Electrospun Polymer Nanofibers Reinforced by Tannic Acid/Fe+++ Complexes
†
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of PVA Stock Solutions and TA-Fe+++ Stock Suspension
2.2.2. Preparation and Characterization of Spinning Solutions
2.2.3. Electrospinning
2.2.4. Fiber Characterization
2.2.5. Statistical Analysis
3. Results and Discussion
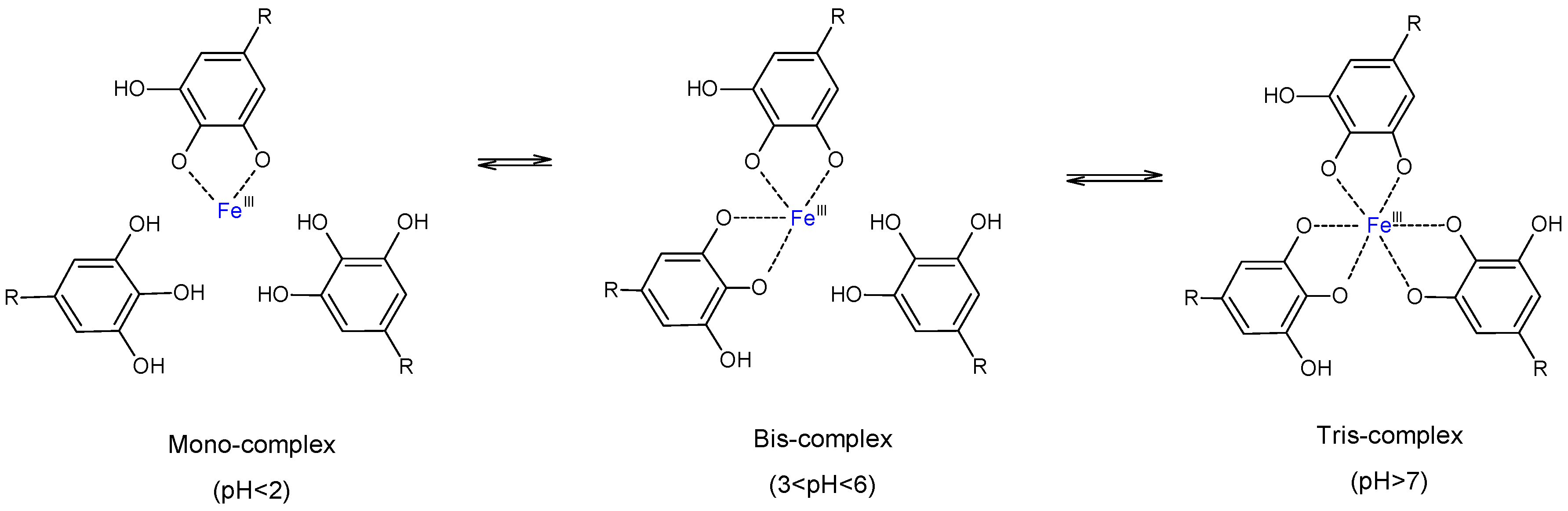
3.1. Formation of TA-Fe+++ Complexes
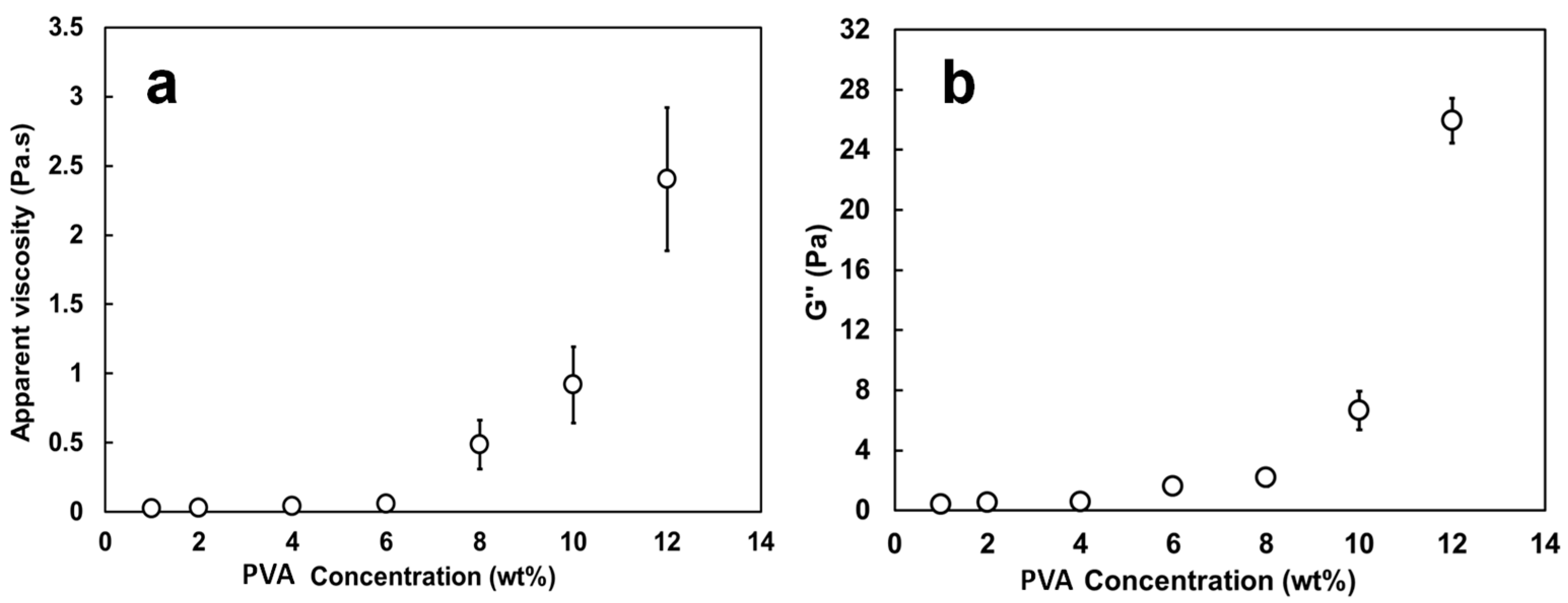
3.2. Characterization of the Spinning Solutions
3.3. Electrospinning
3.4. Mechanical Properties of the Fibrous Mats
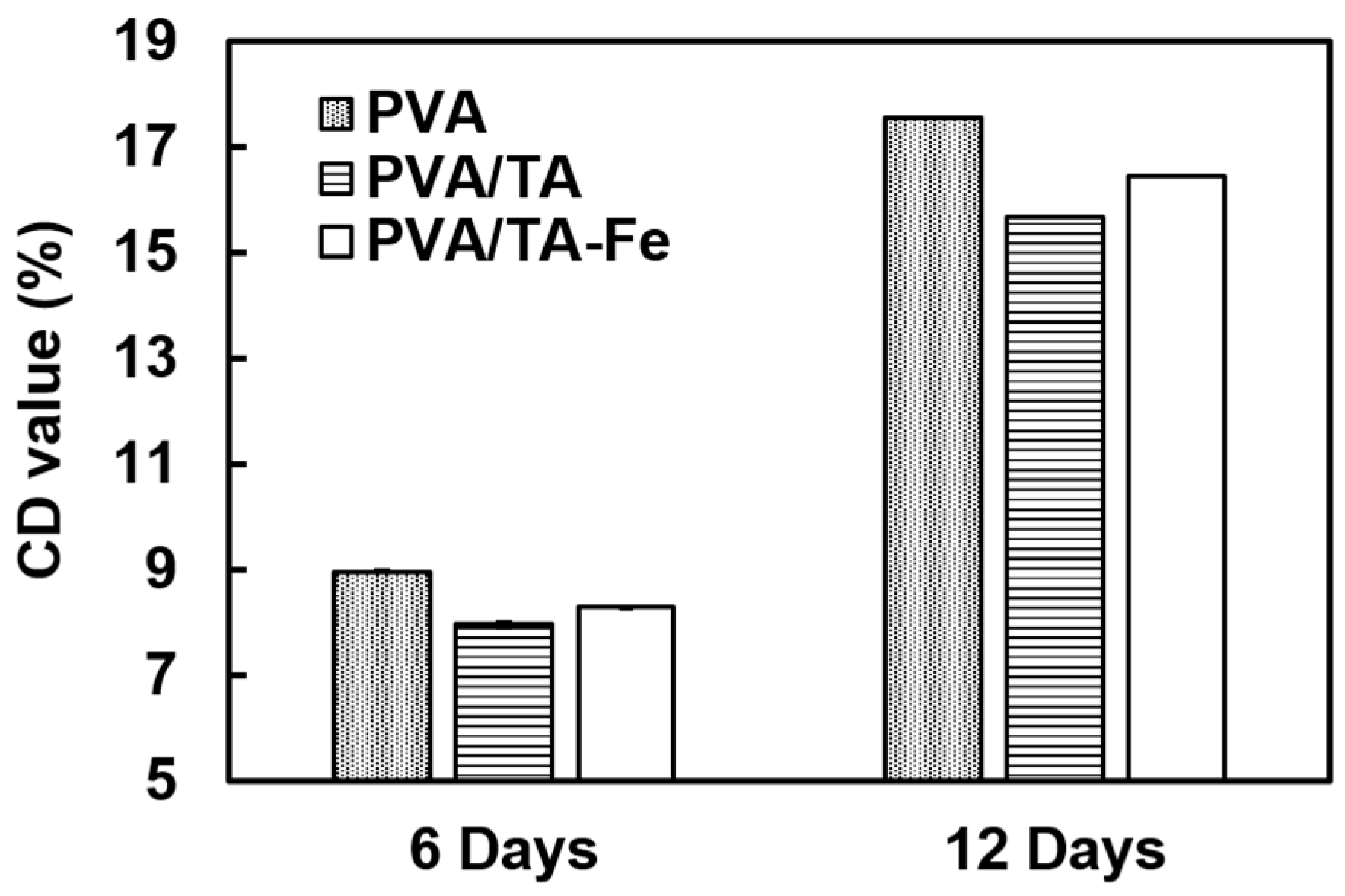
3.5. Antioxidant Activity Assay in Sunflower Oil
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bhushani, J.A.; Anandharamakrishnan, C. Electrospinning and electrospraying techniques: Potential food based applications. Trends Food Sci. Technol. 2014, 38, 21–33. [Google Scholar] [CrossRef]
- Fernandez, A.; Torres-Giner, S.; Lagaron, J.M. Novel route to stabilization of bioactive antioxidants by encapsulation in electrospun fibers of zein prolamine. Food Hydrocoll. 2009, 23, 1427–1432. [Google Scholar] [CrossRef]
- Mohammadzadehmoghadam, S.; Dong, Y.; Davies, I.J. Recent Progress in Electrospun Nanofibers: Reinforcement Effect and Mechanical Performance. J. Polym. Sci. Part B Polym. Phys. 2015, 53, 1171–1212. [Google Scholar] [CrossRef]
- Kim, J.-S.; Reneker, D.H. Mechanical properties of composites using ultrafine electrospun fibers. Polym. Compos. 1999, 20, 124–131. [Google Scholar] [CrossRef]
- Kriegel, C.; Arrechi, A.; Kit, K.; McClements, D.J.; Weiss, J. Fabrication, functionalization, and application of electrospun biopolymer nanofibers. Crit. Rev. Food Sci. Nutr. 2008, 48, 775–797. [Google Scholar] [CrossRef] [PubMed]
- Greiner, A.; Wendorff, J.H. Electrospinning: A fascinating method for the preparation of ultrathin fibres. Angew. Chem. Int. Ed. 2007, 46, 5670–5703. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.J.; Stoyanov, S.D.; Stride, E.; Pelan, E.; Edirisinghe, M. Electrospinning versus fibre production methods: From specifics to technological convergence. Chem. Soc. Rev. 2012, 41, 4708–4735. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Kim, S.; Oh, D.X.; Masic, A.; Cha, H.J.; Hwang, D.S. Mussel-inspired adhesive protein-based electrospun nanofibers reinforced by Fe(III)-DOPA complexation. J. Mater. Chem. B 2015, 3, 112–118. [Google Scholar] [CrossRef]
- Spearman, S.S.; Rivero, I.V.; Abidi, N. Influence of Polycaprolactone/Polyglycolide Blended Electrospun Fibers on the Morphology and Mechanical Properties of Polycaprolactone. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Deng, L.; Eichhorn, S.J.; Kao, C.-C.; Young, R.J. The Effective Young’s Modulus of Carbon Nanotubes in Composites. ACS Appl. Mater. Interfaces 2011, 3, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Jose, M.V.; Thomas, V.; Xu, Y.; Bellis, S.; Nyairo, E.; Dean, D. Aligned bioactive multi-component nanofibrous nanocomposite scaffolds for bone tissue engineering. Macromol. Biosci. 2010, 10, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Deng, Y. Increased Mechanical Properties of Aligned and Isotropic Electrospun PVA Nanofiber Webs by Cellulose Nanowhisker Reinforcement. Macromol. Res. 2012, 20, 76–83. [Google Scholar] [CrossRef]
- Hagerman, A.E.; Riedl, K.M.; Jones, G.A.; Sovik, K.N.; Ritchard, N.T.; Hartzfeld, P.W.; Riechel, T.L. High molecular weight plant polyphenolics (tannins) as biological antioxidants. J. Agric. Food Chem. 1998, 46, 1887–1892. [Google Scholar] [CrossRef]
- Valcic, S.; Muders, A.; Jacobsen, N.E.; Liebler, D.C.; Timmermann, B.N. Antioxidant chemistry of green tea catechins. Identification of products of the reaction of (−)-epigallocatechin gallate with peroxyl radicals. Chem. Res. Toxicol. 1999, 12, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.-T.; Wong, T.Y.; Wei, C.-I.; Huang, Y.-W.; Lin, Y. Tannins and human health: A review. Crit. Rev. Food Sci. Nutr. 1998, 38, 421–464. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, İ.; Huyut, Z.; Elmastaş, M.; Aboul-Enein, H.Y. Radical scavenging and antioxidant activity of tannic acid. Arab. J. Chem. 2010, 3, 43–53. [Google Scholar] [CrossRef]
- Rahim, M.A.; Ejima, H.; Cho, K.L.; Kempe, K.; Müllner, M.; Best, J.P.; Caruso, F. Coordination-driven multistep assembly of metal–polyphenol films and capsules. Chem. Mater. 2014, 26, 1645–1653. [Google Scholar]
- Thitiwongsawet, P.; Wichapeng, N.; Waiyaroj, A. Electrospun polycaprolactone fiber mats as carriers for tannic acid. Adv. Mat. Res. 2013, 774, 896–900. [Google Scholar] [CrossRef]
- Zhou, B.; Jin, X.; Li, J.; Xu, W.; Liu, S.; Li, Y.; Li, B. Vacuum-assisted layer-by-layer electrospun membranes: Antibacterial and antioxidative applications. RSC Adv. 2014, 4, 54517–54524. [Google Scholar] [CrossRef]
- Kiechel, M.A.; Schauer, C.L. Non-covalent crosslinkers for electrospun chitosan fibers. Carbohyd. Polym. 2013, 95, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Andjelkovic, M.; Van Camp, J.; De Meulenaer, B.; Depaemelaere, G.; Socaciu, C.; Verloo, M.; Verhe, R. Iron-chelation properties of phenolic acids bearing catechol and galloyl groups. Food Chem. 2006, 98, 23–31. [Google Scholar] [CrossRef]
- Sample, C.C.S. Hydrogen-bonded Layer-by-Layer Assembly of Poly(vinyl alcohol) and Tannic Acid; Massachusetts Institute of Technology: Cambridge, MA, USA, 2014. [Google Scholar]
- Ejima, H.; Richardson, J.J.; Liang, K.; Best, J.P.; van Koeverden, M.P.; Such, G.K.; Cui, J.; Caruso, F. One-step assembly of coordination complexes for versatile film and particle engineering. Science 2013, 341, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Ping, Y.; Ejima, H.; Alt, K.; Meissner, M.; Richardson, J.J.; Yan, Y.; Peter, K.; von Elverfeldt, D.; Hagemeyer, C.E.; et al. Engineering Multifunctional Capsules through the Assembly of Metal–Phenolic Networks. Angew. Chem. Int. Ed. 2014, 53, 5546–5551. [Google Scholar] [CrossRef] [PubMed]
- Rošic, R.; Pelipenko, J.; Kristl, J.; Kocbek, P.; Bester-Rogac, M.; Baumgartner, S. Physical characteristics of poly(vinyl alcohol) solutions in relation to electrospun nanofiber formation. Eur. Polym. J. 2013, 49, 290–298. [Google Scholar] [CrossRef]
- Rwei, S.-P.; Huang, C.-C. Electrospinning PVA solution-rheology and morphology analyses. Fibers Polym. 2012, 13, 44–50. [Google Scholar] [CrossRef]
- Son, W.K.; Youk, J.H.; Lee, T.S.; Park, W.H. Effect of pH on electrospinning of poly(vinyl alcohol). Mater. Lett. 2005, 59, 1571–1575. [Google Scholar]
- Camerlo, A.; Vebert-Nardin, C.; Rossi, R.M.; Popa, A.-M. Fragrance encapsulation in polymeric matrices by emulsion electrospinning. Eur. Polym. J. 2013, 49, 3806–3813. [Google Scholar] [CrossRef]
- Kayaci, F.; Uyar, T. Encapsulation of vanillin/cyclodextrin inclusion complex in electrospun polyvinyl alcohol (PVA) nanowebs: Prolonged shelf-life and high temperature stability of vanillin. Food Chem. 2012, 133, 641–649. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Yue, T.; Lee, T.-C. Development of Pleurocidin-poly(vinyl alcohol) electrospun antimicrobial nanofibers to retain antimicrobial activity in food system application. Food Control 2015, 54, 150–157. [Google Scholar] [CrossRef]
- Sousa, A.M.M.; Souza, H.K.S.; Uknalis, J.; Liu, S.-C.; Gonçalves, M.P.; Liu, L. Electrospinning of agar/PVA aqueous solutions and its relation with rheological properties. Carbohydr. Polym. 2015, 115, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, A.E.; Roozen, J.P. Effect of plant extracts on the oxidative stability of sunflower oil and emulsion. Food Chem. 1999, 64, 323–329. [Google Scholar] [CrossRef]
- Royston, J.P. An extension of Shapiro and Wilk’s W test for normality to large samples. Appl. Stat. 1982, 31, 115–124. [Google Scholar] [CrossRef]
- Ramakrishna, S.; Fujihara, K.; Teo, W.-E.; Lim, T.-C.; Ma, Z. An Introduction to Electrospinning and Nanofibers; World Scientific Publishing Co. Ltd.: Singapore, 2005. [Google Scholar]
- Shenoy, S.L.; Bates, W.D.; Frisch, H.L.; Wnek, G.E. Role of chain entanglements on fiber formation during electrospinning of polymer solutions: Good solvent, non-specific polymer–polymer interaction limit. Polymer 2005, 46, 3372–3384. [Google Scholar] [CrossRef]
- Bercea, M.; Morariu, S.; Rusu, D. In situ gelation of aqueous solutions of entangled poly(vinyl alcohol). Soft Matter 2013, 9, 1244–1253. [Google Scholar] [CrossRef]
- Deitzel, J.M.; Kleinmeyer, J.; Harris, D.; Tan, N.C.B. The effect of processing variables on the morphology of electrospun nanofibers and textiles. Polymer 2001, 42, 261–272. [Google Scholar] [CrossRef]
- Huang, Z.-M.; Zhang, Y.; Ramakrishna, S.; Lim, C. Electrospinning and mechanical characterization of gelatin nanofibers. Polymer 2004, 45, 5361–5368. [Google Scholar] [CrossRef]
- Lancuški, A.; Vasilyev, G.; Putaux, J.-L.; Zussman, E. Rheological Properties and Electrospinnability of High-Amylose Starch in Formic Acid. Biomacromolecules 2015, 16, 2529–2536. [Google Scholar] [CrossRef] [PubMed]
- Cai, N.; Dai, Q.; Wang, Z.; Luo, X.; Xue, Y.; Yu, F. Toughening of electrospun poly(L-lactic acid) nanofiber scaffolds with unidirectionally aligned halloysite nanotubes. J. Mat. Sci. 2015, 50, 1435–1445. [Google Scholar] [CrossRef]

| Sample ID. | (PVA) (wt %) | (TA) (mg/mL) | (Fe+++) (mg/mL) | Final pH | TA-Fe+++ Complexes Preferentially Formed in Solution |
|---|---|---|---|---|---|
| 4 wt % PVA/TA-Fe+++ (II) | 4 | 0.2 | 0.05 | 5.7 | bi-complexes |
| 6 wt % PVA/TA-Fe+++ (II) | 6 | 0.2 | 0.05 | 5.5 | bi-complexes |
| 8 wt % PVA/TA-Fe+++ (II) | 8 | 0.2 | 0.05 | 5.5 | bi-complexes |
| 10 wt % PVA/TA-Fe+++ (II) | 10 | 0.2 | 0.05 | 5.5 | bi-complexes |
| 12 wt % PVA/TA-Fe+++ (II) | 12 | 0.2 | 0.05 | 5.5 | bi-complexes |
| 8 wt % PVA | 8 | 0 | 0 | 5.5 | n.a. |
| 8 wt % PVA/TA | 8 | 0.2 | 0 | 5.5 | n.a. |
| 8 wt % PVA/TA-Fe+++ (III) 1 | 8 | 0.2 | 0.05 | 7.4 | tri-complexes |
| 8 wt % PVA/TA-Fe+++ (I) 2 | 8 | 0.2 | 0.05 | 2.0 | mono-complexes |
| Sample ID. | TS (MPa) | EB (%) | FDS (nm) | Fiber Morphology |
|---|---|---|---|---|
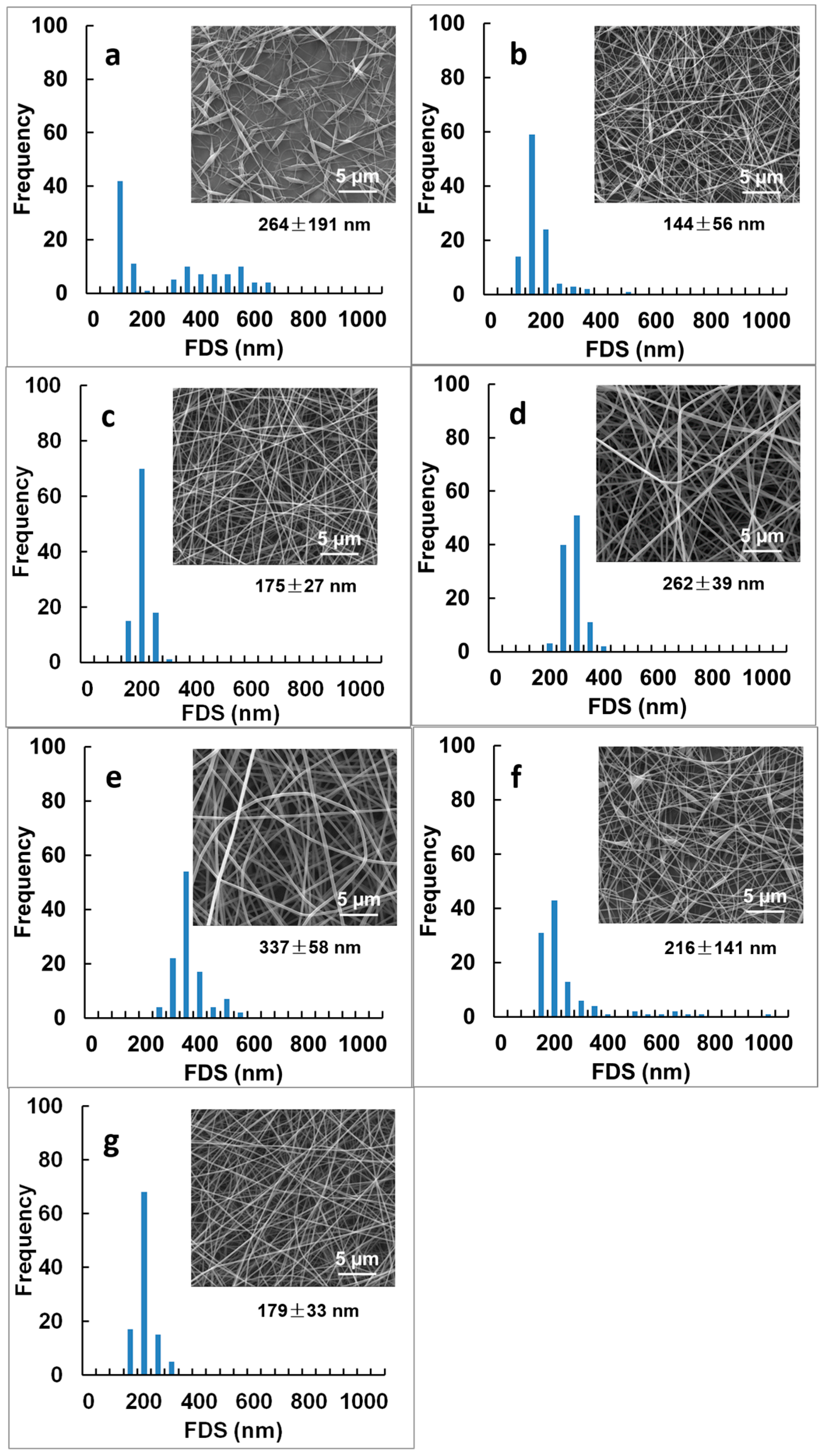
| 4 wt % PVA/TA-Fe+++ (II) | n.d. | n.d. | 264 ± 191 a,e,f | discontinuous fibers w/defects |
| 6 wt % PVA/TA-Fe+++ (II) | n.d. | n.d. | 144 ± 56 a | fibers with defects |
| 8 wt % PVA/TA-Fe+++ (II) | 31.4 ± 2.5 a | 23.9 ± 5.1 a | 175 ± 27 b | fibers |
| 10 wt % PVA/TA-Fe+++ (II) | 17.4 ± 3.8 b,c | 24.2 ± 5.4 a,b | 262 ± 39 c | fibers |
| 12 wt % PVA/TA-Fe+++ (II) | 13.8 ± 2.8 b | 41.5 ± 2.3 c | 337 ± 58 d | fibers |
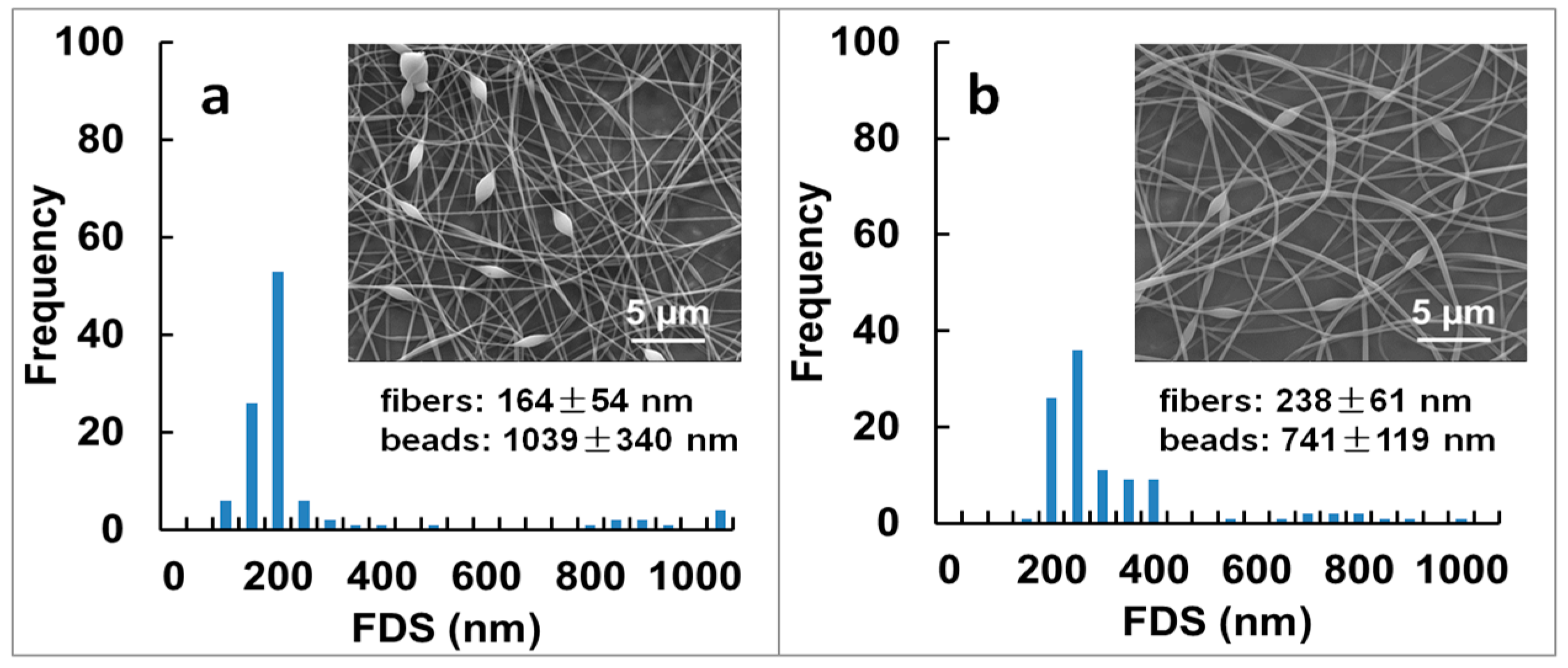
| 8 wt % PVA | 18.6 ± 4.2 d | 11.5 ± 4.1 d,e | 216 ± 141 e | beaded fibers |
| 8 wt % PVA/TA | 22.5 ± 3.3 c,d | 17.0 ± 6.9 b,d | 179 ± 33 e | fibers |
| 8 wt % PVA/TA-Fe+++ (III) | 26.4 ± 3.1 d | 15.9 ± 3.6 b,d | 246 ± 280 e | beaded fibers |
| 8 wt % PVA/TA-Fe+++ (I) | 5.7 ± 1.4 e | 4.0 ± 1.2 e | 291 ± 171 f | beaded fibers |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.; Sousa, A.M.M.; Thomas-Gahring, A.; Fan, X.; Jin, T.; Li, X.; Tomasula, P.M.; Liu, L.
Electrospun Polymer Nanofibers Reinforced by Tannic Acid/Fe+++ Complexes
. Materials 2016, 9, 757.
https://doi.org/10.3390/ma9090757
Yang W, Sousa AMM, Thomas-Gahring A, Fan X, Jin T, Li X, Tomasula PM, Liu L.
Electrospun Polymer Nanofibers Reinforced by Tannic Acid/Fe+++ Complexes
. Materials. 2016; 9(9):757.
https://doi.org/10.3390/ma9090757
Yang, Weiqiao, Ana M. M. Sousa, Audrey Thomas-Gahring, Xuetong Fan, Tony Jin, Xihong Li, Peggy M. Tomasula, and LinShu Liu.
2016. "Electrospun Polymer Nanofibers Reinforced by Tannic Acid/Fe+++ Complexes
" Materials 9, no. 9: 757.
https://doi.org/10.3390/ma9090757








