Diversity, Vertical Stratification and Co-Occurrence Patterns of the Mycetophilid Community among Eastern Hemlock, Tsuga canadensis (L.) Carrière, in the Southern Appalachians
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites and Experimental Design
2.2. Sampling
2.3. Preserving and Identifying Specimens
2.4. Data Analysis
3. Results
3.1. Species Diversity, Abundance, and Richness
| Genus | Species | Author | Collecting Method * | Number of Specimens |
|---|---|---|---|---|
| Boletina | tricincta | Loew | B, BC, H | 23 |
| Bolitophila | cinerea | Meigen | B, BC, T | 24 |
| Bolitophila | disjuncta | Loew | B, T | 32 |
| Bolitophila | hybrida | Meigen | B, BC, H | 17 |
| Docosia | dichroa | Loew | B, T | 33 |
| Dynatosoma | fulvidum | Coquillett | B, H, T | 113 |
| Dynatosoma | placidum | Johannsen | B, BC, H | 45 |
| Lasiosoma | fasciata | Say | B, H, T | 19 |
| Leia | bivittata | Say | B, BC, T | 25 |
| Leia | decora | Loew | B, BC, H, T | 175 |
| Leptomorphus | subcaerulea | (Coquillett) | B, BC, H | 16 |
| Mycetophila | fallax | Loew | H, T | 18 |
| Mycetophila | quatuornotata | Loew | BC, T | 13 |
| Mycetophila | punctata | Meigen | BC, T | 46 |
| Mycomya | vulgaris | Garrett | B, H, T | 19 |
| Orfelia | genualis | (Johannsen) | BC, T | 39 |
| Phronia | bicolor | Dziedzicki | H, T | 45 |
| Phronia | braueri | Dziedzicki | BC, H, T | 16 |
| Phronia | cinerascens | Winnertz | B, H, T | 28 |
| Phronia | conformis | (Walker) | B, BC, H, T | 44 |
| Saigusaia | cincta | (Johannsen) | B, BC, H, T | 52 |
| Synapha | tibialis | (Coquillett) | B, BC, H, T | 118 |
| Zygomyia | ornata | Loew | B, H, T | 19 |
| Zygomyia | ignobillis | Loew | BC, H, T | 11 |
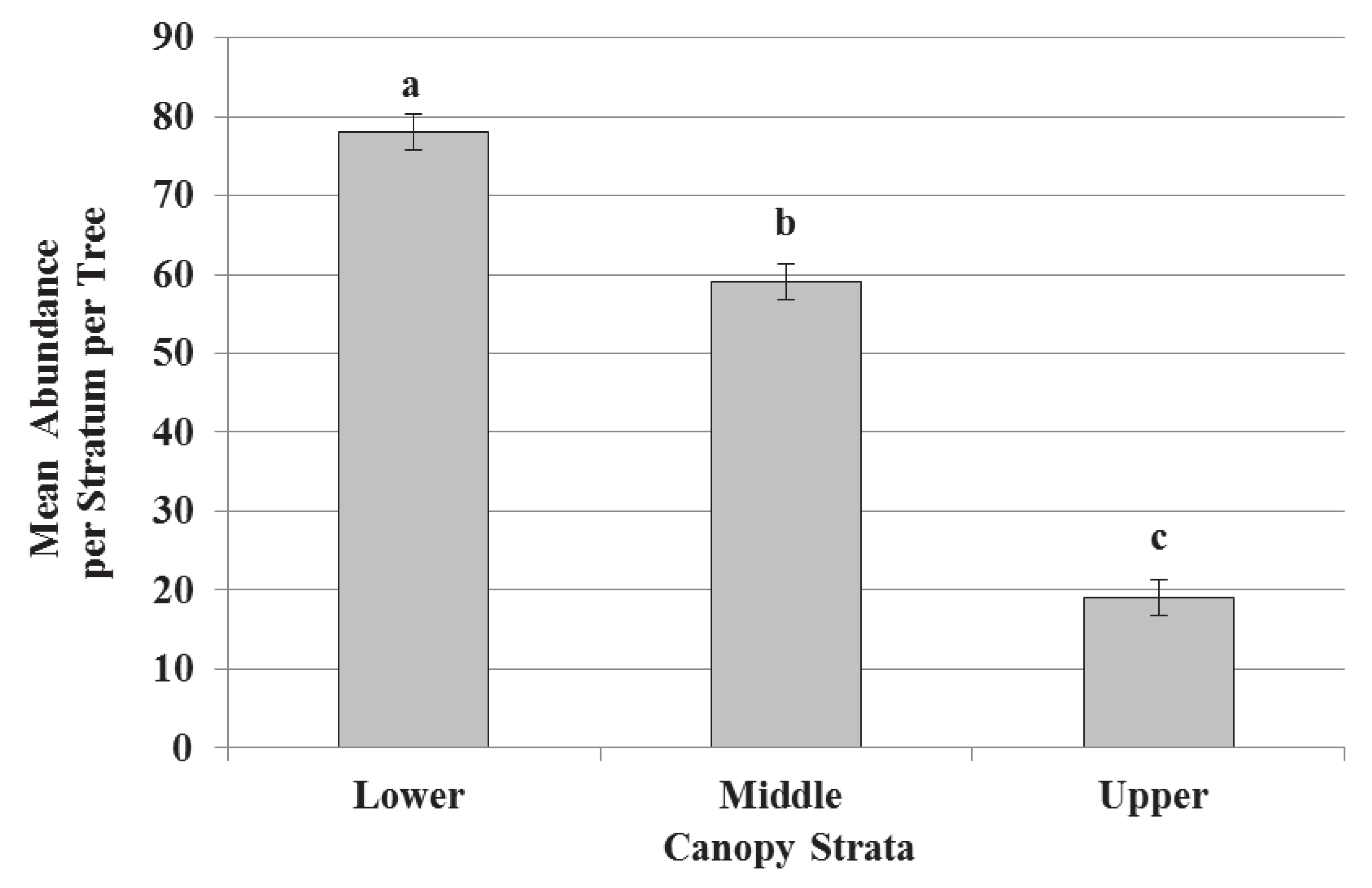
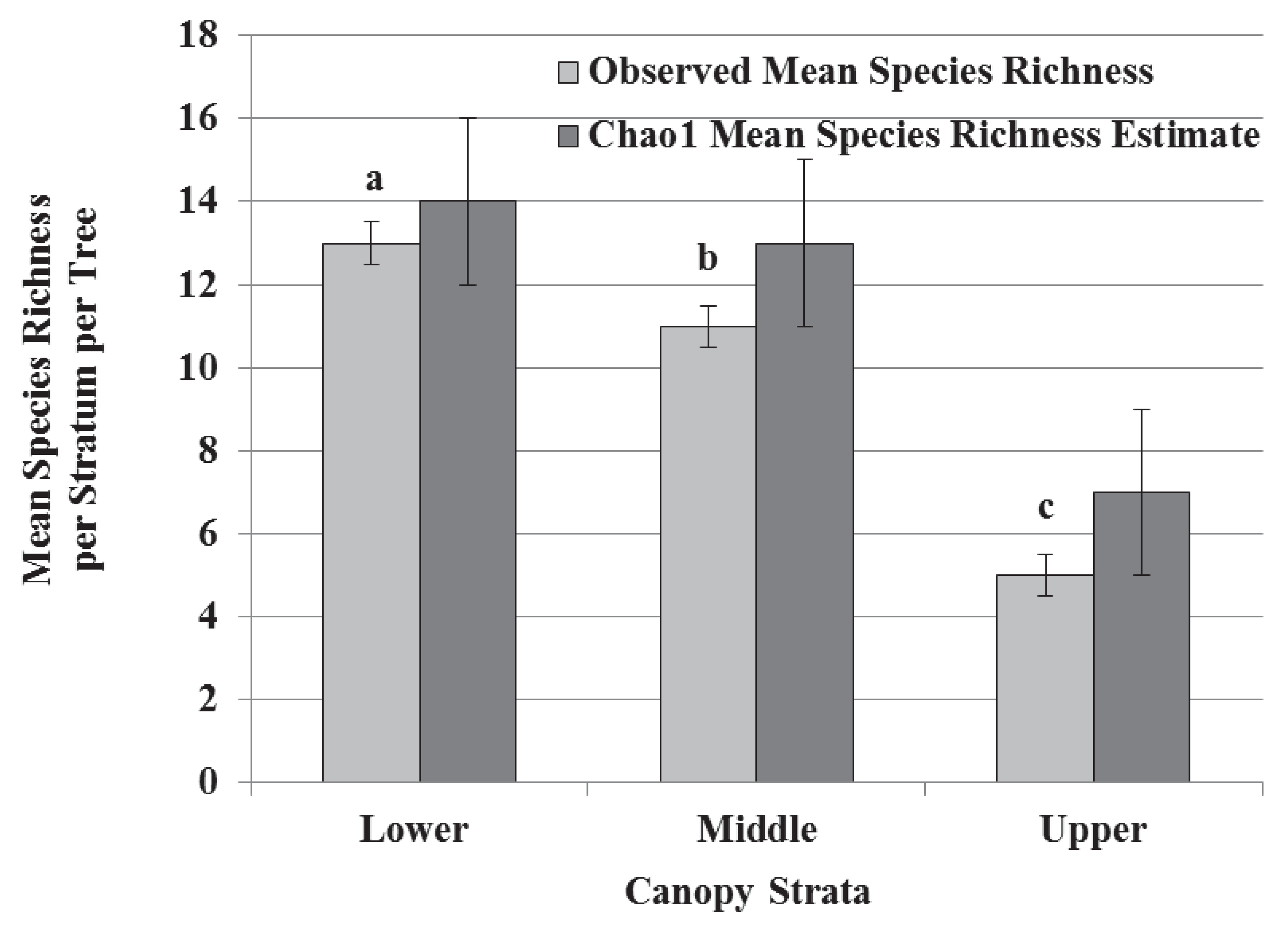
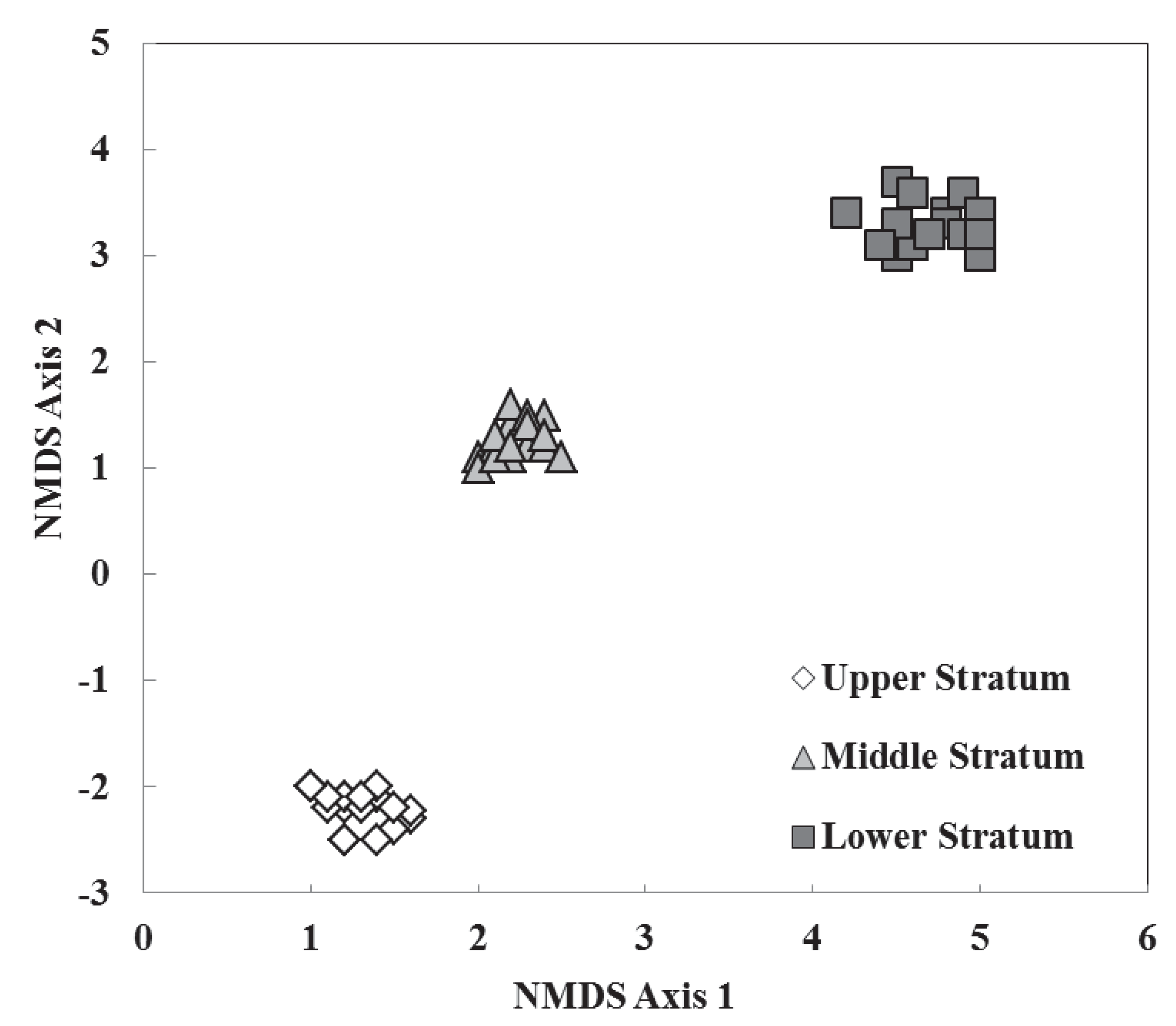
3.2. Species Composition, Vertical Stratification, and Co-Occurrence Pattern
4. Discussion
5. Conclusions
Acknowledgments
Conflict of Interest
References
- Vockeroth, J.R. Mycetophilidae. In Manual of Nearctic Diptera Volume 1; Research Branch Agriculture Canada Monograph No. 27; McAlpine, J.F., Peterson, B.V., Shewell, G.E., Teskey, H.J., Vockeroth, J.R., Wood, D.M., Eds.; Canadian Government Publishing Centre: Quebec, Canada, 1981; pp. 223–246. [Google Scholar]
- Triplehorn, C.A.; Johnson, N.F. Introduction to the Study of Insects, 7th ed; Thomson Brooks/Cole Press: Belmont, CA, USA, 2004; p. 718. [Google Scholar]
- Didham, R.K. Dipteran Tree-Crown Assemblages in a Diverse Southern Temperate Rainfores. In Canopy Arthropods; Stork, N.E., Adis, J., Didham, R.K., Eds.; Chapma and Hall: London, UK, 1997; pp. 551–561. [Google Scholar]
- Godman, R.M.; Lancaster, K. Tsuga canadensis (L.) Carr. Eastern Hemlock. In Silvics of North America: Volume 1, Conifers, Agricultural Handbook 654; Burns, R.M., Honkala, B.H., Eds.; U.S. Department of Agriculture, Forest Service: Washington, DC, USA, 1990; pp. 604–612. [Google Scholar]
- Ward, J.S.; Montgomery, M.E.; Cheah, C.A.S.-J.; Onken, B.P.; Cowles, R.S. Eastern Hemlock Forests: Guidelines to Minimize the Impacts of Hemlock Woolly Adelgid; U.S. Department of Agriculture, Forest Service: Morgantown, WV, USA, 2004; p. 27. [Google Scholar]
- Erwin, T.L. Measuring Arthropod Biodiversity in the Tropical Rainforest Canopy. In Forest Canopies; Lowman, M.D., Nadkarni, N.M., Eds.; Academic Press: San Diego, CA, USA, 1995; pp. 109–127. [Google Scholar]
- Winchester, N.N. Canopy Arthropods of Coastal Sitka Spruce Trees on Vancouver Island, British Columbia, Canada. In Canopy Arthropods; Stork, N.E., Adis, J., Didham, R.K., Eds.; Chapman and Hall: London, UK, 1997; pp. 151–168. [Google Scholar]
- Buck, S.; Lambdin, P.; Paulsen, D.; Grant, J.; Saxton, A. Checklist of insect species associated with eastern hemlock in the Great Smoky Mountains National Park and environs. Tennessee Acad. Sci. 2005, 80, 1–10. [Google Scholar]
- Dilling, C.I.; Lambdin, P.L.; Grant, J.F.; Buck, S.L. Insect guild structure associated with eastern hemlock in the southern Appalachians. Environ. Entomol. 2007, 36, 1408–1414. [Google Scholar] [CrossRef]
- Dilling, C.I.; Lambdin, P.L.; Grant, J.F.; Rhea, J.R. Community response of non-target phytophagous and transient insects associated with eastern hemlock, Tsuga canadensis (L.) Carrière, to imidacloprid and horticultural oil treatments. Environ. Entomol. 2009, 38, 53–66. [Google Scholar]
- Coots, C.I.; Lambdin, P.L.; Grant, J.F.; Rhea, J.R.; Mockford, E.L. Vertical stratification and co-occurrence patterns of the Psocoptera community associated with eastern hemlock, Tsuga canadensis (L.) Carrière, in the Southern Appalachians. Forests 2011, 3, 127–136. [Google Scholar]
- Wallace, M.S.; Hain, F.P. Field surveys and evaluation of native and established predators of the hemlock woolly adelgid (Homoptera: Adelgidae) in the southeastern United States. Environ. Entomol. 2000, 29, 638–644. [Google Scholar]
- Daubenmire, R.F. Factors favoring the persistence of a relic association of eastern hemlock in Indiana. Butler Univ. Bot. Stud. 1931, 2, 29–32. [Google Scholar]
- Friesner, R.C.; Potzger, J.E. Studies in forest ecology, II. The ecological significance of Tsuga candensis in Indiana. Butler Univ. Bot. Stud. 1932, 2, 133–149. [Google Scholar]
- Friesner, R.C.; Potzger, J.E. Climax conditions and ecological status of Pinus strobus, Taxus canadensis, and Tsuga canadensis in the Pine Hills region of Indiana. Butler Univ. Bot. Stud. 1934, 3, 65–83. [Google Scholar]
- Friesner, R.C.; Potzger, J.E. Soil moisture and the nature of the Tsuga and Tsuga—Pinus forest association in Indiana. Butler Univ. Bot. Stud. 1936, 3, 207–209. [Google Scholar]
- Friesner, R.C.; Potzger, J.E. Survival of hemlock seedlings in a relic colony under forest conditions. Butler Univ. Bot. Stud. 1944, 6, 102–115. [Google Scholar]
- Hough, A.F. Frost pockets and other microclimates in forests of the northern Allegheny Plateau. Ecology 1945, 26, 230–250. [Google Scholar]
- Moore, B.; Richards, H.M.; Gleason, H.A.; Stout, A.B. Hemlock and itsenvironment. Field records. New York Bot. Gard. Bull. 1924, 12, 325–350. [Google Scholar]
- Oosting, H.J.; Hess, D.W. Microclimate and relict stand of Tsuga canadensis in the lower Peidmont of North Carolina. Ecology 1956, 37, 28–39. [Google Scholar] [CrossRef]
- Shreve, F. Soil temperature in redwood and hemlock forests. 1927. Bull. Torrey Bot. Club 1927, 54, 649–656. [Google Scholar] [CrossRef]
- Basset, Y.; Hammond, P.M.; Barrios, H.; Holloways, J.D.; Miller, S.E. Vertical Stratification of Arthropod Assemblages. In Arthropods of Tropical Forests; Bassett, Y., Novotny, V., Miller, S.E., Kitching, R.L., Eds.; Cambridge University Press: Cambridge, UK, 2003; pp. 17–27. [Google Scholar]
- Hakeem, A. Effect of Imidacloprid on the Predatory Arthropod Guild on Eastern Hemlock, Tsuga canadensis (L.) Carrière, in the Southern Appalachians.
- Eyre, F. Forest Cover Types of the United States and Canada; Society of American Foresters: Washington, DC, USA, 1980; p. 156. [Google Scholar]
- Johannsen, O.A. The fungus gnats of North America. The Mycetophilidae of North America. Part I. Bull. ME Agric. Exp. Stn. 1909, 172, 209–276. [Google Scholar]
- Johannsen, O.A. The fungus gnats of North America. The Mycetophilidae of North America. Part II. Bull. Me Agric. Exp. Stn. 1910, 180, 125–192. [Google Scholar]
- Johannsen, O.A. The fungus gnats of North America. The Mycetophilidae of North America. Part III. Bull. ME Agric. Exp. Stn. 1911, 196, 249–328. [Google Scholar]
- Johannsen, O.A. The fungus gnats of North America. The Mycetophilidae of North America. Part IV. Bull. ME Agric. Exp. Stn. 1912, 200, 57–146. [Google Scholar]
- SAS Institute, SAS User’s Guide: Statistics, Version 9.1; SAS Institute: Cary, NC, USA, 2005; p. 201.
- Colwell, R.K. EstimateS: Statistical Estimation of Species Richness and Shared Species from Samples, Version 5; User’s Guide and Application. Available online: http://viceroy.eeb.uconn.edu/estimates (accessed on 31 May 2012).
- Clarke, K.R.; Gorley, R.N. PRIMER Version 6: User Manual/Tutorial; PRIMER-E: Plymouth, UK, 2006; p. 156. [Google Scholar]
- Stone, L.; Roberts, A. The checkerboard score and species distributions. Oecologia 1990, 85, 74–79. [Google Scholar] [CrossRef]
- Gotelli, N. Null model analysis of species co-occurrence patterns. Ecology 2000, 81, 2606–2621. [Google Scholar] [CrossRef]
- Gotelli, N.J.; Entsminger, G.L. Swap and fill algorithms in null model analysis: Rethinking the Knight’s Tour. Oecologia 2001, 129, 281–291. [Google Scholar] [CrossRef]
- Ulyshen, M.D. Arthropod vertical stratification in temperate deciduous forests: Implications for conservation management. For. Ecol. Manag. 2011, 261, 1479–1489. [Google Scholar] [CrossRef]
- Gotelli, N.J.; McGill, B.J. Null versus neutral models: What’s the difference? Ecography 2006, 29, 793–800. [Google Scholar] [CrossRef]
- Gotelli, N.J.; McCabe, D.J. Species co-occurrence: A meta-analysis of J. M. Diamond’s assembly rules. Ecology 2002, 83, 2091–2096. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Coots, C.; Lambdin, P.; Grant, J.; Rhea, R. Diversity, Vertical Stratification and Co-Occurrence Patterns of the Mycetophilid Community among Eastern Hemlock, Tsuga canadensis (L.) Carrière, in the Southern Appalachians. Forests 2012, 3, 986-996. https://doi.org/10.3390/f3040986
Coots C, Lambdin P, Grant J, Rhea R. Diversity, Vertical Stratification and Co-Occurrence Patterns of the Mycetophilid Community among Eastern Hemlock, Tsuga canadensis (L.) Carrière, in the Southern Appalachians. Forests. 2012; 3(4):986-996. https://doi.org/10.3390/f3040986
Chicago/Turabian StyleCoots, Carla, Paris Lambdin, Jerome Grant, and Rusty Rhea. 2012. "Diversity, Vertical Stratification and Co-Occurrence Patterns of the Mycetophilid Community among Eastern Hemlock, Tsuga canadensis (L.) Carrière, in the Southern Appalachians" Forests 3, no. 4: 986-996. https://doi.org/10.3390/f3040986
APA StyleCoots, C., Lambdin, P., Grant, J., & Rhea, R. (2012). Diversity, Vertical Stratification and Co-Occurrence Patterns of the Mycetophilid Community among Eastern Hemlock, Tsuga canadensis (L.) Carrière, in the Southern Appalachians. Forests, 3(4), 986-996. https://doi.org/10.3390/f3040986




