1. Introduction
According to FAO, approximately 400 million ha are affected by sodic or saline soils [
1]. Among the recommendations for applied research by an FAO expert committee were development of salt-tolerant crop varieties and the use of
Salicornia,
Atriplex,
Salvadora and
Prosopis as a good alternative in salt-affected areas [
2]. Nitrogen fixing trees of the genus
Prosopis show promise for the rehabilitation of saline soils in subtropical regions because of their high salt tolerance and because they have economically useful products that can provide the economic incentive to drive the restoration of saline lands. The value of
Prosopis is illustrated by the fact that in Argentina more than 100,000 tons of
Prosopis logs were harvested annually and processed for furniture, flooring, door, window and shutter fabrication prior to about 2003 [
3]. In 2004, the Argentinean Secretariat of the Environment and Sustainable Development, which includes the Forestry Department, issued a report which decried the deforestation and degradation of Argentine native forests, and in particular forests in the Chaco ecological zone where
Prosopis alba (Griseb.) is located [
4]. Several of the largest
P. alba lumber, flooring, and furniture companies have ceased operations due to the severity of this degradation [
5]. Flour from the milled pods is also being developed for human food uses [
6]. In the USA there are numerous markets that offer mesquite (
Prosopis glandulosa Torr. or
P. velutina Woot.) lumber at prices greater than US$ 2,000 m−3. Felker and Guevara [
3] calculated that a sawn lumber price of US$ 800 m−3 will be required to achieve an internal rate of return of 11% for well managed plantations of genetically unimproved stock. Further, plantations on moderately saline soils (
Various studies have reported high salinity tolerance for different
Prosopis species [
8,
9,
10,
11,
12]. A recent study compared the survival and growth of
Prosopispallida (Humb. & Bonpl. ex Willd.) Kunth from Peru and
P. alba from Argentina in a greenhouse setting in which the salinity was increased from 10 to 45 dS m−1 over a period of four months and maintained at 45 dS m−1 for an additional two months [
13].
From the more than 2,000 seedlings from 25 half-sib families in this hydroponic selection system that evaluated a broad based collection of African, Peruvian, Chilean and Argentinean
Prosopis [
13], 21
P. alba seedlings, from nine Argentine
P. alba half-sib families that grew in the highest salinity treatment (45 dS m−1) were repotted and clonally propagated to establish a clonal seed orchard on the grounds of the Universidad Catolica de Santiago del Estero field station in Fernandez, Argentina. None of the Peruvian accessions in this trial with high salt tolerances were evaluated due to their lack of cold hardiness in Argentina. The soil was slightly saline (EC 5.15 to 7.3 dS m−1) but it had pH values ranging from 8.9 to 10.2. These pH values are far more alkaline than a pH value of 8.0 where P, Mn, B, Cu and Zn availability begins to severely decline [
14].
To capitalize on the high salt tolerance of these seedlings, it will be necessary to multiply them asexually. While rapid and reliable propagation by mini-grafting is possible [
15], this will not be useful since the rootstocks would come from genetic stock with unknown salt tolerance.
Interspecific hybridization frequently occurs among
Prosopis species [
16].
In spite of the fact that specimens were selected of
P. alba mother trees in accordance with Burkart [
17], great segregation in morphological characters was observed in the progeny. As described in other studies [
18], some clones have finely divided leaflets typical of the
P.alba mother tree while other clones have much larger and more widely spaced leaflets typical of
P. vinallilo (little vinal). Some taxonomists have suggested
P. vinallilo could be a hybrid between
P.alba and
P.ruscifolia [
17]. The weedy
P.ruscifolia, with its 15 cm long thorns, is more common in the area where the
P.alba was collected.
The main objective of this study was to determine if the greenhouse hydroponic screening assay was a useful genotype selection tool. Thus regressions between height growth in the greenhouse versus biomass estimates for five year old trees in the field were examined. Additionally it was important to examine these clones in a multi-year field trial for other positive and negative characteristics that could not have arisen in the highly controlled greenhouse environment. An unexpected result that required analyses/interpretation was the presence of interspecific hybrid progeny possessing superior tolerance to soils with pH 10.3.
2. Methodology
The previous hydroponic system evaluated 25 accessions of
Prosopis from Peru (
P. pallida), West Africa (
P.juliflora), Chile (
P. alba/nigra) and Argentina (
P. alba,
P. flexuosa,
P. ruscifolia) in a randomized complete block design (RCBD) with four replicates. The Peruvian
P. pallida species are not adaptable to Argentina due to lack of cold hardiness and
P. alba is more amenable to lumber production than the
P. flexuosa and
P. ruscifolia evaluated in the hydroponic system. Thus for the clonal seed orchard described in this communication, we only selected clones from nine open pollinated half-sibling families of
P. alba designated O1 to O9 that originated from a highly saline area (floodplain of the Rio Saladillo in Province of Santiago del Estero, Argentina 28°52’36.85”S: 63°58’46.36”W). An example of one of these
P. alba trees near a home is presented in Figure 1. These mother trees fell within Burkart’s [
16] delineation of
P.alba. A flow diagram of the genetic selection process is illustrated in Figure 2.
Each replicate in the hydroponic screening trial consisted of a row of 16 seedlings. The codes for the clones are as previously described [
12], e.g.,
O7B4P6(V) is half-sib family O7, block 4 and plant 6 of 16, and V to indicate a morphology similar to
P. vinalillo. Due to lack of growth, no plants were selected from family O1 or O5 and only one plant was selected from family O2, O4, and O9. After the seedlings in the previous trial were kept for about four weeks at each of the 10, 25, 35 and 45 dS m−1 salinities [
13], they were subjected to two additional months in the same 45 dS m−1 experimental condition. Those plants showing a very healthy appearance and/or a new live apical meristem were chosen for further experimentation. Overall plant growth was limited under this salinity level due to the small size of the root cell volume (50 cm tall plants in five cm deep cavity) that made them root bound. Only 21 of the 576 (3.6%) best plants from the
P.alba replicated portion of this trial were selected for further evaluation and asexually propagated by rooting cuttings using bottom heat, water soluble salts of plant hormones and
Agrobacteriumrhizogenes that can insert the indoleacetic acid synthesizing gene into plant roots and stimulate rooting [
19]. The three check varieties in this field trial were seedlings originated form “plus trees”, located near the Forestry Experiment Station of the Universidad Catolica de Santiago del Estero, in Fernandez, Argentina, that were used for seed for the one million seedlings produced per year for sale to establish plantations.
Figure 1.
Figure 1. One of the P. alba mother trees (O7) collected near a home in the highly saline floodplain of the Rio Saladillo, Province of Santiago del Estero, Argentina whose seeds were used in the hydroponic salinity selection system in the greenhouse.
Figure 1.
Figure 1. One of the P. alba mother trees (O7) collected near a home in the highly saline floodplain of the Rio Saladillo, Province of Santiago del Estero, Argentina whose seeds were used in the hydroponic salinity selection system in the greenhouse.
Figure 2.
Figure 2. Flow diagram from the original collection of seed from trees in the saline areas of the Rio Saladillo, Argentina, to establishment of 21 clones in five replicates on high pH soils in Fernandez, Argentina.
Figure 2.
Figure 2. Flow diagram from the original collection of seed from trees in the saline areas of the Rio Saladillo, Argentina, to establishment of 21 clones in five replicates on high pH soils in Fernandez, Argentina.
Some of the progeny had intermediate leaf and thorn morphology between the
P. alba female parent and a putative unknown
P. ruscifolia male parent that is much more abundant in this region than
P. alba and that only grows next to homes where this highly palatable species can be protected from herbivores. The seedlings with intermediate leaf morphology were presumed to be
P. vinalillo and were designated by a V after the codes. The great differences in leaf/thorn morphologies of these three “species” can be seen in
Figure 3.
Figure 3.
Figure 3. Leaf, thorn and inflorescence morphology of thornless P. alba from seed (left), thornless putative P. vinalillo (O7B4P6V) with larger and more widely spaced leaflets (center) and putative P. ruscifolia (vinal) (right). Note that there are no thorns on these P. alba or putative P. vinallilo but long thorns on the putative P. ruscifolia. The ruler is in cm.
Figure 3.
Figure 3. Leaf, thorn and inflorescence morphology of thornless P. alba from seed (left), thornless putative P. vinalillo (O7B4P6V) with larger and more widely spaced leaflets (center) and putative P. ruscifolia (vinal) (right). Note that there are no thorns on these P. alba or putative P. vinallilo but long thorns on the putative P. ruscifolia. The ruler is in cm.
Due to the small size of these plants, only enough rooted cuttings were available to establish five single tree replications in a RCBD at the Universidad Catolica de Santiago del Estero Forestry Experiment Station in Fernandez, Argentina. This planting served as a clonal seed orchard, clonal evaluation trial and source of material for full sib crosses. This trial was established on 13 January 2004 (summer rainy season in Argentina) and five years after the 2–3 mm diameter rooted cuttings were planted. On 25 August 2009, measurements of height and diameter were made. Biomass estimates were obtained using a previously published model [
7]: log(fresh wt) = −1.1085 + 2.7027 × log(diameter), where fresh weight and diameter are in kg and cm, respectively. After planting the trees were hand watered. The plants were flood irrigated every winter with 100 mm of water. The rainfall measured at the Research station in Fernandez was 709 mm in 2004, 402 mm in 2005, 750 mm in 2006, 674 mm in 2007, 678 mm in 2008 and 602 mm in 2009. Each plant was protected from rabbit damage by placing spiny branches around the plants. For the first three years, the insecticides cypermethrin and Lambda-cyhalothrin were used three times in the summer at rates on the label to control psyllids that are the leaf sucking insects. Weeds were controlled by mowing, hoeing, and spraying 1.5% glyphosate at the base of the trees. Samples for soil pH and salinity were taken at the 0–30 and 60–90 cm depths in each of the five blocks in August 2009. Soil samples were taken from various locations in the block and pooled for a single sample per depth per block. Pruning of the trees began in 2005.
The Instituto Nacional de Tecnologia Agricultura (INTA) (Argentine federal government agricultural service) in Santiago del Estero, Argentina measured the electrical conductivity on a suspension of 10 g of dry soil and 25 mL of water and the pH was measured on a saturated paste using the method of Richards [
20].
In plant breeding, probably the two most important breeding factors, besides the genetic control (i.e., that a trait is heritable) are: (a) a high yield of the economic trait of interest (wood, grain, etc.) and (b) uniformity to facilitate harvest and to maintain product quality standards in the market. It is possible to have two families or clones with the same mean economic yield but that greatly differ in uniformity. For example clone A could have moderate photosynthetic assimilation over all blocks but not be susceptible to low yields in some blocks due to an unfavorable edaphic factor, i.e., low P, pH, water logging, etc., while clone B could have higher photosynthetic assimilation than clone A on favorable blocks but lower yields on blocks with unfavorable edaphic factors. Uniformity is typically measured by the variance or standard error of the mean value (i.e., SEM) for a given genotype, or equivalently by obtaining confidence intervals (CIs). Here, it is desirable to have the smallest SEMs values or range of CIs.
Thus, in this study, as a first stage, a linear mixed model was fitted for the responses height, diameter and biomass, based on the following linear model:
yij = µ + Blocki + Clonej + eij
where
µ corresponds to the overall mean,
Blocki to the fixed effect of block,
Clonej to the fixed effect of clone, and
eij is the residual term for the observation on the
ith block
jth clone, with
eij ~ N(0,σ
j 2). In addition, a different residual variance was estimated for each of the clones. A likelihood ratio test [
21] was used to test for this homogeneity of variances. Means and SEMs were predicted for each of the genotypes and 95% confidence intervals were obtained based on the means of the five blocks.
In order to estimate genetic variance components, the above model was re-fitted assuming the Clone term as a random effect, with Clonej ~ N(0, σclone 2) . The expression to estimate broad-sense (clonal mean) heritability, H2, corresponded to:
where
![Forests 03 00095 i001]()
is the variance component associated with the clones and
![Forests 03 00095 i002]()
is the mean residual variance obtained over all clones, and an approximate standard error was obtained using the delta method. All models were fitted using the software ASReml v. 3.0 [
21].
3. Results
The soil pH and salinity of this trial are shown in Table 1 where it can be seen that the salinity was far less than the 45 dS m−1 level that was used to select the initial seedlings. In contrast, the pH values were extreme, ranging from 8.9 in block 1 to 10.2 in block 5. The pH values for the 60–90 cm depth ranged from 0.9 to 0.2 pH units lower than the surface 0–30 cm. Due to the gradient in soil pH, the mean height, diameter and biomass of all blocks in addition to the values for block 5 which had the most extreme pH values is presented.
Table 1.
Table 1. Soil electrical conductivity and pH values for each block and their average in the field trial of P. alba clones selected for growth at high salinities.
Table 1.
Table 1. Soil electrical conductivity and pH values for each block and their average in the field trial of P. alba clones selected for growth at high salinities.
| Depth (cm) | Block 1 | Block 2 | Block 3 | Block 4 | Block 5 |
|---|
| Conductivity dS m−1 |
| 0–30 | 6.90 | 7.45 | 7.10 | 7.30 | 5.15 |
| 60–90 | 5.75 | 6.45 | 11.35 | 5.85 | 10.10 |
| Average | 6.33 | 6.95 | 9.23 | 6.58 | 7.63 |
| pH |
| 0–30 | 8.90 | 9.10 | 9.70 | 10.11 | 10.20 |
| 60–90 | 9.80 | 9.90 | 10.20 | 10.30 | 10.40 |
| Average | 9.35 | 9.50 | 9.95 | 10.21 | 10.30 |
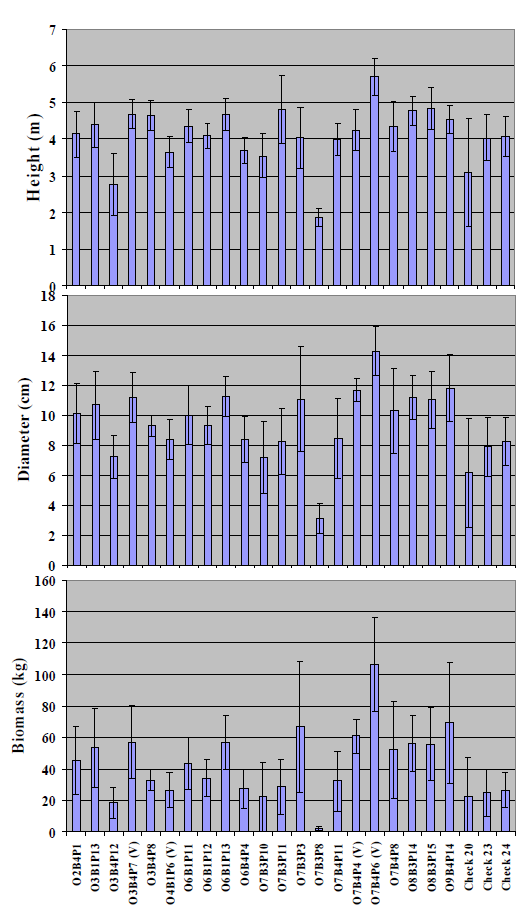
Figure 4 presents the mean diameter, biomass and height growth values for all five blocks per genotype. Since biomass is exponentially related to diameter, greater differences among the clones are seen in biomass than diameter or height. In relation to the overall mean values (
Figure 4), almost all of the clones had a mean height greater than 4 m, with the exception of one clone that was more than 5 m tall, which is exceptional at this high pH. One important observation is that the variability in biomass resulting from progeny of the same mother tree (within O3, O7,
etc. ) is apparently large. As observed by the individual 95% confidence intervals (as discussed below), there were 10 clones
i.e., O2B4P1, O3B1P13, O3B4P7(V), O6B1P13, O7B3P3, O7B4P4(V), 07B4P6(V), O8B3P14, O8B3P15 and O9B4P14 that had significantly greater biomass than the three check varieties. At the end of five years, the most rapidly growing clone O7B4P6(V) had a biomass of 106 kg per tree, which was more than four times the mean of the three checks,
i.e., 24.4 kg.
In comparing the mean values for the overall diameter, height and biomass (
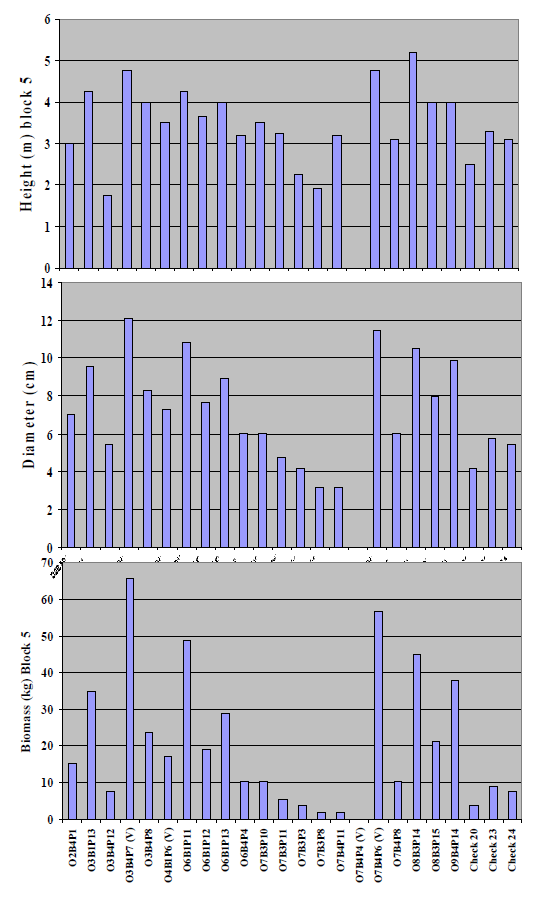
Figure 4) to the individual values for block 5 (
Figure 5), where the most extreme pH values occurred, the comparative performance of two putative
P. vinallilo (
i.e., O3B4P17(V) and O7B4P6(V)), and the
P.alba clones O6B1P11, O8B3P14 and O9B4P14 over the three
P. alba check half-sib families was especially notable.
Figure 4.
Figure 4. Means of five blocks for height (m), basal diameter (cm), and biomass (kg) of 21 clones from P. alba parents selected for growth in a greenhouse hydroponic system at 45 dS m−1 electrical conductivities. Note the clones are arranged by families O2, O3, etc. Biomass values were estimated with regression equations as described in the text. The error bars are 95% confidence intervals.
Figure 4.
Figure 4. Means of five blocks for height (m), basal diameter (cm), and biomass (kg) of 21 clones from P. alba parents selected for growth in a greenhouse hydroponic system at 45 dS m−1 electrical conductivities. Note the clones are arranged by families O2, O3, etc. Biomass values were estimated with regression equations as described in the text. The error bars are 95% confidence intervals.
Figure 5.
Figure 5. Individual values for block 5, which had most extreme pH value (i.e.,average 10.3), for height (m), basal diameter (cm), and biomass (kg) of 21 clones from P. alba parents selected for growth in a greenhouse hydroponic system at 45 dS m−1 electrical conductivities. Biomass values were estimated with regression equations as described in the text.
Figure 5.
Figure 5. Individual values for block 5, which had most extreme pH value (i.e.,average 10.3), for height (m), basal diameter (cm), and biomass (kg) of 21 clones from P. alba parents selected for growth in a greenhouse hydroponic system at 45 dS m−1 electrical conductivities. Biomass values were estimated with regression equations as described in the text.
Table 2 shows the
p-values of evaluating the significance of clonal differences together with the observed broad-sense heritability. For all traits analyzed, there are significant differences among the clones, and the amount of genetic control of these traits, as indicated by the heritability is moderate, where the largest values correspond to total height. Therefore, relevant genetic gains can be obtained by selecting and vegetatively propagating the best genotypes.
Table 2.
Table 2. P-values of evaluating the significance of fixed clonal effects and broad-sense heritability for biomass, diameter and height for 21 clones from P. alba mother trees tested on an alkaline site in Argentina. These were obtained from two different fitted linear models. Standard errors are presented in parenthesis.
Table 2.
Table 2. P-values of evaluating the significance of fixed clonal effects and broad-sense heritability for biomass, diameter and height for 21 clones from P. alba mother trees tested on an alkaline site in Argentina. These were obtained from two different fitted linear models. Standard errors are presented in parenthesis.
| Biomass | Diameter | Height |
|---|
| Clone | 0.030 | |
In order to study further the uniformity, and therefore variability, of the different clones, a simple linear regression was obtained between the estimated residual variance, as a response, and the predicted mean, as an explanatory variable. This was done for each of the traits using all 21 clones.
No significant relationships, which were tested by fitting a simple linear regression, were obtained for total height and diameter, with correlations of −0.089 and −0.051, respectively. However, a significant association (
p = 0.003), was detected for biomass with a correlation of 0.587. This result, when all clones are pooled, indicates that as the mean biomass of a given clone gets larger an increase on the variability is observed. Similar results have been reported in other biological situations [
22]. The model fitted in this case was (SEM clonal Biomass) = 3.42 + 0.1272 × (mean clonal Biomass).
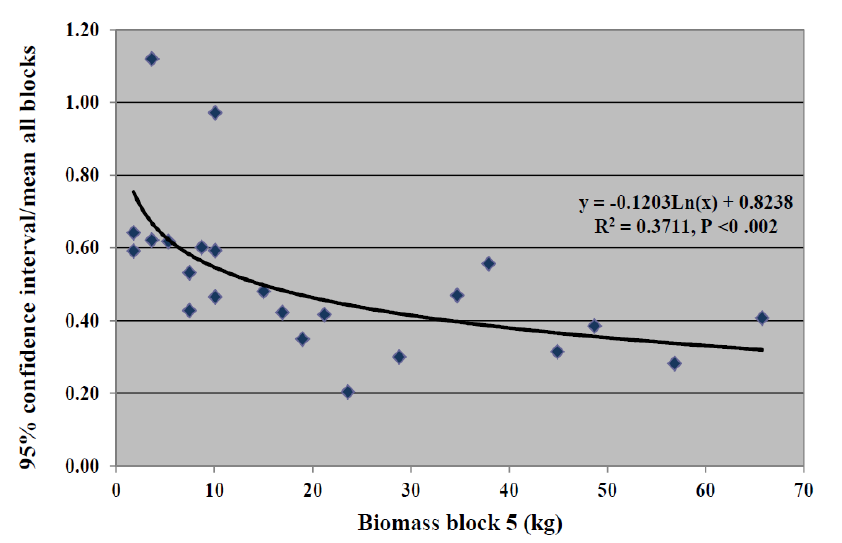
With regard to evaluating clones for uniformity of response on sites with contrasting edaphic factors, it was hypothesized that if a clone existed that did not decrease in biomass on block 5 with the greatest edaphic stress, then it would be expected to have a lower coefficient of variation. To test this, we have plotted the 95% confidence intervals (for the five blocks for each clone), divided by the mean of the five blocks, against the mean biomass of block 5, as shown in
Figure 6 below.
Figure 6 shows that clones, which had the greatest biomass in the block with the most adverse edaphic conditions, also had the smallest confidence interval equivalent of the coefficient of variation. This significant relationship (
pFigure 4, had a lower SE than other clones with values around 60 kg because this clone was not depressed as much in yield in block 5 as the others.
Figure 6 suggests that several clones, such as O7B4P6(V), will not only have greater biomass than other clones but also greater uniformity.
Figure 6.
Figure 6. Growth of P. alba and putative P. vinallilo clones in block 5 with highest soil pH of 10.3 versus 95% confidence intervals/mean biomass of all five blocks.
Figure 6.
Figure 6. Growth of P. alba and putative P. vinallilo clones in block 5 with highest soil pH of 10.3 versus 95% confidence intervals/mean biomass of all five blocks.
Regardless of the interpretation whether or not the variances can be pooled, the next step, as described below, would seem to be to make crosses between the high biomass producing putative P. vinallilo clone (O7B4P6V) and P.alba clones to examine segregation ratios and recombination frequencies.
Simple linear regressions were examined between the growth in greenhouse salinity selection system to five year old growth in the field. For
P. alba trees over all five blocks this correlation had an R2 of 0.108 and for
P.vinallilo the correlationwas 0.445. This information is presented graphically in Figure 7 for block 5 that had the most severe soil pH. For
P.alba, there was a weak positive correlation (R2 = 0.262) that for 17 values would be significant at a level of 5%. In contrast, for putative
P. vinallilo, there was a negative correlation with an R2 of 0.938 that is significant. However, with only four values for the putative
P. vinallilo the negative correlation between growth at high salinity in the greenhouse, and high pH/moderate salinity in the field is not very clarifying. There is a possibility that selection at high salinity may be useful for selection at high pH values. At least the four
P. alba clones with the greatest growth at pH 10 were also the ones with the greatest growth at the high salinity values,
i.e., selection of
P. alba clones with >3 cm growth in the sand culture identified the
P. alba with the greatest growth in the field. What is not presented here are the growth of the other 97% of the trees that had severe dieback, dead apical meristems, or were entirely dead and thus not evaluated in this field trial [
13]. Given the several fold increase in biomass of some of the clones over the three check varieties for the means of all five blocks, and the even larger increase in biomass over the checks in block 5, it is clear that the greenhouse screen was successful in identifying superior salt tolerant clones. Apparently whether the greenhouse seedlings had, 1, 2 or 3 cm growth was not as important as just having a healthy live apical meristem.
Figure 7.
Figure 7. Regressions between Prosopis biomass at the end of five years growth in field on high pH soils for block 5 versus growth and the height growth (in cm) for the last two months in a greenhouse hydroponic system at 45 dS m−1.
Figure 7.
Figure 7. Regressions between Prosopis biomass at the end of five years growth in field on high pH soils for block 5 versus growth and the height growth (in cm) for the last two months in a greenhouse hydroponic system at 45 dS m−1.
As described earlier there was great variability among the clones for ability to produce roots from cuttings [
18]. Fortunately, some of the highest biomass producing clones also had high rooting percentages. For example, in block 5 the biomass and rooting percentage for the test clones were: O3B1P13 (35 kg/100%), O3B4P7(V) (66 kg/40%), O6B1P11 (49 kg/60%), O6B1P13 (29 kg/100%), O7B4P6(V) (57 kg/100%), O8B3P14 (45 kg/100%) and O9B4P14 (38 kg/60%).
Five of the clones (O3B4P7(V), O6B1P11, 07B4P6(V), O8B3P14 and O9B4P14) in the high pH block 5 had diameters of approximately 10 cm or greater. The largest diameters were obtained by the putative
P. vinallilo clones
i.e., 11 cm for O7B4P6(V) and 12 cm for O3B4P7(V). This corresponds to an annual increment diameter growth of about 2 cm, for these trees that were planted five years earlier as several mm diameter rooted cuttings. Felker and Guevara [
2] examined the rates of internal returns for
Prosopis plantations as a function of level of genetic improvement and management in which they assumed that trees would be harvested when they attained 40 cm in diameter at 10 × 10 m spacing. The diameter growth of 2 cm year−1 reported in this paper corresponded to a rotation age of 20 years. For the Argentina scenario, assuming a wholesale price of US$ 800 m−3 for the sawn lumber, Felker and Guevara [
3] calculated an internal rate of return of 11.8% for a 24 year rotation and 19.8% for a 17 year rotation. Thus, the diameter growth reported in this paper would correspond to an internal rate of return of about 15%.



 is the variance component associated with the clones and
is the variance component associated with the clones and  is the mean residual variance obtained over all clones, and an approximate standard error was obtained using the delta method. All models were fitted using the software ASReml v. 3.0 [21].
is the mean residual variance obtained over all clones, and an approximate standard error was obtained using the delta method. All models were fitted using the software ASReml v. 3.0 [21].