Operation of Thin-Film Electrolyte Metal-Supported Solid Oxide Fuel Cells in Lightweight and Stationary Stacks: Material and Microstructural Aspects
Abstract
:1. Introduction
2. Results and Discussion
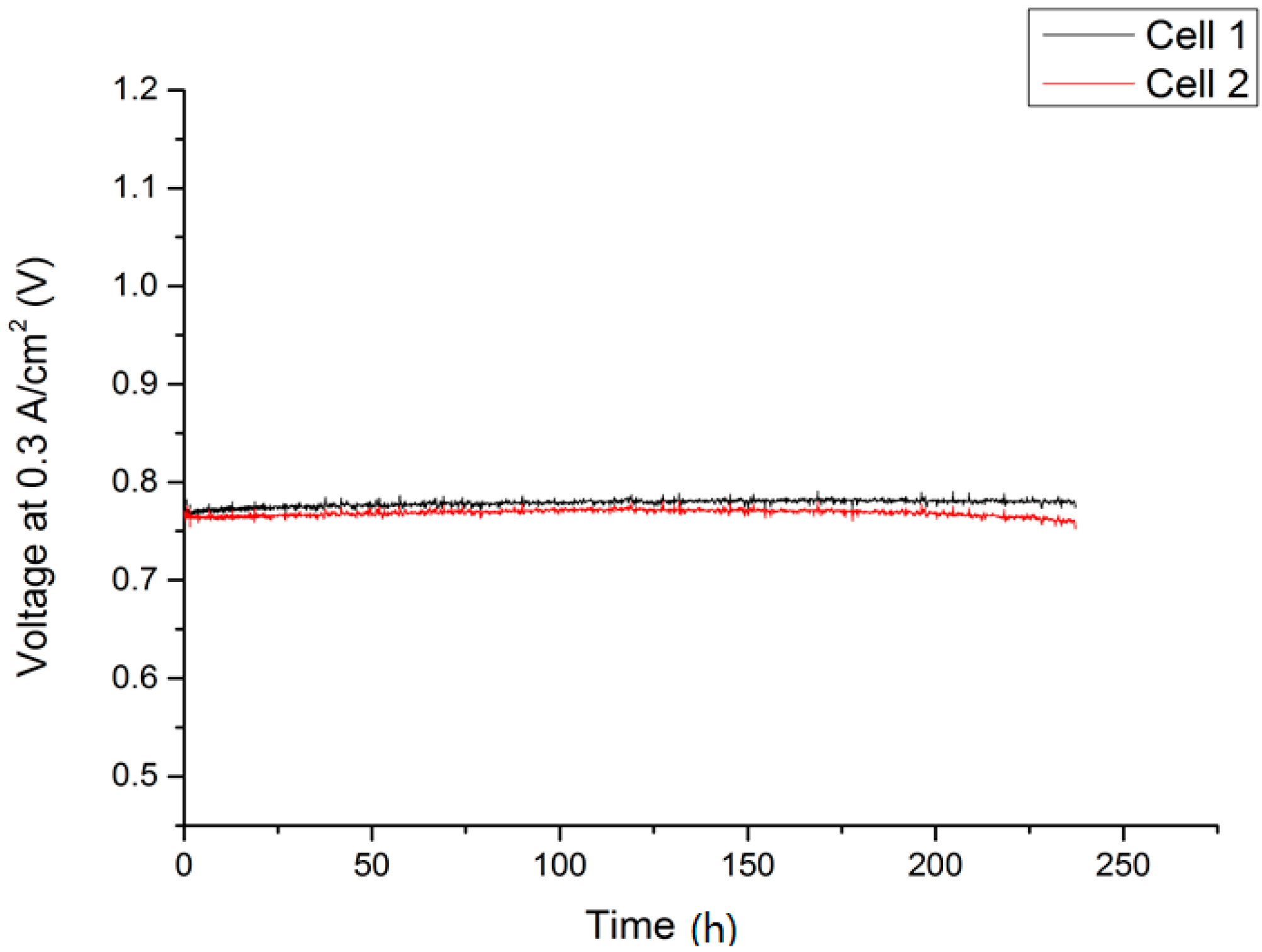
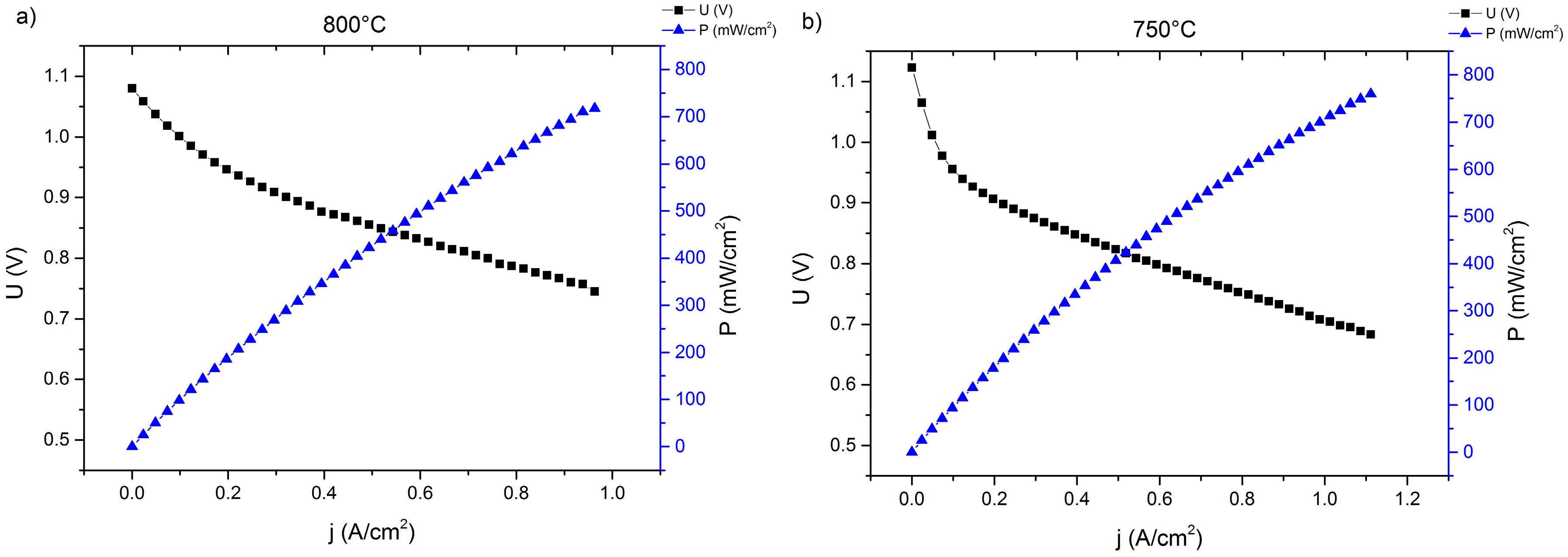
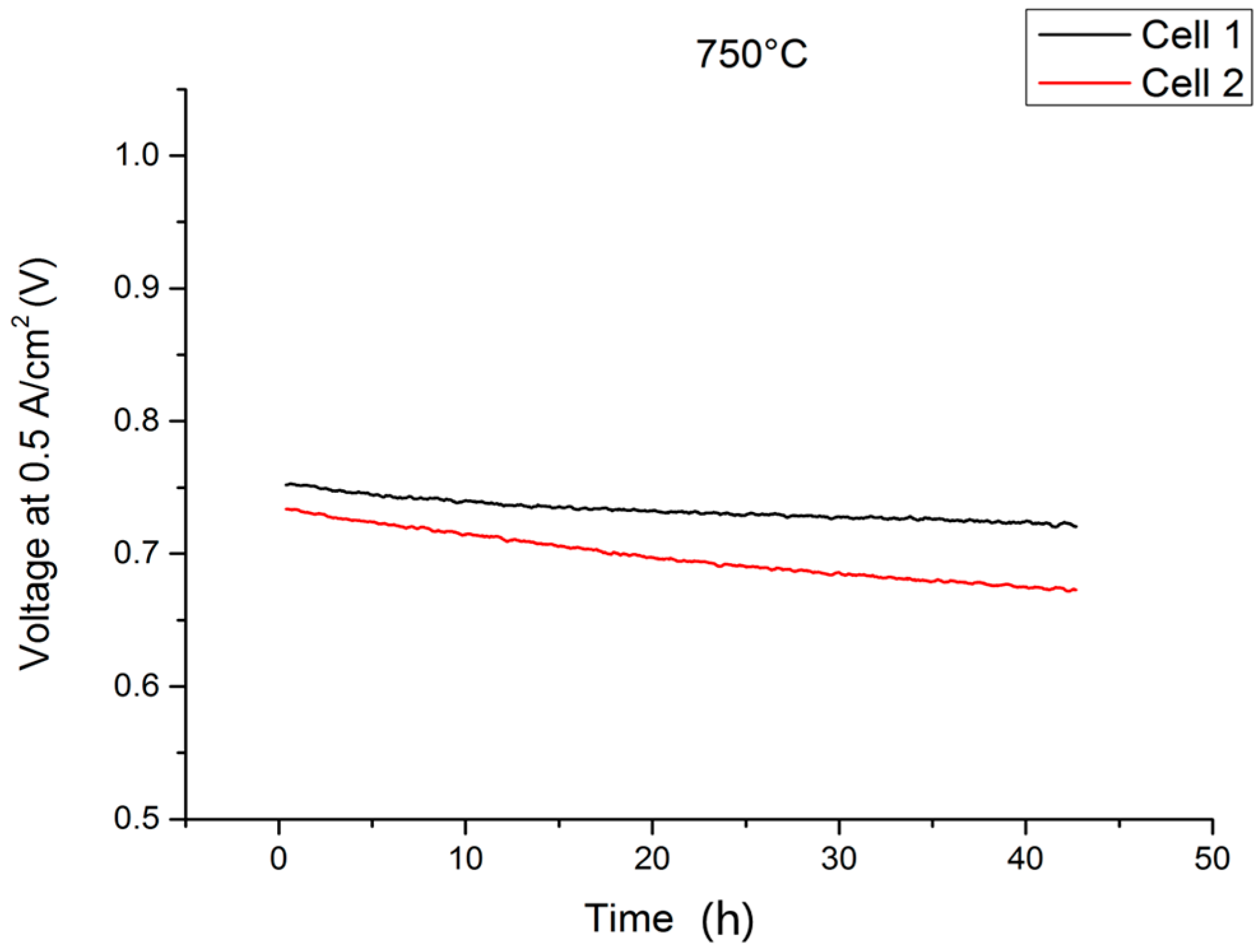
2.1. Single-Cell Test
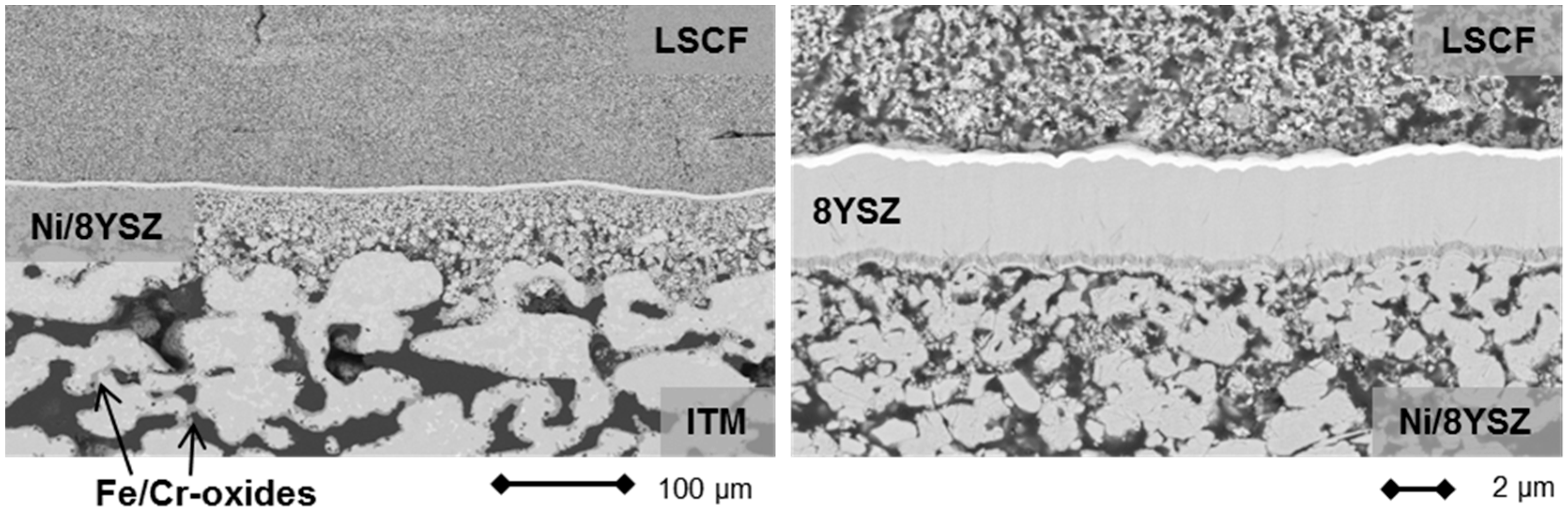
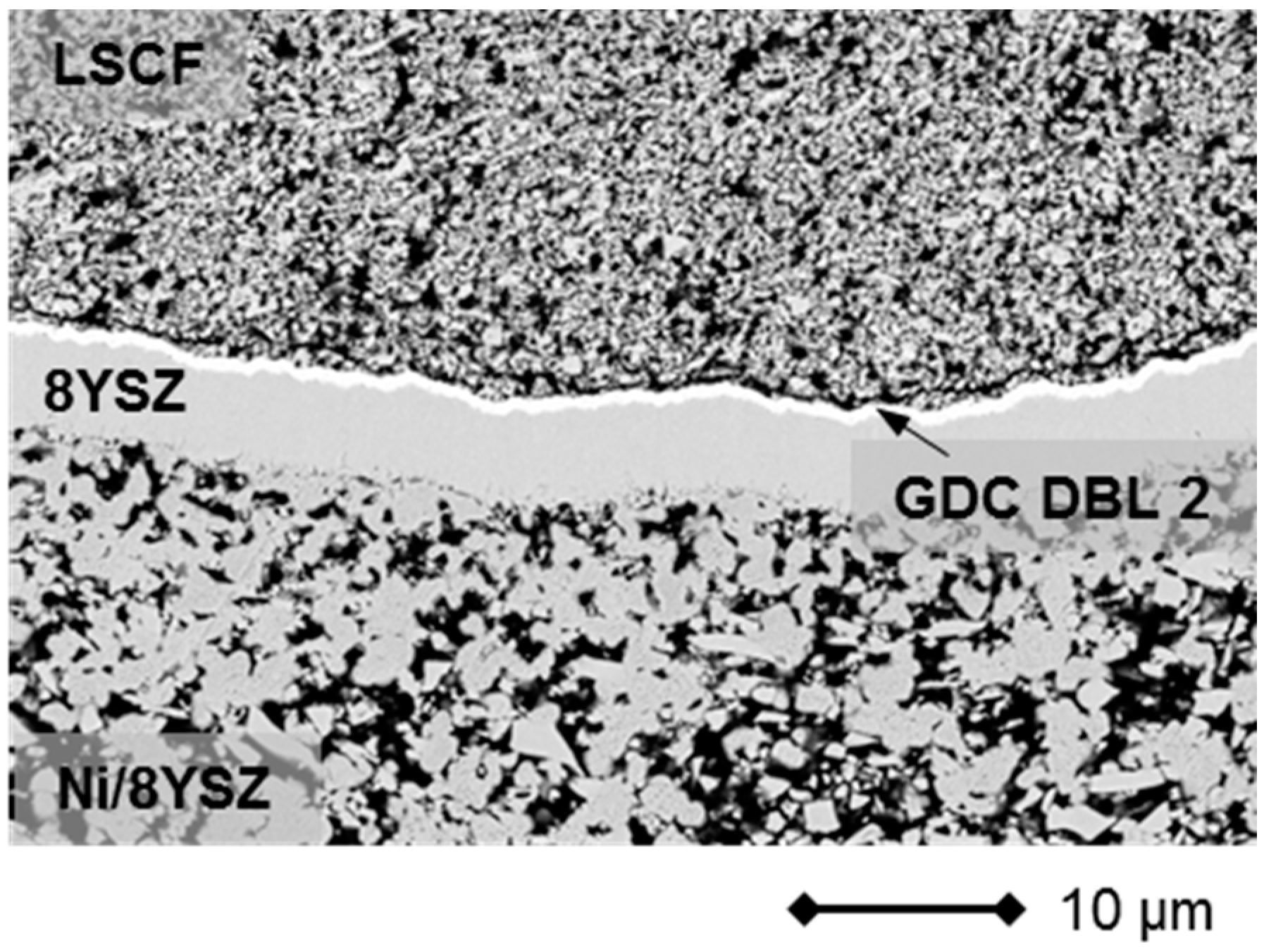
2.2. Lightweight Cassette Stack
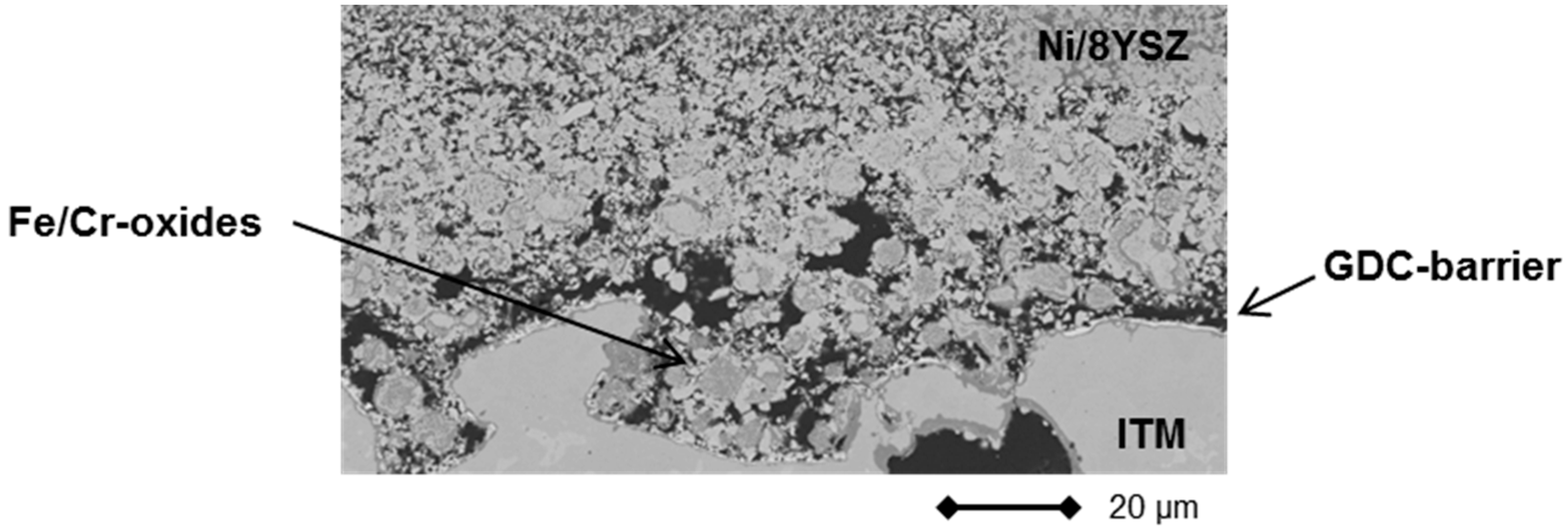
2.3. Stationary Stack
3. Materials and Methods
3.1. Cell Design and Manufacturing
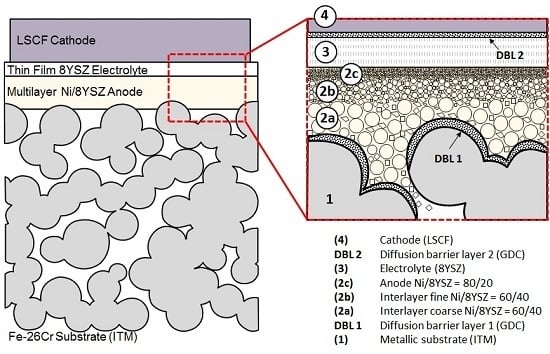
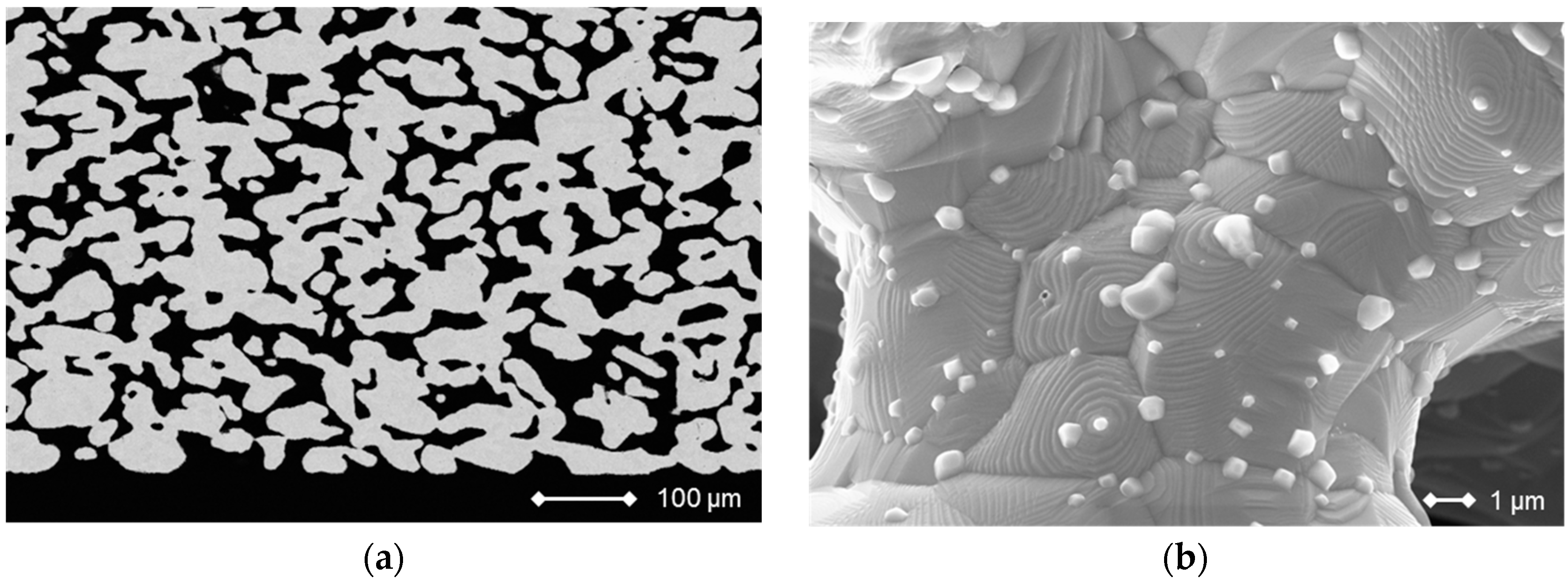
3.1.1. Substrate
3.1.2. Fuel-Side Electrode
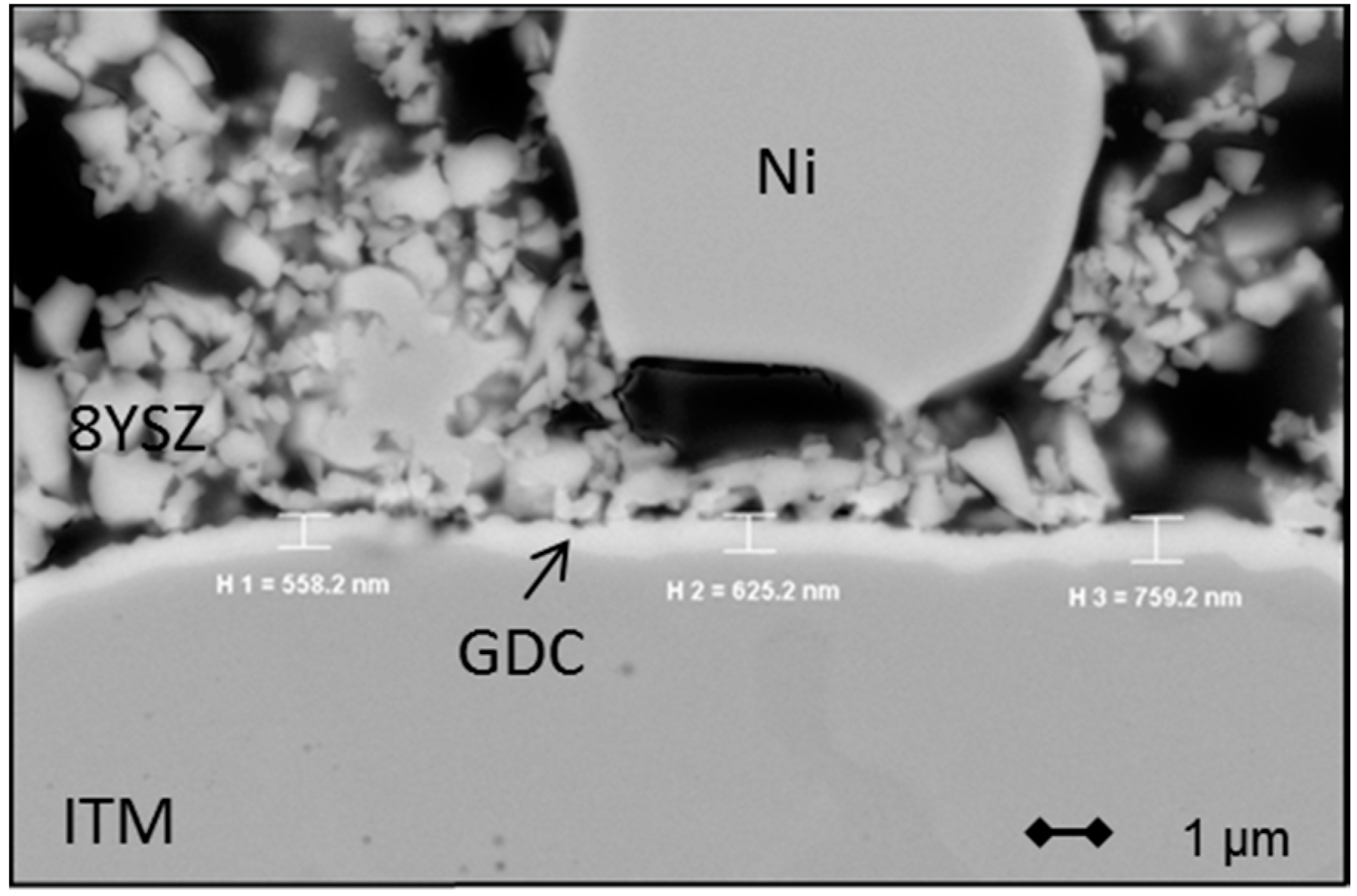
3.1.3. Electrolyte Membrane
3.1.4. Air-Side Electrode
3.1.5. Stack Assembly
3.1.6. Electron Microscopy
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Steele, B.C.H.; Heinzel, A. Materials for fuel-cell technologies. Nature 2001, 414, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Singhal, S.C. Solid oxide fuel cells for stationary, mobile, and military applications. Solid State Ion. 2002, 152, 405–410. [Google Scholar] [CrossRef]
- Stambouli, A.B.; Traversa, E. Solid oxide fuel cells (SOFCS): A review of an environmentally clean and efficient source of energy. Renew. Sustain. Energy Rev. 2002, 6, 433–455. [Google Scholar] [CrossRef]
- Goodenough, J.B. Oxide-ion electrolytes. Annu. Rev. Mater. Res. 2003, 33, 91–128. [Google Scholar] [CrossRef]
- Brett, D.J.L.; Atkinson, A.; Brandon, N.P.; Skinner, S.J. Intermediate temperature solid oxide fuel cells. Chem. Soc. Rev. 2008, 37, 1568–1578. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Hui, R.; Roller, J. Cathode materials for solid oxide fuel cells: A review. J. Solid State Electrochem. 2010, 14, 1125–1144. [Google Scholar] [CrossRef]
- Fergus, J.W. Metallic interconnects for solid oxide fuel cells. Mater. Sci. Eng. A 2005, 397, 271–283. [Google Scholar] [CrossRef]
- Quadakkers, W.J. Metallic materials in solid oxide fuel cells. Mater. Res. 2004, 7, 203–208. [Google Scholar] [CrossRef]
- Menzler, N.; Tietz, F.; Uhlenbruck, S.; Buchkremer, H.; Stöver, D. Materials and manufacturing technologies for solid oxide fuel cells. J. Mater. Sci. 2010, 45, 3109–3135. [Google Scholar] [CrossRef]
- Tietz, F.; Mai, A.; Stöver, D. From powder properties to fuel cell performance—A holistic approach for SOFC cathode development. Solid State Ion. 2008, 179, 1509–1515. [Google Scholar] [CrossRef]
- Singhal, S.C. Advances in solid oxide fuel cell technology. Solid State Ion. 2000, 135, 305–313. [Google Scholar] [CrossRef]
- Steele, B.C.H. Materials for IT-SOFC stacks: 35 years R&D: The inevitability of gradualness? Solid State Ion. 2000, 134, 3–20. [Google Scholar]
- Tucker, M.C. Progress in metal-supported solid oxide fuel cells: A review. J. Power Sources 2010, 195, 4570–4582. [Google Scholar] [CrossRef]
- Tucker, M.C.; Lau, G.Y.; Jacobson, C.P.; DeJonghe, L.C.; Visco, S.J. Stability and robustness of metal-supported SOFCs. J. Power Sources 2008, 175, 447–451. [Google Scholar] [CrossRef]
- Ansar, A.; Szabo, P.; Arnold, J.; Ilhan, Z.; Soysal, D.; Costa, R.; Zagst, A.; Gindrat, M.; Franco, T. Metal supported solid oxide fuel cells and stacks for auxilary power units—Progress, challenges and lessons learned. ECS Trans. 2011, 35, 147–155. [Google Scholar]
- Villarreal, I.; Jacobson, C.; Leming, A.; Matus, Y.; Visco, S.; De Jonghe, L. Metal-supported solid oxide fuel cells. Electrochem. Solid State Lett. 2003, 6, A178–A179. [Google Scholar] [CrossRef]
- Brandon, N.P.; Corcoran, D.; Cummins, D.; Duckett, A.; El-Khoury, K.; Haigh, D.; Leah, R.; Lewis, G.; Maynard, N.; McColm, T.; et al. Development of metal supported solid oxide fuel cells for operation at 500–600 °C. J. Mater. Eng. Perform. 2004, 13, 253–256. [Google Scholar] [CrossRef]
- Tucker, M.C.; Lau, G.Y.; Jacobson, C.P.; DeJonghe, L.C.; Visco, S.J. Performance of metal-supported SOFCs with infiltrated electrodes. J. Power Sources 2007, 171, 477–482. [Google Scholar] [CrossRef]
- Blennow, P.; Hjelm, J.; Klemensø, T.; Persson, A.; Brodersen, K.; Srivastava, A.; Frandsen, H.; Lundberg, M.; Ramousse, S.; Mogensen, M. Development of planar metal supported SOFC with novel cermet anode. ECS Trans. 2009, 25, 701–710. [Google Scholar]
- Huang, Q.-A.; Wang, B.; Qu, W.; Hui, R. Impedance diagnosis of metal-supported SOFCs with SDC as electrolyte. J. Power Sources 2009, 191, 297–303. [Google Scholar] [CrossRef]
- Rodriguez-Martinez, L.M.; Otaegi, L.; Alvarez, M.; Rivas, M.; Gomez, N.; Zabala, A.; Arizmendiarrieta, N.; Antepara, I.; Urriolabeitia, A.; Olave, M.; et al. Degradation studies on tubular metal supported SOFC. ECS Trans. 2009, 25, 745–752. [Google Scholar]
- Blennow, P.; Hjelm, J.; Klemensø, T.; Ramousse, S.; Kromp, A.; Leonide, A.; Weber, A. Manufacturing and characterization of metal-supported solid oxide fuel cells. J. Power Sources 2011, 196, 7117–7125. [Google Scholar] [CrossRef] [Green Version]
- Matus, Y.B.; De Jonghe, L.C.; Jacobson, C.P.; Visco, S.J. Metal-supported solid oxide fuel cell membranes for rapid thermal cycling. Solid State Ion. 2005, 176, 443–449. [Google Scholar] [CrossRef]
- Lang, M.; Szabo, P.; Ilhan, Z.; Cinque, S.; Franco, T.; Schiller, G. Development of solid oxide fuel cells and short stacks for mobile application. J. Fuel Cell Sci. Technol. 2007, 4, 384–391. [Google Scholar] [CrossRef]
- Franco, T.; Brandner, M.; Rüttinger, M.; Kunschert, G.; Venskutonis, A.; Sigl, L. Recent development aspects of metal supported thin-film SOFC. ECS Trans. 2009, 25, 681–688. [Google Scholar]
- Franco, T.; Haydn, M.; Weber, A.; Schafbauer, W.; Blum, L.; Packbier, U.; Roehrens, D.; Menzler, N.H.; Rechberger, J.; Venskutonis, A.; et al. The status of metal-supported SOFC development and industrialization at plansee. ECS Trans. 2013, 57, 471–480. [Google Scholar] [CrossRef]
- Haydn, M.; Ortner, K.; Franco, T.; Uhlenbruck, S.; Menzler, N.H.; Stöver, D.; Bräuer, G.; Venskutonis, A.; Sigl, L.S.; Buchkremer, H.-P.; et al. Multi-layer thin-film electrolytes for metal supported solid oxide fuel cells. J. Power Sources 2014, 256, 52–60. [Google Scholar] [CrossRef]
- Rüttinger, M.; Mücke, R.; Franco, T.; Büchler, O.; Menzler, N.H.; Venskutonis, A. Metal-supported cells with comparable performance to anode-supported cells in short-term stack environment. ECS Trans. 2011, 35, 259–268. [Google Scholar]
- Celik, I.B.; Pakalapati, S.R. From a Single cell to a stack modeling. In Modeling Solid Oxide Fuel Cells: Methods, Procedures and Techniques; Bove, R., Ubertini, S., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2008; pp. 123–182. [Google Scholar]
- Blum, L.; Buchkremer, H.P.; Gross, S.; Gubner, A.; de Haart, L.G.J.; Nabielek, H.; Quadakkers, W.J.; Reisgen, U.; Smith, M.J.; Steinberger-Wilckens, R.; et al. Solid oxide fuel cell development at forschungszentrum juelich. Fuel Cells 2007, 7, 204–210. [Google Scholar] [CrossRef]
- Rojek, V.; Roehrens, D.; Brandner, M.; Menzler, N.H.; Guillon, O.; Opitz, A.K.; Bram, M. Development of high performance anodes for metal-supported fuel cells. ECS Trans. 2015, 68, 1297–1307. [Google Scholar] [CrossRef]
- Kim, K.J.; Choi, G.M. Phase stability and oxygen non-stoichiometry of Gd-doped ceria during sintering in reducing atmosphere. J. Electroceramics 2015, 35, 1–7. [Google Scholar] [CrossRef]
- Jeong, J.; Baek, S.-W.; Bae, J. A diesel-driven, metal-based solid oxide fuel cell. J. Power Sources 2014, 250, 98–104. [Google Scholar] [CrossRef]
- Blennow, P.; Sudireddy, B.R.; Persson, Å.H.; Klemensø, T.; Nielsen, J.; Thydén, K. Infiltrated SrTiO3: Fecr-based anodes for metal-supported SOFC. Fuel Cells 2013, 13, 494–505. [Google Scholar] [CrossRef]
- Choi, J.H.; Lee, T.; Choi, M.; Yoo, Y.-S.; Baek, S.-W.; Bae, J. Long-term performance of anode-supported SOFC integrated with metal interconnect by joining process. Int. J. Hydrogen Energy 2010, 35, 4285–4291. [Google Scholar] [CrossRef]
- Venskutonis, A.; Kunschert, G.; Mueller, E.; Hoehle, H.-M. P/M processing and coating technologies for fabrication of interconnect for stationary and mobile SOFC applications. ECS Trans. 2007, 7, 2109–2115. [Google Scholar]
- Vasechko, V.; Pećanac, G.; Kuhn, B.; Malzbender, J. Mechanical properties of porous ITM alloy. Int. J. Hydrogen Energy 2016, 41, 562–569. [Google Scholar] [CrossRef]
- Megel, S.; Girdauskaite, E.; Sauchuk, V.; Kusnezoff, M.; Michaelis, A. Area specific resistance of oxide scales grown on ferritic alloys for solid oxide fuel cell interconnects. J. Power Sources 2011, 196, 7136–7143. [Google Scholar] [CrossRef]
- Kim, K.J.; Kim, S.J.; Choi, G.M. Y0.08Sr0.88TiO3–CeO2 composite as a diffusion barrier layer for stainless-steel supported solid oxide fuel cell. J. Power Sources 2016, 307, 385–390. [Google Scholar] [CrossRef]
- Brandner, M.; Bram, M.; Froitzheim, J.; Buchkremer, H.P.; Stöver, D. Electrically conductive diffusion barrier layers for metal-supported SOFC. Solid State Ion. 2008, 179, 1501–1504. [Google Scholar] [CrossRef]
- Yan, Y.; Bateni, R.; Harris, J.; Kesler, O. Fabrication of reactive element oxide coatings on porous ferritic stainless steel for use in metal-supported solid oxide fuel cells. Surf. Coat. Technol. 2015, 272, 415–427. [Google Scholar] [CrossRef]
- Jung, T.; Kälber, T.; Heide, V.v.d. Gas flow sputtering of oxide coatings: Practical aspects of the process. Surf. Coat. Technol. 1996, 86, 218–224. [Google Scholar] [CrossRef]
- Roehrens, D.; Han, F.; Haydn, M.; Schafbauer, W.; Sebold, D.; Menzler, N.H.; Buchkremer, H.P. Advances beyond traditional SOFC cell designs. Int. J. Hydrogen Energy 2015, 40, 11538–11542. [Google Scholar] [CrossRef]
- Keuter, T.; Roehrens, D.; Menzler, N.H.; Vaßen, R. Redox-stable high-performance thin-film solid oxide fuel cell. ECS Trans. 2015, 68, 2001–2009. [Google Scholar] [CrossRef]
- Mai, A.; Haanappel, V.A.C.; Uhlenbruck, S.; Tietz, F.; Stöver, D. Ferrite-based perovskites as cathode materials for anode-supported solid oxide fuel cells: Part I. Variation of composition. Solid State Ion. 2005, 176, 1341–1350. [Google Scholar] [CrossRef]
- Mai, A.; Haanappel, V.A.C.; Tietz, F.; Stöver, D. Ferrite-based perovskites as cathode materials for anode-supported solid oxide fuel cells: Part II. Influence of the CGO interlayer. Solid State Ion. 2006, 177, 2103–2107. [Google Scholar] [CrossRef]
- Tai, L.W.; Nasrallah, M.M.; Anderson, H.U.; Sparlin, D.M.; Sehlin, S.R. Structure and electrical properties of La1−xSrxCo1−yFeyO3. Part 1. The system La0.8Sr0.2Co1−yFeyO3. Solid State Ion. 1995, 76, 259–271. [Google Scholar] [CrossRef]
- Gross, S.M.; Koppitz, T.; Remmel, J.; Reisgen, U. Glass-Ceramic Materials of the System BaO-CaO-SiO2 as Sealants for Sofc Applications. In Advances in Solid Oxide Fuel Cells: Ceramic Engineering and Science Proceedings; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; pp. 239–245. [Google Scholar]





© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roehrens, D.; Packbier, U.; Fang, Q.; Blum, L.; Sebold, D.; Bram, M.; Menzler, N. Operation of Thin-Film Electrolyte Metal-Supported Solid Oxide Fuel Cells in Lightweight and Stationary Stacks: Material and Microstructural Aspects. Materials 2016, 9, 762. https://doi.org/10.3390/ma9090762
Roehrens D, Packbier U, Fang Q, Blum L, Sebold D, Bram M, Menzler N. Operation of Thin-Film Electrolyte Metal-Supported Solid Oxide Fuel Cells in Lightweight and Stationary Stacks: Material and Microstructural Aspects. Materials. 2016; 9(9):762. https://doi.org/10.3390/ma9090762
Chicago/Turabian StyleRoehrens, Daniel, Ute Packbier, Qingping Fang, Ludger Blum, Doris Sebold, Martin Bram, and Norbert Menzler. 2016. "Operation of Thin-Film Electrolyte Metal-Supported Solid Oxide Fuel Cells in Lightweight and Stationary Stacks: Material and Microstructural Aspects" Materials 9, no. 9: 762. https://doi.org/10.3390/ma9090762