone Forests in the West; Island Press: Washington, DC, USA, 2005. [Google Scholar]
- Skinner, C.N.; Taylor, A.H. Southern Cascades bioregion. In Fire in California’s Ecosystems; Sugihara, N.G., van Wagtendonk, J.W., Fites-Kaufman, J., Shaffer, K.E., Those, A.E., Eds.; University of California Press: Berkeley, CA, USA, 2006; pp. 195–224. [Google Scholar]
- Oliver, W.W. Ecological Research at Blacks Mountain Experimental Forest in Northeastern California; USDA Forest Service General Technical Report PSW-GTR-179; USDA Forest Service Pacific Southwest Research Station: Berkeley, CA, USA, 2000. [Google Scholar]
- Peck, R.W.; Niwa, C.G. Longer-term effects of selective thinning on microarthropod communities in a late-successional coniferous forest. Environ. Entomol. 2005, 34, 646–655. [Google Scholar] [CrossRef]
- Oliver, W.W.; Powers, R.F. Blacks Mountain, California: Interdisciplinary field research in ponderosa pine. J. For. 1998, 96, 4–9. [Google Scholar]
- Barrett, J.W.; McDonald, P.M.; Ronco, F., Jr.; Ryker, R.A. Interior ponderosa pine. In Forest Cover Types of the United States and Canada; Eyre, F.H., Ed.; Society of American Foresters: Washington, DC, USA, 1980; pp. 114–115. [Google Scholar]
- McCulloch, C.E.; Searle, S.R. Generalized, Linear, and Mixed Models; John Wiley & Sons, Inc.: New York, NY, USA, 2001. [Google Scholar]
- The R Development Core Team. Available online: http://www.r-project.org/ (accessed on 17 October 2012).
- Ihaka, R.; Gentleman, R. R: A Language for data analysis and graphics. J. Comp. Graph. Stat. 1996, 5, 299–314. [Google Scholar]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S; Springer-Verlag: New York, NY, USA, 2002. [Google Scholar]
- Magurran, A.E. Ecological Diversity and Its Measurement; Princeton University Press: Princeton, NJ, USA, 1988. [Google Scholar]
- Krebs, C.J. Ecological Methodology, 2nd ed; Benjamin/Cummings: Menlo Park, CA, USA, 1999. [Google Scholar]
- Gotelli, N.J.; Colwell, R.K. Quantifying biodiversity: Procedures and pitfalls in the measurement and comparison of species richness. Ecol. Lett. 2001, 4, 397–391. [Google Scholar] [CrossRef]
- Palmer, M.W. The estimation of species richness by extrapolation. Ecology 1990, 71, 1195–1199. [Google Scholar] [CrossRef]
- Camargo, J.A. On measuring species evenness and other associated parameters of community structure. Oikos 1995, 74, 538–542. [Google Scholar] [CrossRef]
- Dufrêne, M.; Legendre, P. Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar]
- Zimmerman, G.M.; Goetz, H.; Mielke, P.W. Use of an improved statistical method for group comparisons to study effects of prairie fire. Ecology 1985, 66, 606–611. [Google Scholar] [CrossRef]
- McCune, B.; Mefford, M.J. Multivariate Analysis of Ecological Data, version 4.0; MjM Software: Gleneden Beach, OR, USA, 1999. [Google Scholar]
- Oksanen, J.; Kindt, R.; O’Hara, B. Community Ecology Package, version 1.6-9. Available online: http://www.r-project.org. (accessed on 17 October 2012).
- Venables, W.N.; Ripley, B.D. Package MASS. Available online: http://www.r-project.org. (accessed on 17 October 2012).
- Cole, L.; Bradford, M.A.; Shaw, P.J.A.; Bardgett, R.D. The abundance, richness and functional role of soil meso- and macrofauna in temperate grassland—A case study. Appl. Soil Ecol. 2006, 33, 186–198. [Google Scholar]
- Apigian, K.O.; Dahlsten, D.L.; Stephens, S.L. Fire and fire surrogate treatment effects on leaf litter arthropods in a western Sierra Nevada mixed-conifer forest. For. Ecol. Manag. 2006, 221, 110–122. [Google Scholar] [CrossRef]
- Stephens, S.L.; Fry, D.L.; Franco-Vizcaino, E.; Collins, B.M.; Moghaddas, J.M. Coarse woody debris and canopy cover in an old-growth Jeffrey pine-mixed conifer forest from the Sierra San Pedro Mártir, Mexico. For. Ecol. Manag. 2007, 240, 87–95. [Google Scholar] [CrossRef]
- Ferrenberg, S.M.; Schwilk, D.W.; Knapp, E.E.; Groth, E.; Keeley, J.E. Fire decreases arthropod abundance but increases diversity: Early and late season prescribed fire effects in a Sierra Nevada mixed-conifer forest. Fire Ecol. 2006, 2, 79–102. [Google Scholar] [CrossRef]
- Malmström, A.; Persson, T.; Ahlström, K.; Gongalsky, K.B.; Bengtsson, J. Dynamics of soil meso- and macrofauna during a 5-year period after clear-cut burning in a boreal forest. Appl. Soil Ecol. 2009, 43, 61–74. [Google Scholar] [CrossRef]
- Kim, J.W.; Jung, C. Abundance of soil microarthropods associated with forest fire severity in Samcheok, Korea. J. Asia-Pac. Entomol. 2008, 11, 77–81. [Google Scholar]
- Jung, C.; Kim, J.W.; Marquardt, T.; Kaczmarek, S. Species richness of soil gamasid mites (Acari: Mesostigmata) in fire-damaged mountain sites. J. Asia-Pac. Entomol. 2010, 13, 233–237. [Google Scholar]
- Marra, J.L.; Edmonds, R.L. Soil arthropod responses to different patch types in a mixed-conifer forest of the Sierra Nevada. For. Sci. 2005, 51, 255–265. [Google Scholar]
- Malmström, A. Temperature tolerance in soil microarthropods: Simulation of forest-fire heating in the laboratory. Pedobiologia 2008, 51, 419–426. [Google Scholar]
- Gongalsky, K.B.; Malmström, A.; Zaitsev, A.S.; Shakhab, S.V.; Bengtsson, J.; Persson, T. Do burned areas recover from inside? An experiment with soil fauna in a heterogeneous landscape. Appl. Soil Ecol. 2012, 59, 73–86. [Google Scholar] [CrossRef]
- Lindberg, N.; Bengtsson, J. Population responses of oribatid mites and collembolans after drought. Appl. Soil Ecol. 2005, 28, 163–175. [Google Scholar] [CrossRef]
- Berch, S.M.; Battigelli, J.P.; Hope, G.D. Responses of soil mesofauna communities and oribatid mite species to stie preparation treatments in high-elevation cutblocks in southern British Columbia. Pedobiologia 2007, 51, 23–32. [Google Scholar] [CrossRef]
- Hallin, W.E. The Application of Unit-Area Control in the Management of Ponderosa-Jeffrey Pine at Blacks Mountain Experimental Forest; USDA Technical Bulletin 1191; USDA: Washington, DC, USA, 1959. [Google Scholar]
- Oliver, W.W.; Ryker, R.A. Pinus ponderosa Dougl. ex Laws. In Silvics of North America, Volume 1, Conifers; Agricultural Handbook 654; Burns, R.M., Honkala, B.H., Eds.; USDA Forest Service: Washington, DC, USA, 1990; pp. 413–424. [Google Scholar]
- Alexander, E.B. Ecological Unit Inventory, Blacks Mountain Experimental Forest; Report on file at Redding Silviculture Laboratory; Pacific SW Research Station, USDA Forest Service: Redding, CA, USA, 1993. [Google Scholar]
- Anderson, J.M. Inter- and intra-habitat relationships between woodland Cryptostigmata species diversity and the diversity of soil and litter microhabitats. Oecologia 1978, 32, 341–348. [Google Scholar] [CrossRef]
- Walter, D.W.; Proctor, H.C. Mites: Ecology, Evolution, and Behavior; University of New South Wales Press: Sydney, Australia, 2004. [Google Scholar]
- Colwell, R.K. EstimateS, version 7.5; Statistical Estimation of Species Richness and Shared Species from Samples; University of Connecticut: Storrs, CT, USA. Available online: http://purl.oclc.org/estimates (accessed on 17 October 2012).
- Minchin, P.R. An evaluation of relative robustness of techniques for ecological ordinations. Vegetatio 1987, 71, 145–156. [Google Scholar]
- McCune, B.; Grace, J.B. Analysis of Ecological Communities; MjM Software: Gleneden Beach, OR, USA, 2002. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Camann, M.A.; Lamoncha, K.L.; Gillette, N.E. Oribatid Mite Community Decline Two Years after Low-Intensity Burning in the Southern Cascade Range of California, USA. Forests 2012, 3, 959-985. https://doi.org/10.3390/f3040959
Camann MA, Lamoncha KL, Gillette NE. Oribatid Mite Community Decline Two Years after Low-Intensity Burning in the Southern Cascade Range of California, USA. Forests. 2012; 3(4):959-985. https://doi.org/10.3390/f3040959
Chicago/Turabian StyleCamann, Michael A., Karen L. Lamoncha, and Nancy E. Gillette. 2012. "Oribatid Mite Community Decline Two Years after Low-Intensity Burning in the Southern Cascade Range of California, USA" Forests 3, no. 4: 959-985. https://doi.org/10.3390/f3040959




