Post-Fire Resprouting in New Zealand Woody Vegetation: Implications for Restoration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Resprouting Review
2.2. Field Study Site
2.3. Sampling and Measurements
2.4. Data Analysis
3. Results
3.1. Resprouting Review
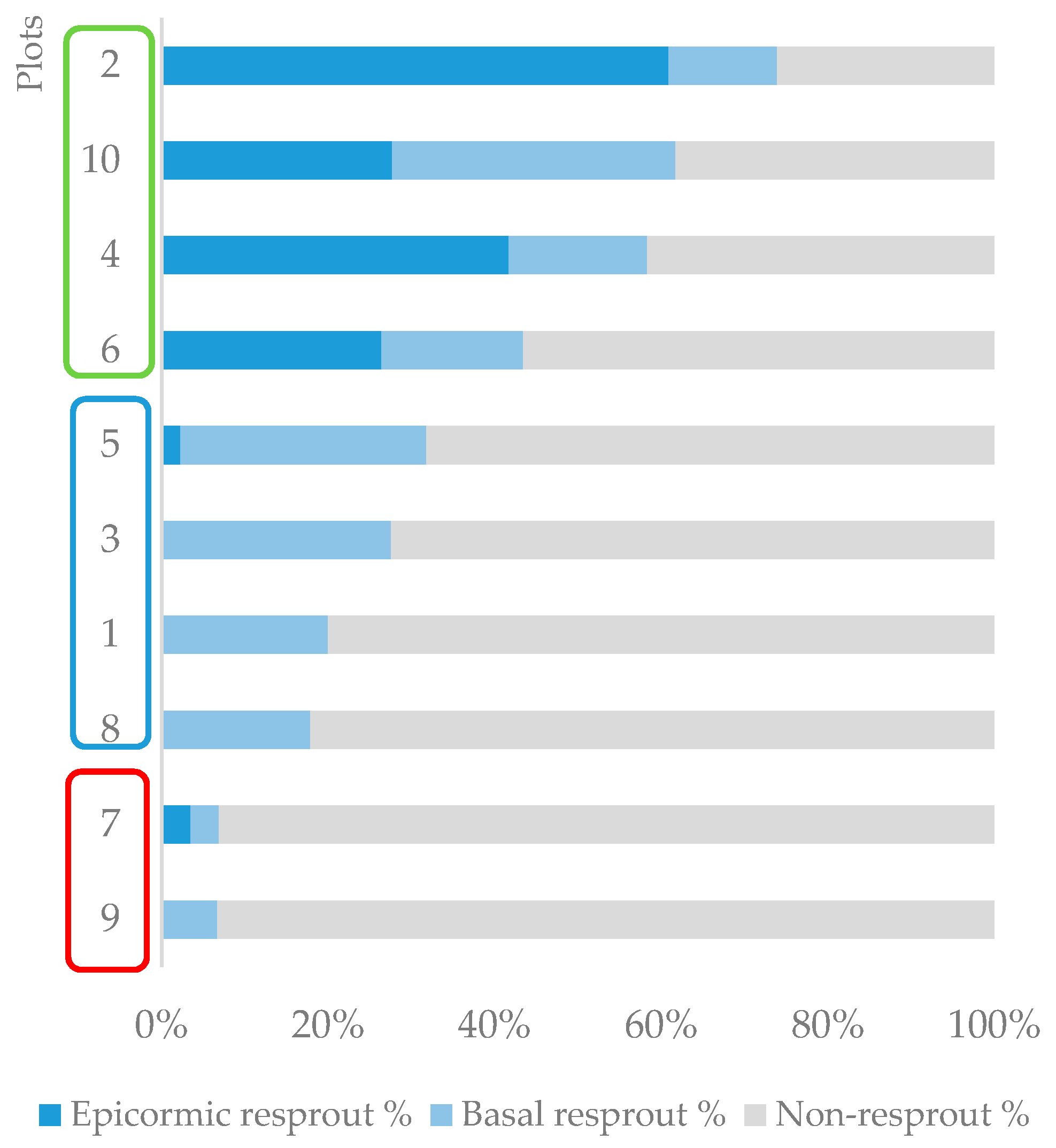
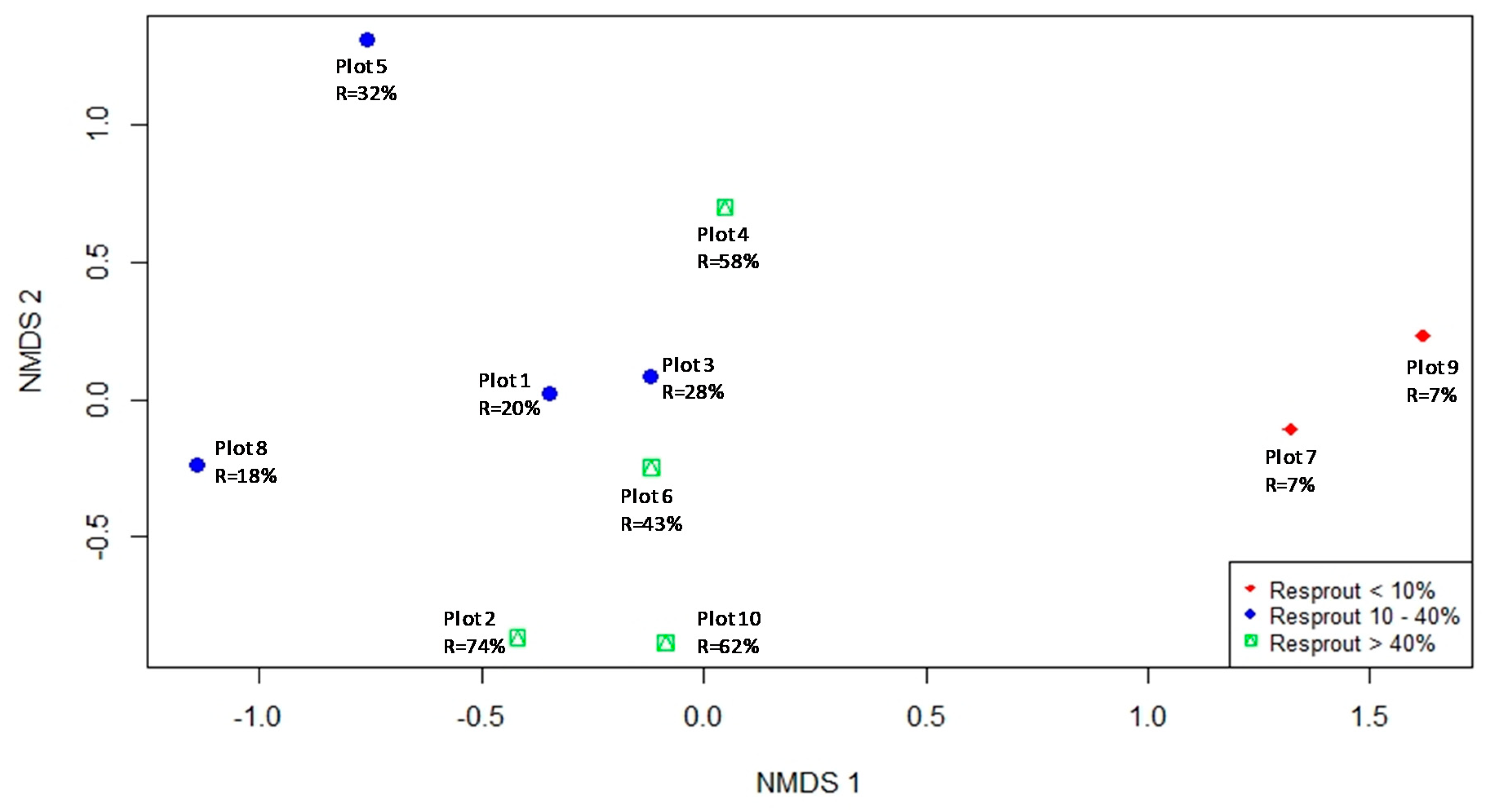
3.2. Field Study—Species’ Responses
3.3. Field Study—Community Response
4. Discussion
4.1. Resprouting Review
4.2. Species Response
4.3. Community Response
4.4. Implications for Restoration
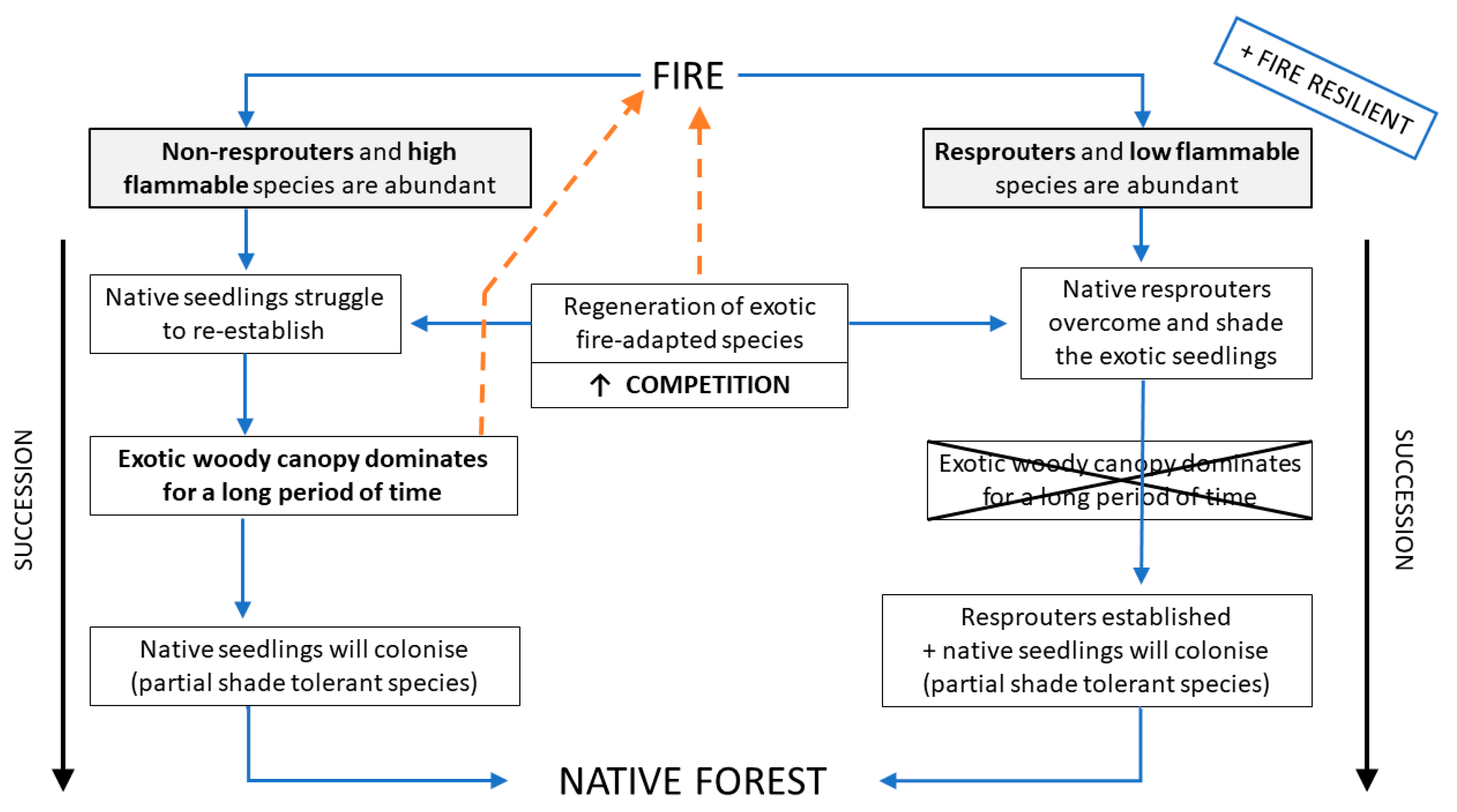
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Family | Species | Life-Form | Burrows (1994) [9] | Wabing (2014) [46] | Glogoski (2017) [70] | Others | Current Research | Comments |
|---|---|---|---|---|---|---|---|---|
| Araliaceae | Meryta sinclairii (Hook.f.) Seem | tree | No [71] | Appears to lack the capacity to resprout after damage [71]. | ||||
| Araliaceae | Pseudopanax arboreus (L.f.) Allan | tree | 100% (n = 7) | Yes [14] | 5% (n = 43) | |||
| Araliaceae | Pseudopanax crassifolius (Sol. ex A.Cunn.) C.Koch | tree | Yes | Yes [14] | No (n = 1) | |||
| Araliaceae | Schefflera digitata J.R.Forst. et G.Forst. | tree | Yes | 100% (n = 2) | Yes (n = 2) | |||
| Araucariaceae | Agathis australis (D.Don) Lindl. | tree | Yes [19] | Resprout ability reported, although the species is considered a fire-sensitive [19]. | ||||
| Argophyllaceae | Corokia cotoneaster Raoul | shrub | Yes | |||||
| Asparagaceae | Cordyline australis (Forst.f.) Endl. * | tree (monoc) | Yes | 100% (n = 20) | Trees often resprouted, even when severely burned (current research). | |||
| Asparagaceae | Cordyline spp. | tree (monoc) | Yes [19] | |||||
| Asteraceae | Olearia avicenniifolia (Raoul) Hook.f. ** | tree | 0% (n = 12) | |||||
| Asteraceae | Olearia colensoi Hook.f. | shrub | No [72] | |||||
| Asteraceae | Olearia ilicifolia Hook.f. | tree | Yes [73] | |||||
| Asteraceae | Olearia rani (A.Cunn.) Druce | tree | Yes [74] | Resprout after tree falls and storm damage, but fire can decrease the possibility of recovery from resproutings [74]. | ||||
| Asteraceae | Ozothamnus leptophyllus (G.Forst.) Breitw. et J.M.Ward | shrub | No | Yes [19,73] | May resprout from the base [73]. | |||
| Corynocarpaceae | Corynocarpus laevigatus J.R.Forst. et G.Forst. | tree | Yes | Yes [71] | Resprouts may grow into new trees when young trees are sawn off at the base [9]. | |||
| Cunoniaceae | Weinmannia racemosa L.f. | tree | Yes | Yes [19] | ||||
| Dicksoniaceae | Dicksonia squarrosa (G.Forst.) Swartz | tree fern | Yes [75] | |||||
| Elaeocarpaceae | Aristotelia fruticosa Hook.f. | tree | Yes | |||||
| Elaeocarpaceae | Aristotelia serrata (J.R.Forst. et G.Forst.) W.R.B.Oliv. | tree | Yes | 100% (n = 9) | Yes [19] | Resprouts may grow into new trees when young trees are sawn off the base [9]. | ||
| Ericaceae | Dracophyllum acerosum Bergg. | tree | No | |||||
| Ericaceae | Dracophyllum longifolium (J.R.Forst et G.Forst.) R.Br. | shrub | No [72] | |||||
| Ericaceae | Dracophyllum subulatum Hook.f. | shrub | No [76] | |||||
| Ericaceae | Dracophyllum spp. | shrub - tree | No [19] | |||||
| Ericaceae | Gaultheria macrostigma (Colenso) D.J.Middleton | shrub | Yes [16] | |||||
| Ericaceae | Gaultheria rupestris (L.f.) D.Don | shrub | Yes [73] | Resprouted from rhizomes [73]. | ||||
| Fabaceae | Sophora microphylla Aiton * | tree | Yes | 25% (n = 4) | Resprouts may grow into new trees, when young trees are sawn off at the base [9]. Usually did not resprout, even when lightly burnt (current research). | |||
| Fabaceae | Sophora prostrata Buchanan | shrub - tree | Yes [15] | 40 plants resprouted and were tagged and 30 were still alive 15 years after the fire [15]. | ||||
| Griseliniaceae | Griselinia littoralis Raoul | tree | Yes | 100% (n = 4) | Yes [71] | 50% (n = 10) | Resprouts when young trees are sawn off at the base [9]. Withstands fire and continues to grow from basal stem sprouts [71]. | |
| Lamiaceae | Teucridium parvifolium (Hook.f.) Kattari et Salmaki | tree | 100% (n = 5) | |||||
| Lamiaceae | Vitex lucens Kirk | tree | Yes [77] | Resprouts after general disturbance, nothing specific about fire response [77]. | ||||
| Lauraceae | Beilschmiedia spp. | tree | Yes [19] | Although the resprout ability, is considered fire-sentive species [19]. | ||||
| Lauraceae | Beilschmiedia tawa (A.Cunn.) Benth. et Hook.f. ex Kirk | tree | Yes | Yes [78] | Resprouts may grow into new trees when young trees are sawn off at the base [9]. Resprouted after volcanic activity [78]. | |||
| Loganiaceae | Geniostoma ligustrifolium A.Cunn. | shrub | Yes [74] | Resprout after tree falls and storm damage, but fire can decrease the possibility of recovery from resprouts [74]. | ||||
| Malvaceae | Hoheria glabrata Sprague et Summerhayes | tree | Yes [73] | Probably can resprout from burnt stumps [73]. | ||||
| Malvaceae | Hoheria sexstylosa Colenso | tree | Yes | Resprouts may grow into new trees when young trees are sawn off at the base [9]. | ||||
| Malvaceae | Plagianthus regius (Poit.) Hochr. ** | tree | 10% (n = 11) | |||||
| Monimiaceae | Hedycarya arborea J.R.Forst. et G.Forst | tree | Yes [71] | Vigorous basal and stem sprouts (general disturbance, nothing specific about fire response) [71]. | ||||
| Myrtaceae | Kunzea robusta de Lange et Toelken | tree | No | 0% (n = 6) | No [19,15] | 0% (n = 39) | Does not resprout, even when lightly burnt (current research). | |
| Myrtaceae | Leptospermum scoparium J.R.Forst. et G.Forst. | tree | No | No | No | Inability to produce basal sprouts after fire [9]. | ||
| Myrtaceae | Metrosideros umbellata Cav. | tree | No [79] | Trees do not recover from forest fires [79]. | ||||
| Myrtaceae | Metrosideros excelsa Sol. ex Gaertn. | tree | No [80] | Small percentage can resprout from epicormic buds after volcanism [80]. | ||||
| Nothofagaceae | Fuscospora cliffortioides (Hook.f.) Heenan et Smissen | tree | No | No [81] | NZ beeches do not resprout after fire [81]. | |||
| Nothofagaceae | Fuscospora solandri (Hook.f.) Heenan et Smissen | tree | No [81] | Virtually all mountain beech trees scorched by fire died within five years [14]. NZ beeches do not resprout after fire [81]. | ||||
| Nothofagaceae | Fuscospora spp. | tree | No [14,19,81] | NZ beeches do not resprout after fire [81]. Even low temperature ground fires often kill most beech trees and seedlings in New Zealand [14,19] | ||||
| Nothofagaceae | Lophozonia menziesii (Hook.f.) Heenan et Smissen | tree | No [19,81] | NZ beeches do not resprout after fire [81]. | ||||
| Onagraceae | Fuchsia excorticata (J.R.Forst. et G.Forst.) L.f. | tree | Yes | 100% (n = 9) | Yes | 100% (n = 9) | Trees burnt to the ground and felled by wind regenerate from resprouts [9]. After fire, trees often resprouted abundantly from the base (current research). | |
| Phyllocladaceae | Phyllocladus alpinus Hook.f. | shrub | Yes | Yes [72] | Epicormic resprout [72]. | |||
| Phyllocladaceae | Phyllocladus spp. | tree | No [19] | |||||
| Pittosporaceae | Pittosporum eugenioides A.Cunn. | tree | 87% (n = 16) | |||||
| Pittosporaceae | Pittosporum tenuifolium Sol. ex Gaertn | tree | Yes [19] | |||||
| Pittosporaceae | Pittosporum tenuifolium/eugenioides | tree | 3% (n = 35) | Two slightly burnt plants resprouted, but the resprouts dried out and died between 5 and 10 months after being burnt (current research). | ||||
| Pittosporaceae | Pittosporum sp. | tree | 100% (n = 2) | |||||
| Plantaginaceae | Veronica salicifolia G.Forst. ** | tree | 0% (n = 14) | |||||
| Plantaginaceae | Veronica spp. (hebe) | tree | No [73] | |||||
| Podocarpaceae | Dacrycarpus dacrydioides (A.Rich.) de Laub. | tree | No [19] | |||||
| Podocarpaceae | Dacrydium cupressinum Lamb. | tree | No [19] | |||||
| Podocarpaceae | Halocarpus bidwillii (Kirk) Quinn | shrub | Yes | |||||
| Podocarpaceae | Halocarpus spp. | tree | No [19] | |||||
| Podocarpaceae | Podocarpus spp. | tree | No | |||||
| Podocarpaceae | Podocarpus totara G.Benn. ex D.Don | tree | 71% (n = 7) | No [19] | No (n = 3) | |||
| Podocarpaceae | Prumnopitys taxifolia (D.Don) de Laub. | tree | No [19] | |||||
| Polygonaceae | Muehlenbeckia astonii Petrie | shrub | Yes [82] | Resprouts after general disturbance, nothing specific about fire response [82]. | ||||
| Primulaceae | Myrsine australis (A.Rich.) Allan | tree | Yes [83] | Trees damaged near the base produce abundant resprouts [9]. Resprouts after wind-storm, and probably resprout from roots after fire [83]. | ||||
| Rhamnaceae | Discaria toumatou Raoul | shrub-tree | Yes | Yes 35–65% | Yes [70,19] | Between approximately 35 and 65%, depending on plant size and time since the fire event [70]. | ||
| Rosaceae | Rubus cissoides A.Cunn. | tree | 100% (n = 1) | |||||
| Rousseaceae | Carpodetus serratus J.R.Forst. et G.Forst. | tree | Yes | 90% (n = 10) | Yes [14] | |||
| Rubiaceae | Coprosma ciliata Hook.f. | shrub | Yes [16] | |||||
| Rubiaceae | Coprosma crassifolia Colenso | shrub | 7 %(n = 163) [84] | |||||
| Rubiaceae | Coprosma dumosa (Cheeseman) G.T.Jane | tree | 100% (n = 1) | |||||
| Rubiaceae | Corposma sp. aff. intertexa G.Simpson | shrub | Yes [17] | Resprouts from the burnt base and from underground rhizomes [17]. | ||||
| Rubiaceae | Coprosma microcarpa Hook.f. | tree | Yes [14] | |||||
| Rubiaceae | Coprosma propinqua A.Cunn | shrub | Yes | 36% (n = 56) [84] Yes [17] | Resprouts from the burnt base and from underground rhizomes [17]. | |||
| Rubiaceae | Coprosma pseudocuneata Garn.-Jones et R.Elder | tree | Yes [14] | |||||
| Rubiaceae | Coprosma rhamnoides A.Cunn. | tree | Yes [14] | |||||
| Rubiaceae | Coprosma spp. | shrub | Yes [9,85] | |||||
| Rubiaceae | Coprosma cf. rubra ** | shrub | 60% (n = 5) | |||||
| Rubiaceae | Coprosma robusta/lucida ** | tree | 64% (n = 33) | Resprouted–basal and epicormic (current research) | ||||
| Rubiaceae | Coprosma serrulata Hook.f. ex Buchanan | shrub | Yes [73] | Resprouted from surviving base [73]. | ||||
| Sapindaceae | Alectryon excelsus Gaertn. | tree | Yes | Resprouts may grow into new trees when young trees are sawn off the base [9]. | ||||
| Violaceae | Melicytus alpinus (Kirk) Garn.-Jones | shrub | Yes | |||||
| Violaceae | Melicytus ramiflorus J.R.Forst. et G.Forst. | tree | Yes | 93 (n = 29) | Yes [19] | 69% (n = 126) | Resprouted–basal and epicormic (current research) | |
| Violaceae | Melicytus macrophyllus A.Cunn. | tree | Yes [74] | Resprouts after tree falls and storm damage, but fire can decrease the possibility of recovery from resprouting [74]. | ||||
| Winteraceae | Pseudowintera colorata (Raoul) Dandy | tree | 77% (n = 57) |
Appendix B






References
- Bowman, D.M.; Balch, J.K.; Artaxo, P.; Bond, W.J.; Carlson, J.M.; Cochrane, M.A.; D’Antonio, C.M.; DeFries, R.S.; Doyle, J.C.; Harrison, S.P. Fire in the Earth system. Science 2009, 324, 481–484. [Google Scholar] [CrossRef]
- Pausas, J.G.; Keeley, J.E. A burning story: The role of fire in the history of life. BioScience 2009, 59, 593–601. [Google Scholar] [CrossRef] [Green Version]
- Bond, W.J.; Midgley, J.J. Ecology of sprouting in woody plants: The persistence niche. Trends Ecol. Evol. 2001, 16, 45–51. [Google Scholar] [CrossRef]
- Vieira, D.L.; Scariot, A. Principles of natural regeneration of tropical dry forests for restoration. Restor. Ecol. 2006, 14, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Vesk, P.A.; Westoby, M. Sprouting ability across diverse disturbances and vegetation types worldwide. J. Ecol. 2004, 92, 310–320. [Google Scholar] [CrossRef]
- Clarke, P.J.; Lawes, M.J.; Murphy, B.P.; Russell-Smith, J.; Nano, C.E.M.; Bradstock, R.; Enright, N.J.; Fontaine, J.B.; Gosper, C.R.; Radford, I.; et al. A synthesis of postfire recovery traits of woody plants in Australian ecosystems. Sci. Total Environ. 2015, 534, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Clarke, P.J.; Lawes, M.J.; Midgley, J.J.; Lamont, B.B.; Ojeda, F.; Burrows, G.E.; Enright, N.J.; Knox, K.J. Resprouting as a key functional trait: How buds, protection and resources drive persistence after fire. New Phytol. 2013, 197, 19–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreira, B.; Tormo, J.; Pausas, J.G. To resprout or not to resprout: Factors driving intraspecific variability in resprouting. Oikos 2012, 121, 1577–1584. [Google Scholar] [CrossRef]
- Burrows, C.J. Do New Zealand forest trees regenerate from sprouts. Canterb. Bot. Soc. J. 1994, 28, 63–68. [Google Scholar]
- Pausas, J.G.; Pratt, R.B.; Keeley, J.E.; Jacobsen, A.L.; Ramirez, A.R.; Vilagrosa, A.; Paula, S.; Kaneakua-Pia, I.N.; Davis, S.D. Towards understanding resprouting at the global scale. New Phytol. 2016, 209, 945–954. [Google Scholar] [CrossRef] [Green Version]
- Bond, W.J.; Midgley, J.J. The evolutionary ecology of sprouting in woody plants. Int. J. Plant Sci. 2003, 164, S103–S114. [Google Scholar] [CrossRef] [Green Version]
- Kitzberger, T.; Perry, G.L.W.; Paritsis, J.; Gowda, J.H.; Tepley, A.J.; Holz, A.; Veblen, T.T. Fire–vegetation feedbacks and alternative states: Common mechanisms of temperate forest vulnerability to fire in southern South America and New Zealand. N. Z. J. Bot. 2016, 54, 247–272. [Google Scholar] [CrossRef] [Green Version]
- Mason, N.W.; Frazao, C.; Buxton, R.P.; Richardson, S.J. Fire form and function: Evidence for exaptive flammability in the New Zealand flora. Plant Ecol. 2016, 217, 645–659. [Google Scholar] [CrossRef]
- Wiser, S.K.; Allen, R.B.; Platt, K.H. Mountain beech forest succession after a fire at Mount Thomas Forest, Canterbury, New Zealand. N. Z. J. Bot. 1997, 35, 505–515. [Google Scholar] [CrossRef]
- Richardson, S.J.; King, S.; Rose, A.B.; McGlone, M.S.; Holdaway, R.J. Post-fire recovery of a dryland forest remnant in the Wither Hills, Marlborough. N. Z. J. Ecol. 2018, 42, 222–228. [Google Scholar] [CrossRef]
- Johnson, P.N. Vegetation recovery after fire on a southern New Zealand peatland. N. Z. J. Bot. 2001, 39, 251–267. [Google Scholar] [CrossRef]
- Timmins, S.M. Wetland vegetation recovery after fire: Eweburn Bog, Te Anau, New Zealand. N. Z. J. Bot. 1992, 30, 383–399. [Google Scholar] [CrossRef]
- Ogden, J.; Basher, L.; McGlone, M.A. Botanical briefing Fire, Forest Regeneration and Links with Early Human Habitation: Evidence from New Zealand. Ann. Bot. 1998, 81, 687–696. [Google Scholar] [CrossRef] [Green Version]
- Perry, G.L.; Wilmshurst, J.M.; McGlone, M.S. Ecology and long-term history of fire in New Zealand. N. Z. J. Ecol. 2014, 38, 157–176. [Google Scholar]
- Cecil, D.J.; Buechler, D.E.; Blakeslee, R.J. Gridded lightning climatology from TRMM-LIS and OTD: Dataset description. Atmos. Res. 2014, 135–136, 404–414. [Google Scholar] [CrossRef] [Green Version]
- Christian, H.J.; Blakeslee, R.J.; Boccippio, D.J.; Boeck, W.L.; Buechler, D.E.; Driscoll, K.T.; Boeck, W.L.; Buechler, D.E.; Driscoll, K.T.; Goodman, S.J.; et al. Global frequency and distribution of lightning as observed from space by the Optical Transient Detector. J. Geophys. Res. Atmos. 2003, 108, ACL-4. [Google Scholar] [CrossRef]
- Wyse, S.V.; Perry, G.L.; O’Connell, D.M.; Holland, P.S.; Wright, M.J.; Hosted, C.L.; Whitelock, S.L.; Geary, I.J.; Maurin, K.J.; Curran, T.J. A quantitative assessment of shoot flammability for 60 tree and shrub species supports rankings based on expert opinion. Int. J. Wildland Fire 2016, 25, 466–477. [Google Scholar] [CrossRef] [Green Version]
- McGlone, M. Polynesian deforestation of New Zealand: A preliminary synthesis. Archaeol. Ocean. 1983, 18, 11–25. [Google Scholar] [CrossRef]
- Stevens, G.; McGlone, M.; McCulloch, B. Prehistoric New Zealand; Royal New Zealand Foundation of the Blind: Auckland, New Zealand, 1995. [Google Scholar]
- Ewers, R.M.; Kliskey, A.D.; Walker, S.; Rutledge, D.; Harding, J.S.; Didham, R.K. Past and future trajectories of forest loss in New Zealand. Biol. Conserv. 2006, 133, 312–325. [Google Scholar] [CrossRef]
- Pawson, E.; Brooking, T. Making a New Land: Environmental Histories of New Zealand; University of Otago Press: Dunedin, New Zealand, 2013. [Google Scholar]
- Wilson, H.D. Plant Life on Banks Peninsula; Manuka Press: Cromwell, New Zealand, 2013. [Google Scholar]
- Wilson, H.D. Natural History of Banks Peninsula; Canterbury University Press: Christchurch, New Zealand, 2009. [Google Scholar]
- Wilson, H.D. Vegetation of Banks Peninsula. In The Natural History of Canterbury, 3rd ed.; Winterbourn, M., Knox, G., Burrows, C., Marsden, I., Eds.; Canterbury University Press: Christchurch, New Zealand, 2008; pp. 251–278. [Google Scholar]
- Wyse, S.V.; Wilmshurst, J.M.; Burns, B.R.; Perry, G.L. New Zealand forest dynamics: A review of past and present vegetation responses to disturbance, and development of conceptual forest models. N. Z. J. Ecol. 2018, 42, 87–106. [Google Scholar] [CrossRef] [Green Version]
- Perry, G.L.; Wilmshurst, J.M.; Ogden, J.; Enright, N.J. Exotic mammals and invasive plants alter fire-related thresholds in southern temperate forested landscapes. Ecosystems 2015, 18, 1290–1305. [Google Scholar] [CrossRef]
- Tepley, A.J.; Thomann, E.; Veblen, T.T.; Perry, G.L.W.; Holz, A.; Paritsis, J.; Kitzberger, T.; Anderson-Teixeira, K.J. Influences of fire–vegetation feedbacks and post-fire recovery rates on forest landscape vulnerability to altered fire regimes. J. Ecol. 2018, 106, 1925–1940. [Google Scholar] [CrossRef] [Green Version]
- Anderson, S.A.; Doherty, J.J.; Pearce, H.G. Wildfires in New Zealand from 1991 to 2007. N. Z. J. For. 2008, 53, 19–22. [Google Scholar]
- Pearce, H.G.; Clifford, V. Fire weather and climate of New Zealand. N. Z. J. For. 2008, 53, 13–18. [Google Scholar]
- Pearce, H.G. The 2017 Port Hills wildfires—A window into New Zealand’s fire future? Aust. J. Disaster Trauma Stud. 2018, 22, 35–50. [Google Scholar]
- Wilson, H.D. Banks Ecological Region Protected Natural Areas Programme Survey Report 21; Department of Conservation: Christchurch, New Zealand, 1992. [Google Scholar]
- Gill, A.M.; Bradstock, R. A national register for the fire responses of plant species. Cunninghamia 1992, 2, 653–660. [Google Scholar]
- McCune, B.; Grace, J.B.; Urban, D.L. Analysis of Ecological Communities; MjM Software Design: Lincoln, OR, USA, 2002; Volume 28. [Google Scholar]
- Legendre, P.; Legendre, L.F. Numerical Ecology; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Burrows, C.J. Fruit types and seed dispersal modes of woody plants in Ahuriri Summit Bush, Port Hills, western Banks Peninsula, Canterbury, New Zealand. N. Z. J. Bot. 1994, 32, 169–181. [Google Scholar] [CrossRef]
- Oksanen, J. Multivariate analysis of ecological communities in R: Vegan tutorial. R Package Vers. 2011, 1, 1–43. [Google Scholar]
- Veblen, T.T.; Donoso, C.; Kitzberger, T.; Rebertus, A.J. Ecology of southern Chilean and Argentinean Nothofagus forests. In The Ecology and Biogeography of Nothofagus Forests; Veblen, T.T., Hill, R.S., Read, J., Eds.; Yale University Press: New Heaven, CT, USA, 1996; pp. 93–353. [Google Scholar]
- Read, J.; Brown, M.J. Ecology of Australian Nothofagus forests. In The Ecology and Biogeography of Nothofagus Forests; Veblen, T.T., Hill, R.S., Read, J., Eds.; Yale University Press: New Heaven, CT, USA, 1996; pp. 131–181. [Google Scholar]
- Pausas, J.G.; Keeley, J.E. Evolutionary ecology of resprouting and seeding in fire-prone ecosystems. New Phytol. 2014, 204, 55–65. [Google Scholar] [CrossRef]
- Keeley, J.E.; Pausas, J.G.; Rundel, P.W.; Bond, W.J.; Bradstock, R.A. Fire as an evolutionary pressure shaping plant traits. Trends Plant Sci. 2011, 16, 406–411. [Google Scholar] [CrossRef] [Green Version]
- Wabnig, T. Case Study at Hinewai-Reserve: Post-fire Assessment of the Resprouting Ability of some New Zealand Native Woody Plants. Master’s Thesis, Natural Resources Management and Ecological Engineering, Lincoln University, Lincoln, New Zealand, 2014. [Google Scholar]
- Nicholson, A.; Prior, L.D.; Perry, G.L.W.; Bowman, D.M.J.S. High post-fire mortality of resprouting woody plants in Tasmanian Mediterranean-type vegetation. Int. J. Wildland Fire 2017, 26, 532. [Google Scholar] [CrossRef]
- Kauffman, J.B. Survival by sprouting following fire in tropical forests of the eastern Amazon. Biotropica 1991, 23, 219–224. [Google Scholar] [CrossRef]
- Clarke, P.; Knox, K.J.; Campbell, M.L.; Copeland, L.M. Post-fire recovery of woody plants in the New England Tableland Bioregion. Cunninghamia 2009, 11, 221–239. [Google Scholar]
- Su, W.-H.; Shi, Z.; Zhou, R.; Zhao, Y.-J.; Zhang, G.-F. The role of fire in the Central Yunnan Plateau ecosystem, southwestern China. For. Ecol. Manag. 2015, 356, 22–30. [Google Scholar] [CrossRef]
- Reyes, O.; Casal, M.; Rego, F.C. Resprouting ability of six Atlantic shrub species. Folia Geobotanica 2009, 44, 19–29. [Google Scholar] [CrossRef]
- Miller, P.; Kauffman, J. Seedling and sprout response to slash-and-burn agriculture in a Tropical Deciduous Forest 1. Biotropica 1998, 30, 538–546. [Google Scholar] [CrossRef]
- Kennard, D.; Gould, K.; Putz, F.; Fredericksen, T.; Morales, F. Effect of disturbance intensity on regeneration mechanisms in a tropical dry forest. For. Ecol. Manag. 2002, 162, 197–208. [Google Scholar] [CrossRef] [Green Version]
- Ellair, D.P.; Platt, W.J. Fuel composition influences fire characteristics and understorey hardwoods in pine savanna. J. Ecol. 2013, 101, 192–201. [Google Scholar] [CrossRef] [Green Version]
- Burrows, C.J. Germination behaviour of seeds of the New Zealand woody species Alectryon excelsus, Corynocarpus laevigatus, and Kunzea ericoides. N. Z. J. Bot. 1996, 34, 489–498. [Google Scholar] [CrossRef]
- Allen, R.; Partridge, T.; Lee, W.; Efford, M. Ecology of Kunzea ericoides (A. Rich.) J. Thompson (kanuka) in east Otago, New Zealand. N. Z. J. Bot. 1992, 30, 135–149. [Google Scholar] [CrossRef]
- Grady, J.M.; Hoffmann, W.A. Caught in a fire trap: Recurring fire creates stable size equilibria in woody resprouters. Ecology 2012, 93, 2052–2060. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, W.A.; Geiger, E.L.; Gotsch, S.G.; Rossatto, D.R.; Silva, L.C.; Lau, O.L.; Haridasan, M.; Franco, A.C. Ecological thresholds at the savanna-forest boundary: How plant traits, resources and fire govern the distribution of tropical biomes. Ecol. Lett. 2012, 15, 759–768. [Google Scholar] [CrossRef]
- Bellingham, P.J.; Sparrow, A.D. Resprouting as a life history strategy in woody plant communities. Oikos 2000, 89, 409–416. [Google Scholar] [CrossRef]
- de Magalhaes, R.M.; Schwilk, D.W. Leaf traits and litter flammability: Evidence for non-additive mixture effects in a temperate forest. J. Ecol. 2012, 100, 1153–1163. [Google Scholar] [CrossRef]
- Van Altena, C.; van Logtestijn, R.; Cornwell, W.; Cornelissen, H. Species composition and fire: Non-additive mixture effects on ground fuel flammability. Front. Plant Sci. 2012, 3, 63. [Google Scholar]
- Wyse, S.V.; Perry, G.L.; Curran, T.J. Shoot-level flammability of species mixtures is driven by the most flammable species: Implications for vegetation-fire feedbacks favouring invasive species. Ecosystems 2018, 21, 886–900. [Google Scholar] [CrossRef] [Green Version]
- Dent, J.M.; Buckley, H.L.; Lustig, A.; Curran, T.J. Flame temperatures saturate with increasing dead material in Ulex europaeus, but flame duration, fuel consumption and overall flammability continue to increase. Fire 2019, 2, 6. [Google Scholar] [CrossRef] [Green Version]
- Mandle, L.; Bufford, J.L.; Schmidt, I.B.; Daehler, C.C. Woody exotic plant invasions and fire: Reciprocal impacts and consequences for native ecosystems. Biol. Invasions 2011, 13, 1815–1827. [Google Scholar] [CrossRef]
- Brooks, M.L.; D’Antonio, C.M.; Richardson, D.M.; Grace, J.B.; Keeley, J.E.; DiTomaso, J.M.; Hobbs, R.J.; Pellant, M.; Pyke, D. Effects of Invasive Alien Plants on Fire Regimes. BioScience 2004, 54, 677. [Google Scholar] [CrossRef] [Green Version]
- Jackson, S.T.; Hobbs, R.J. Ecological restoration in the light of ecological history. Science 2009, 325, 567–569. [Google Scholar] [CrossRef] [Green Version]
- Robinne, F.-N.; Burns, J.; Kant, P.; Flannigan, M.; Kleine, M.; de Groot, B.; Wotton, D. Global Fire Challenges in a Warming World; Occasional Paper No. 32; International Union of Forest Research Organizations: Vienna, Austria, 2018. [Google Scholar]
- Curran, T.; Perry, G.; Wyse, S.; Alam, M. Managing fire and biodiversity in the wildland-urban interface: A role for green firebreaks. Fire 2018, 1, 3. [Google Scholar] [CrossRef] [Green Version]
- Cui, X.; Alam, M.A.; Perry, G.L.; Paterson, A.M.; Wyse, S.V.; Curran, T.J. Green firebreaks as a management tool for wildfires: Lessons from China. J. Environ. Manag. 2019, 233, 329–336. [Google Scholar] [CrossRef]
- Glogoski, D. Vegetation Recovery Following the 2015 Flock Hill Fire, Canterbury Hign Country. Master’s Thesis, University of Canterbury, Christchurch, New Zealand, 2017. [Google Scholar]
- Molloy, B.P.J. Apomixis in indigenous New Zealand woody seed plants and its ecological and wider significance: A working hypothesis. N. Z. J. Ecol. 2019, 43, 1–11. [Google Scholar] [CrossRef]
- Rogers, G.M.; McGlone, M.S. A history of Kaiparoro clearing and the limits of Nothofagus in the northern Tararua Range, New Zealand. N. Z. J. Bot. 1994, 32, 463–482. [Google Scholar] [CrossRef]
- Calder, J.W.; Wardle, P. Succession in subalpine vegetation at Arthur’s Pass, New Zealand. N. Z. Ecol. Soc. 1969, 16, 36–47. [Google Scholar]
- Enright, N.; Cameron, E. The soil seed bank of a kauri (Agathis australis) forest remnant near Auckland, New Zealand. N. Z. J. Bot. 1988, 26, 223–236. [Google Scholar] [CrossRef]
- Heginbotham, M.; Esler, A. Wild vascular plants of the Opotiki—East Cape region North Island, New Zealand. N. Z. J. Bot. 1985, 23, 379–406. [Google Scholar] [CrossRef] [Green Version]
- Rogers, G.; Leathwick, J. North Island seral tussock grasslands. 3. The influence of heather (Calluna vulgaris) on rates of change from tussock grassland to shrubland. N. Z. J. Bot. 1996, 34, 473–487. [Google Scholar] [CrossRef] [Green Version]
- Grubb, P.J.; Bellingham, P.J.; Kohyama, T.S.; Piper, F.I.; Valido, A. Disturbance regimes, gap-demanding trees and seed mass related to tree height in warm temperate rain forests worldwide. Biol. Rev. 2013, 88, 701–744. [Google Scholar] [CrossRef]
- Nicholls, J. Vulcanicity and indigenous vegetation in the Rotorua district. N. Z. Ecol. Soc. 1963, 10, 58–65. [Google Scholar]
- Wardle, P. Biological flora of New Zealand 6. Metrosideros umbellata Cav.[Syn. M. lucida (Forst. f.) A. Rich.](Myrtaceae) Southern rata. N. Z. J. Bot. 1971, 9, 645–671. [Google Scholar] [CrossRef]
- Clarkson, B.D.; Clarkson, B.R. Vegetation decline following recent eruptions on White Island (Whakaari), Bay of Plenty, New Zealand. N. Z. J. Bot. 1994, 32, 21–36. [Google Scholar] [CrossRef]
- Ogden, J.; Stewart, G.; Allen, R. Ecology of New Zealand Nothofagus forests. Ecol. Biogeogr. Nothofagus For. 1996, 3, 25–82. [Google Scholar]
- Wotton, D.M. Seed germination, dormancy and longevity in the endangered shrub Muehlenbeckia astonii (Polygonaceae). N. Z. J. Bot. 2018, 56, 331–341. [Google Scholar] [CrossRef]
- Atkinson, I.A. Successional processes induced by fires on the northern offshore islands of New Zealand. N. Z. J. Ecol. 2004, 28, 181–193. [Google Scholar]
- Evans, K.B. Resprouting of Coprosma Propinqua and Coprosma Crassifolia after fire at Birdlings Flat, Canterbury; Unpublished Undergraduate Report; University of Lincoln: Lincoln, New Zealand, 2014. [Google Scholar]
- Ledgard, N.; Davis, M. Restoration of mountain beech (Nothofagus solandri var. cliffortioides) forest after fire. N. Z. J. Ecol. 2004, 24, 125–135. [Google Scholar]

| Species | N | Origin | Resprout % | Resprout Ability | Flamm. Category | Dispersal |
|---|---|---|---|---|---|---|
| Cordyline australis | 20 | Native | 100 | Strong | Low/Mod | bird |
| Fuchsia excorticata | 9 | Native | 100 | Strong | Low | bird |
| Sambucus nigra * | 15 | Exotic | 87 | Strong | NA | bird |
| Melicytus ramiflorus | 126 | Native | 69 | Intermediate | Low | bird |
| Coprosma robusta/lucida | 33 | Native | 64 | Intermediate | Low/Mod | bird |
| Griselinia littoralis | 11 | Native | 55 | Intermediate | Low | bird |
| Ulex europaeus * | 47 | Exotic | 13 | Weak | Very high | ballistic |
| Plagianthus regius | 11 | Native | 9 | Weak | Very low | gravity/wind |
| Pseudopanax arboreus | 43 | Native | 5 | Weak | Low | bird |
| Pittosporum tenuifolium/eugenioides | 35 | Native | 3 | Weak | Low/Mod | bird |
| Kunzea robusta | 39 | Native | 0 | None | High | wind |
| Olearia avicenniifolia | 12 | Native | 0 | None | NA | wind |
| Veronica salicifolia | 14 | Native | 0 | None | NA | wind |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teixeira, A.M.C.; Curran, T.J.; Jameson, P.E.; Meurk, C.D.; Norton, D.A. Post-Fire Resprouting in New Zealand Woody Vegetation: Implications for Restoration. Forests 2020, 11, 269. https://doi.org/10.3390/f11030269
Teixeira AMC, Curran TJ, Jameson PE, Meurk CD, Norton DA. Post-Fire Resprouting in New Zealand Woody Vegetation: Implications for Restoration. Forests. 2020; 11(3):269. https://doi.org/10.3390/f11030269
Chicago/Turabian StyleTeixeira, Ana M. C., Timothy J. Curran, Paula E. Jameson, Colin D. Meurk, and David A. Norton. 2020. "Post-Fire Resprouting in New Zealand Woody Vegetation: Implications for Restoration" Forests 11, no. 3: 269. https://doi.org/10.3390/f11030269





