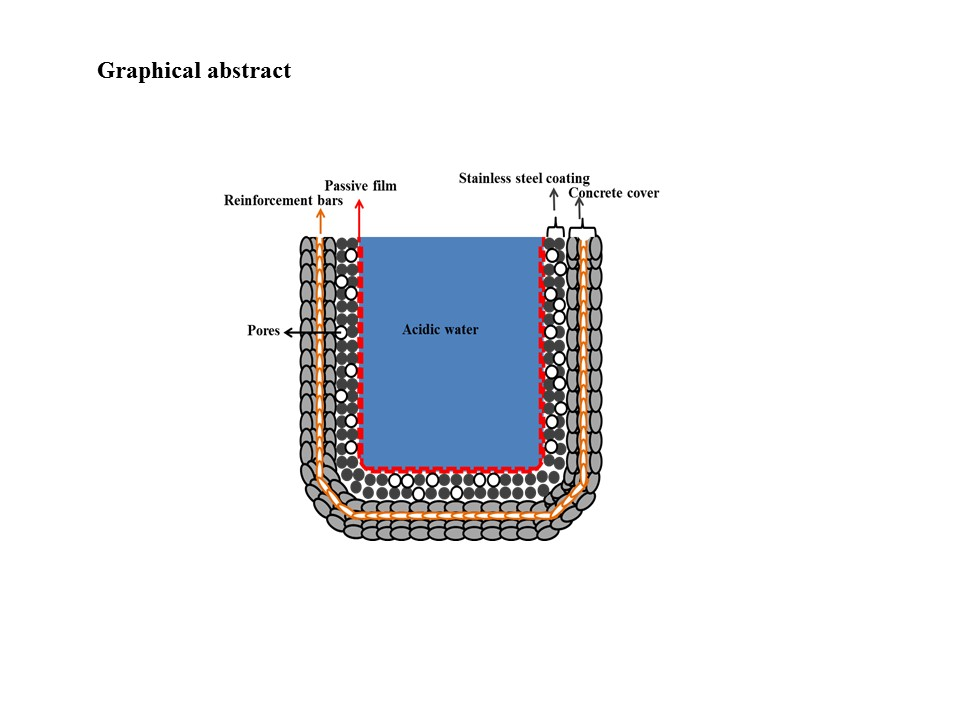
Protection of Reinforced Concrete Structures of Waste Water Treatment Reservoirs with Stainless Steel Coating Using Arc Thermal Spraying Technique in Acidified Water
Abstract
:1. Introduction
2. Experimental Procedure
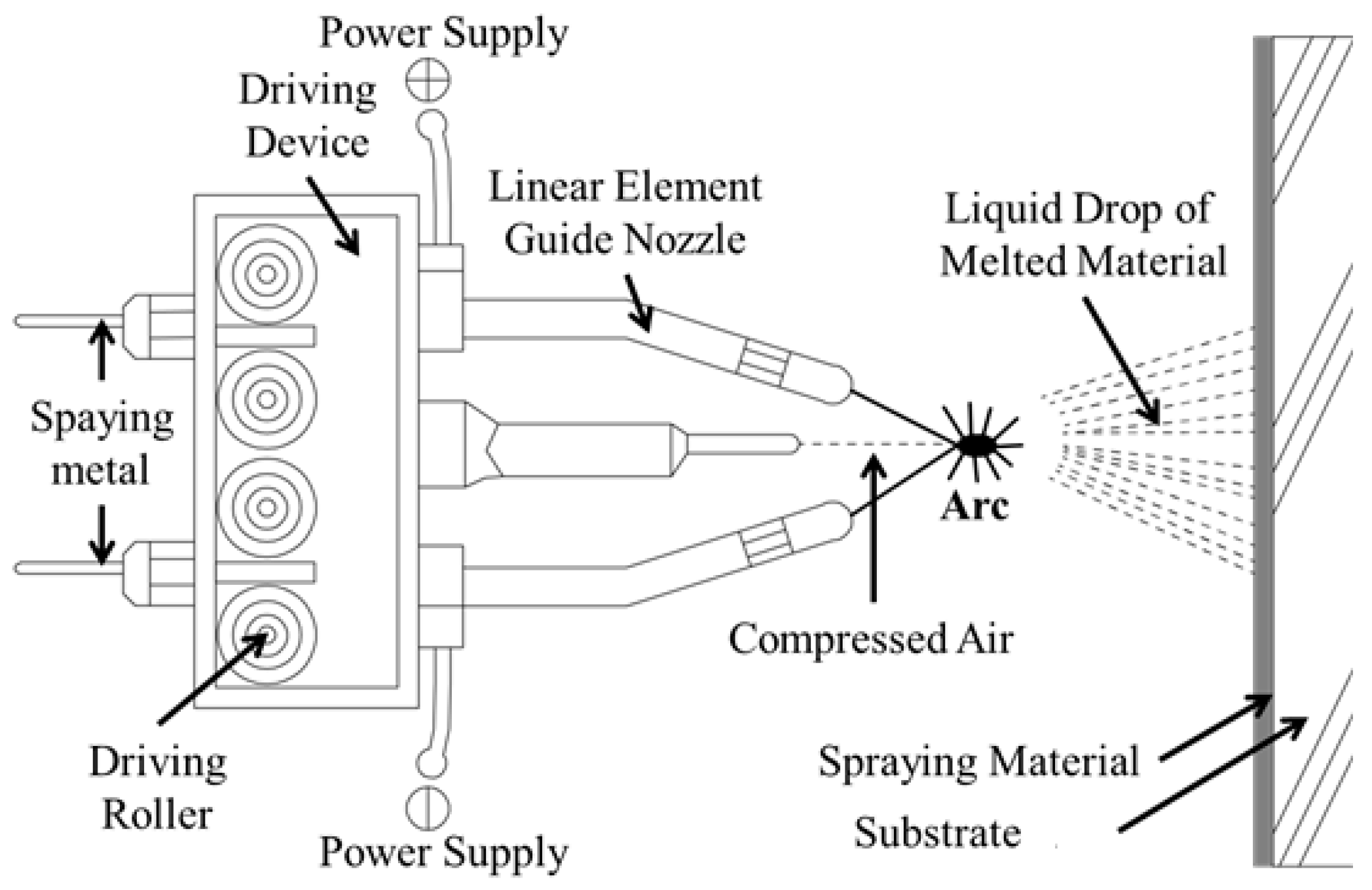
2.1. Process of Coating
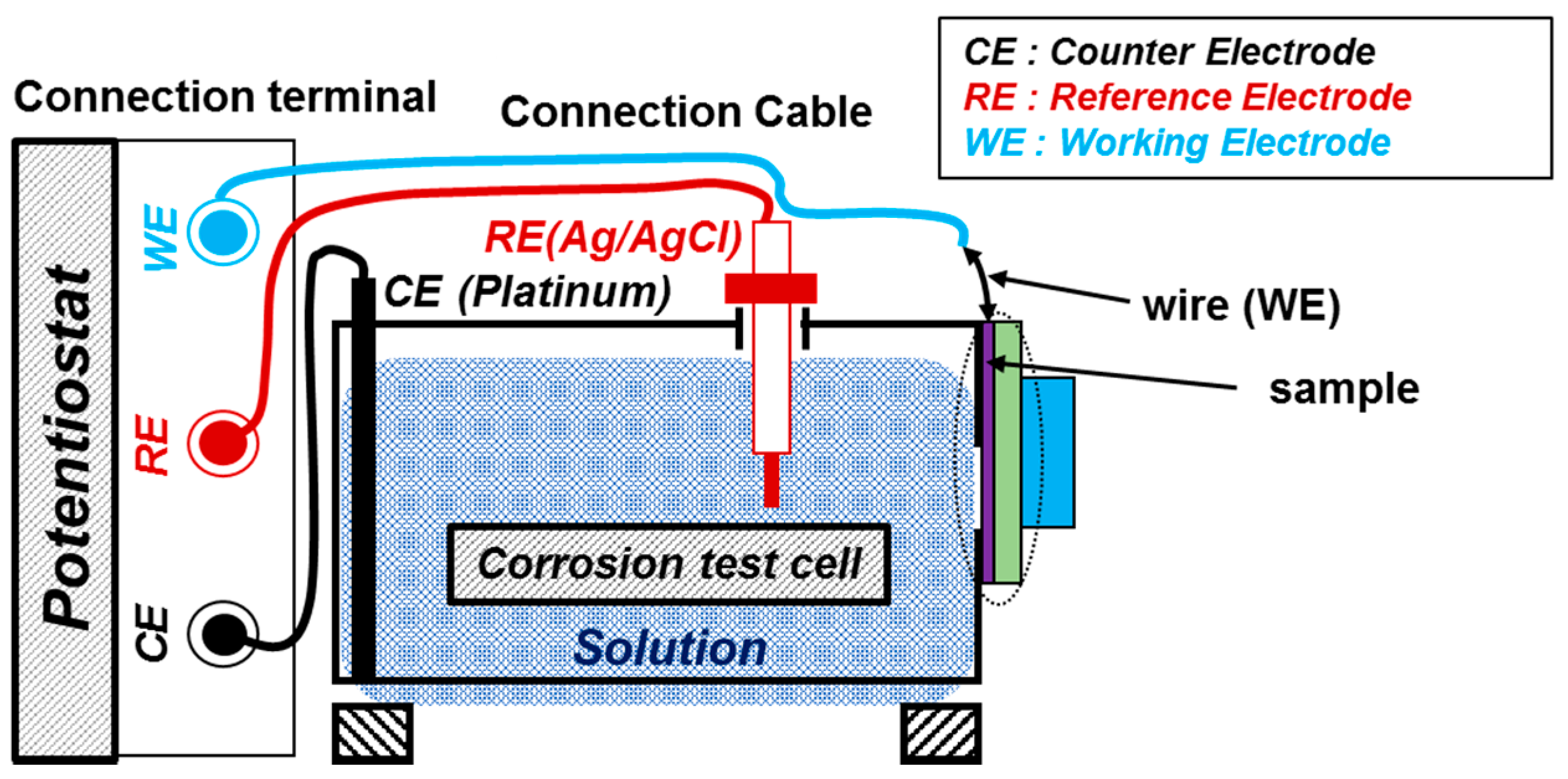
2.2. Electrochemical Experiments
2.3. Characterization of Coating
3. Results
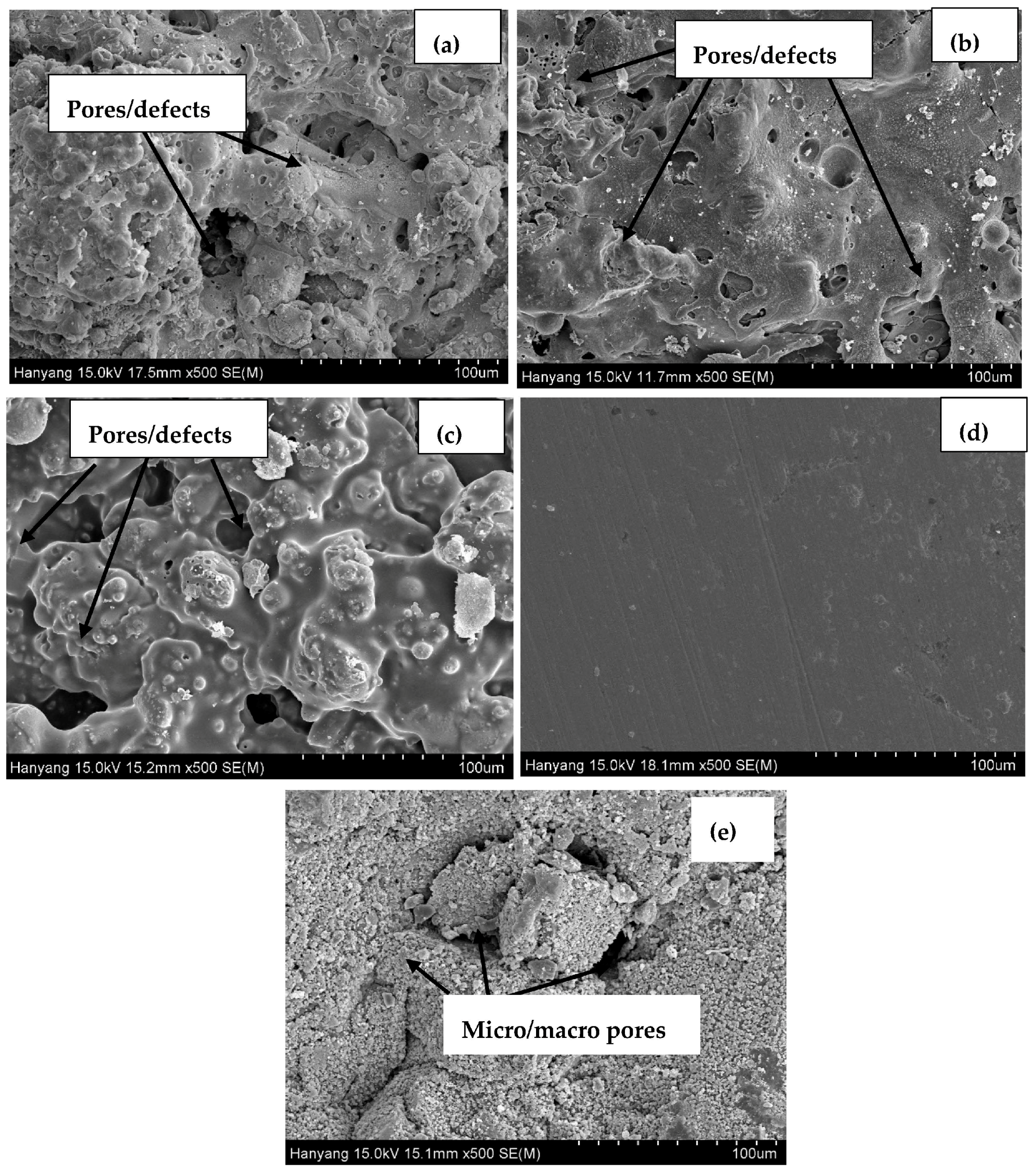
3.1. Morphology of Coatings, Plate, and Concrete Surface
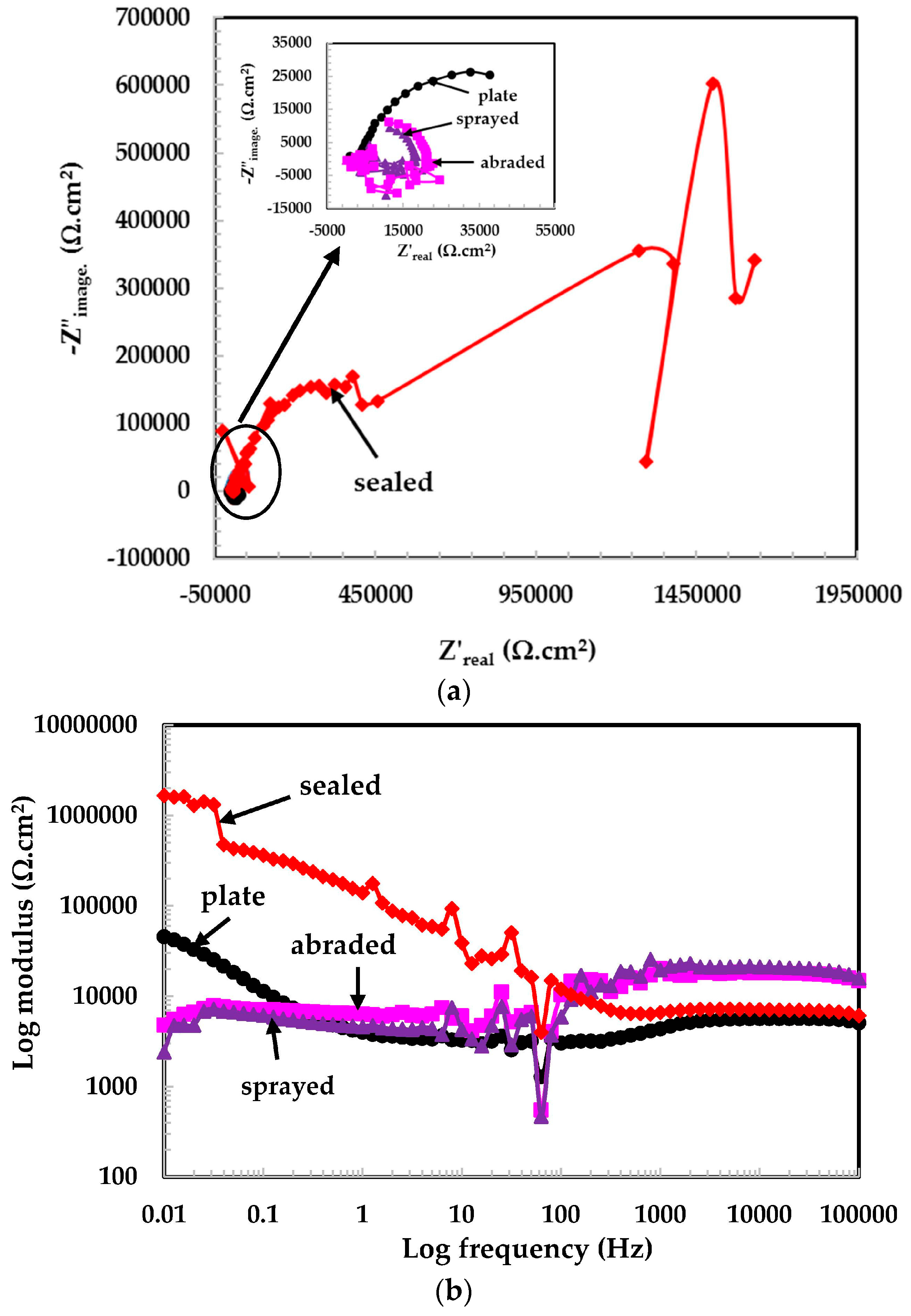
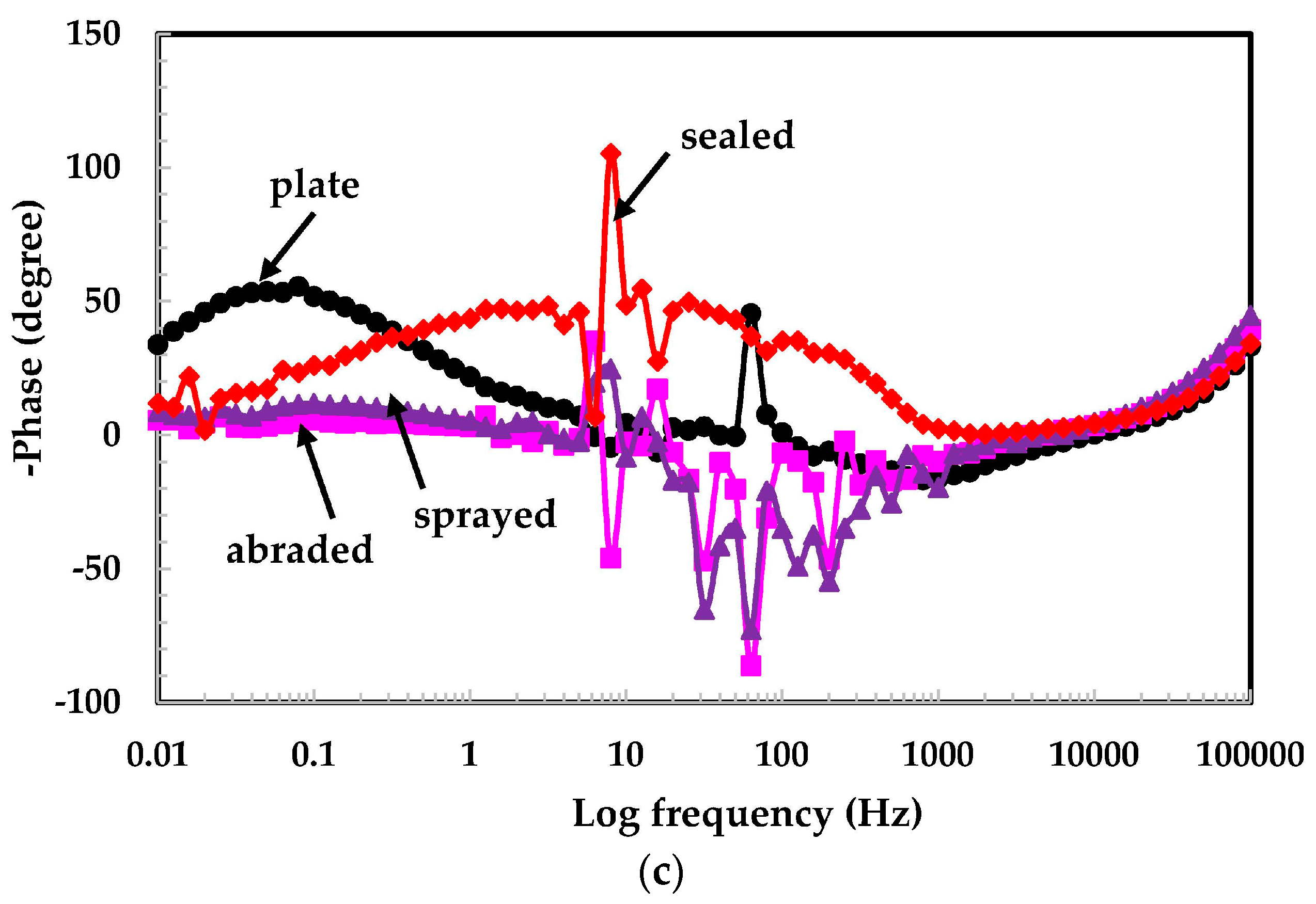
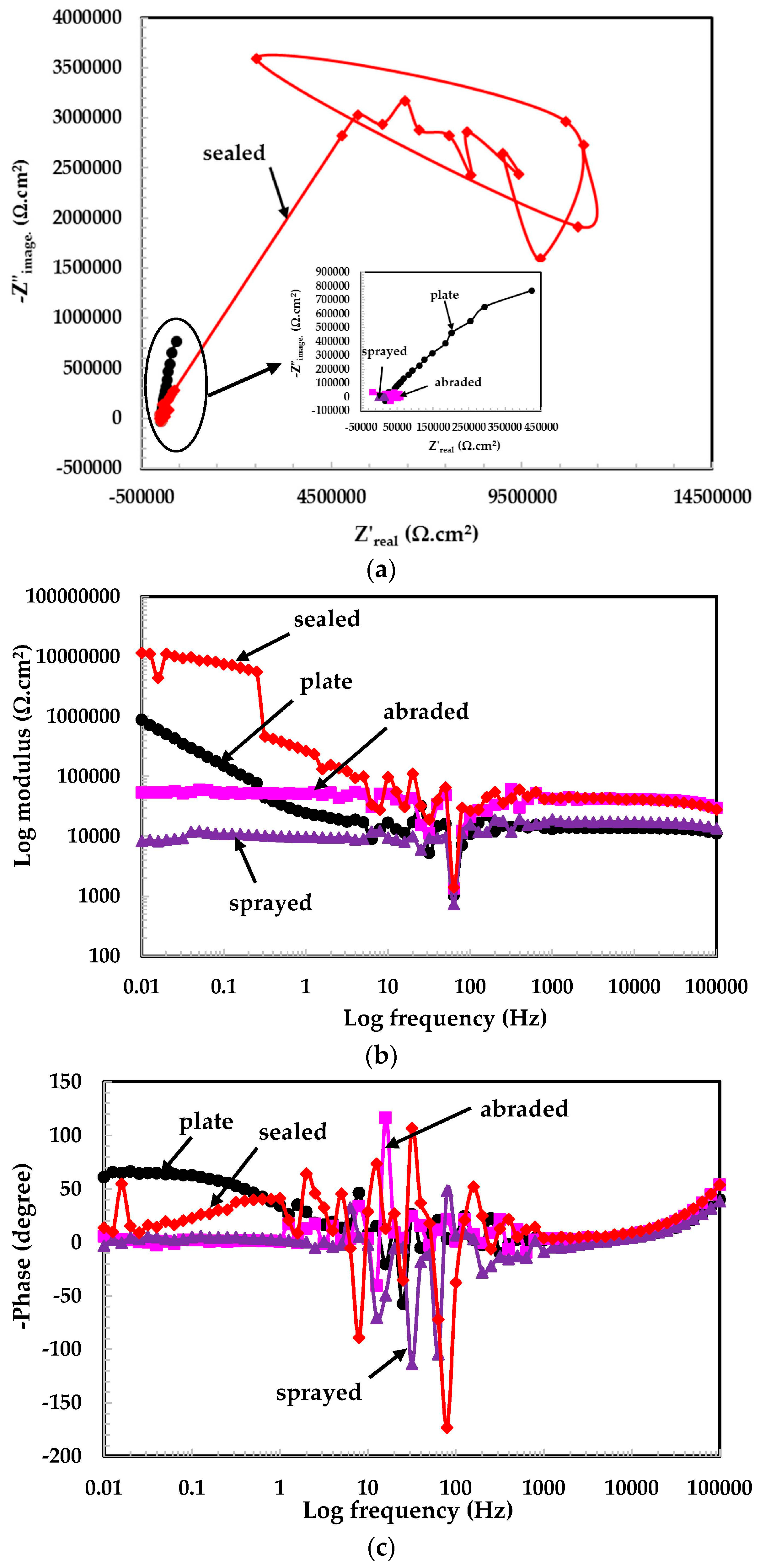
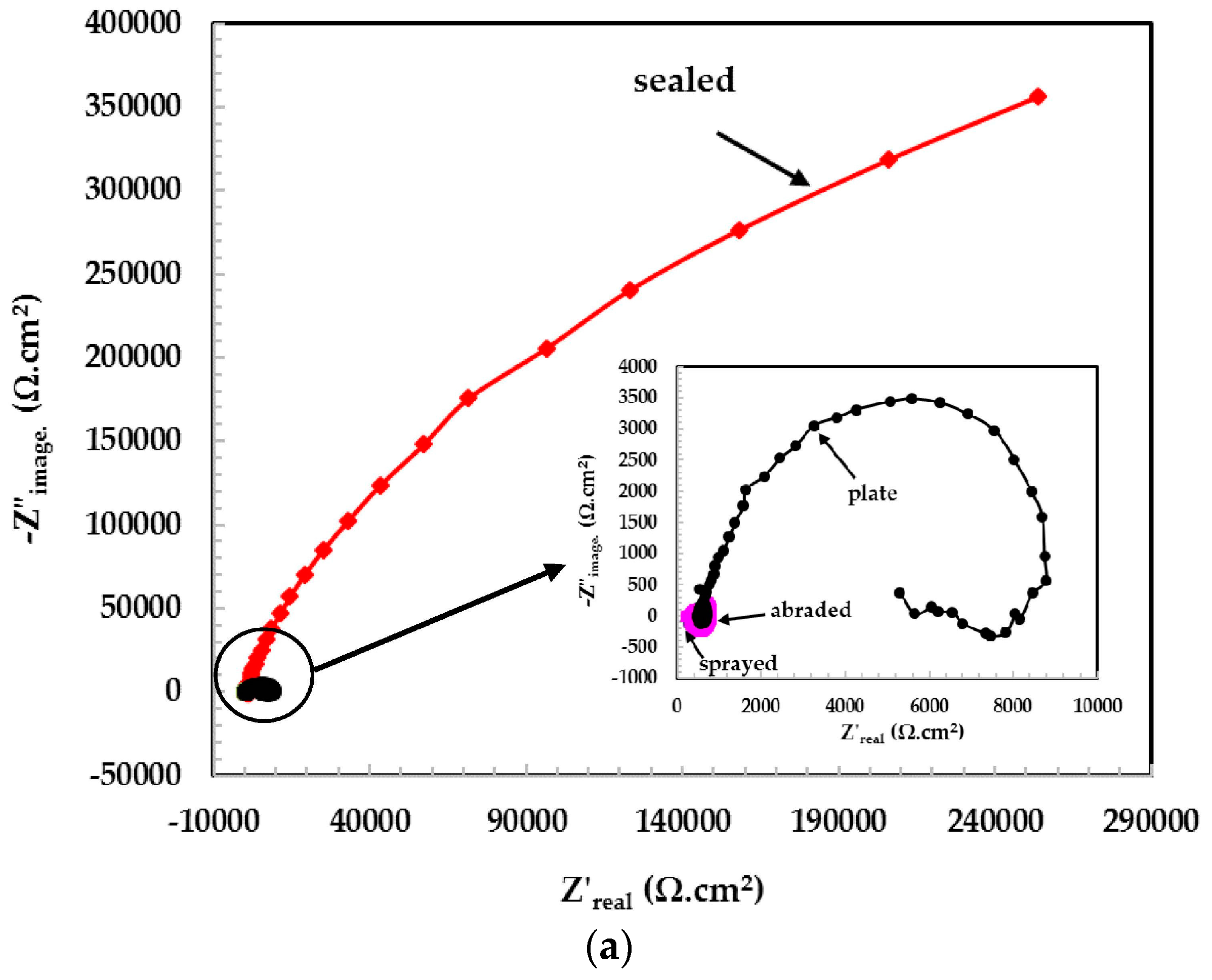
3.2. Electrochemical Experiments for Different pH Solutions
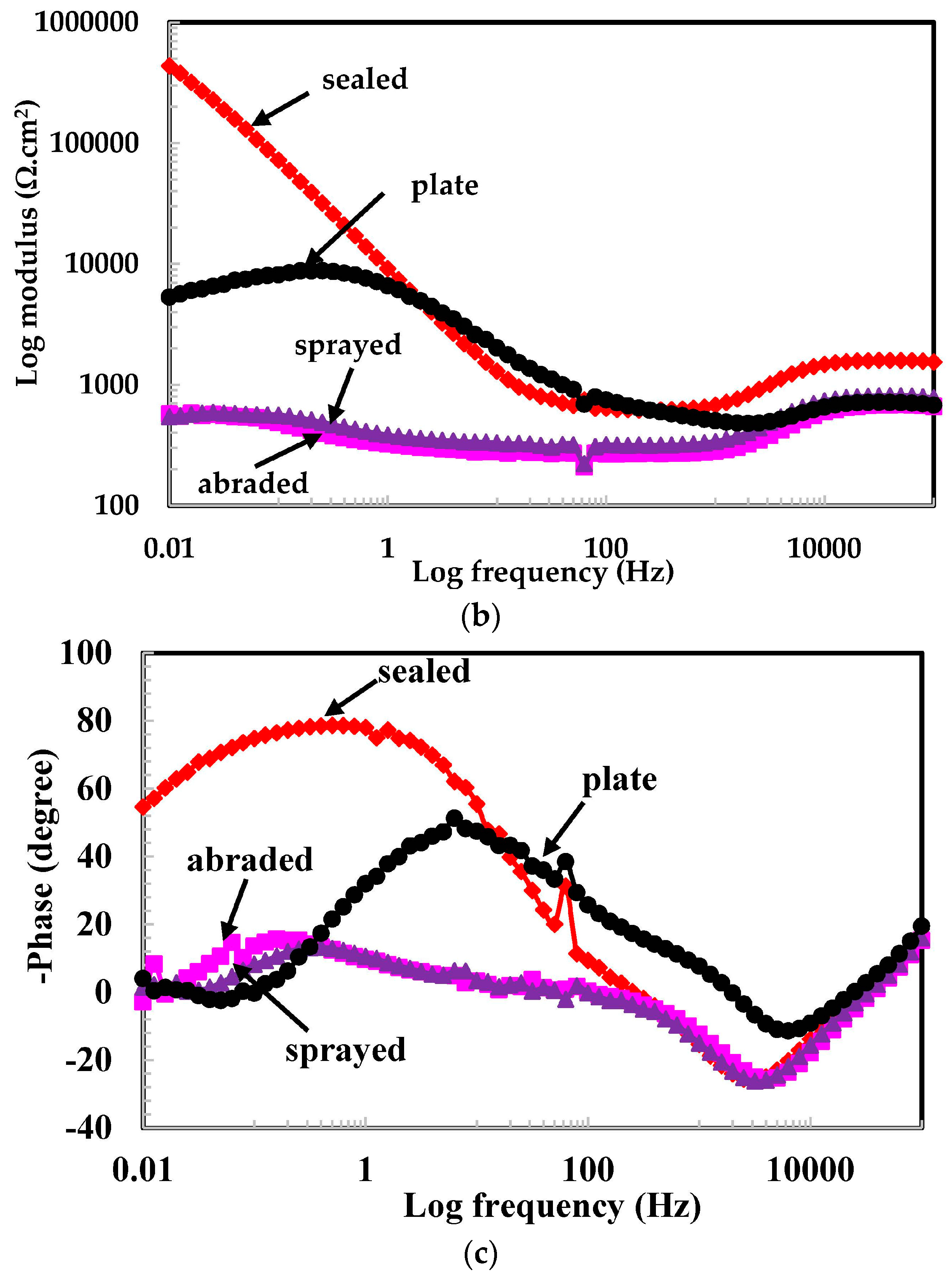
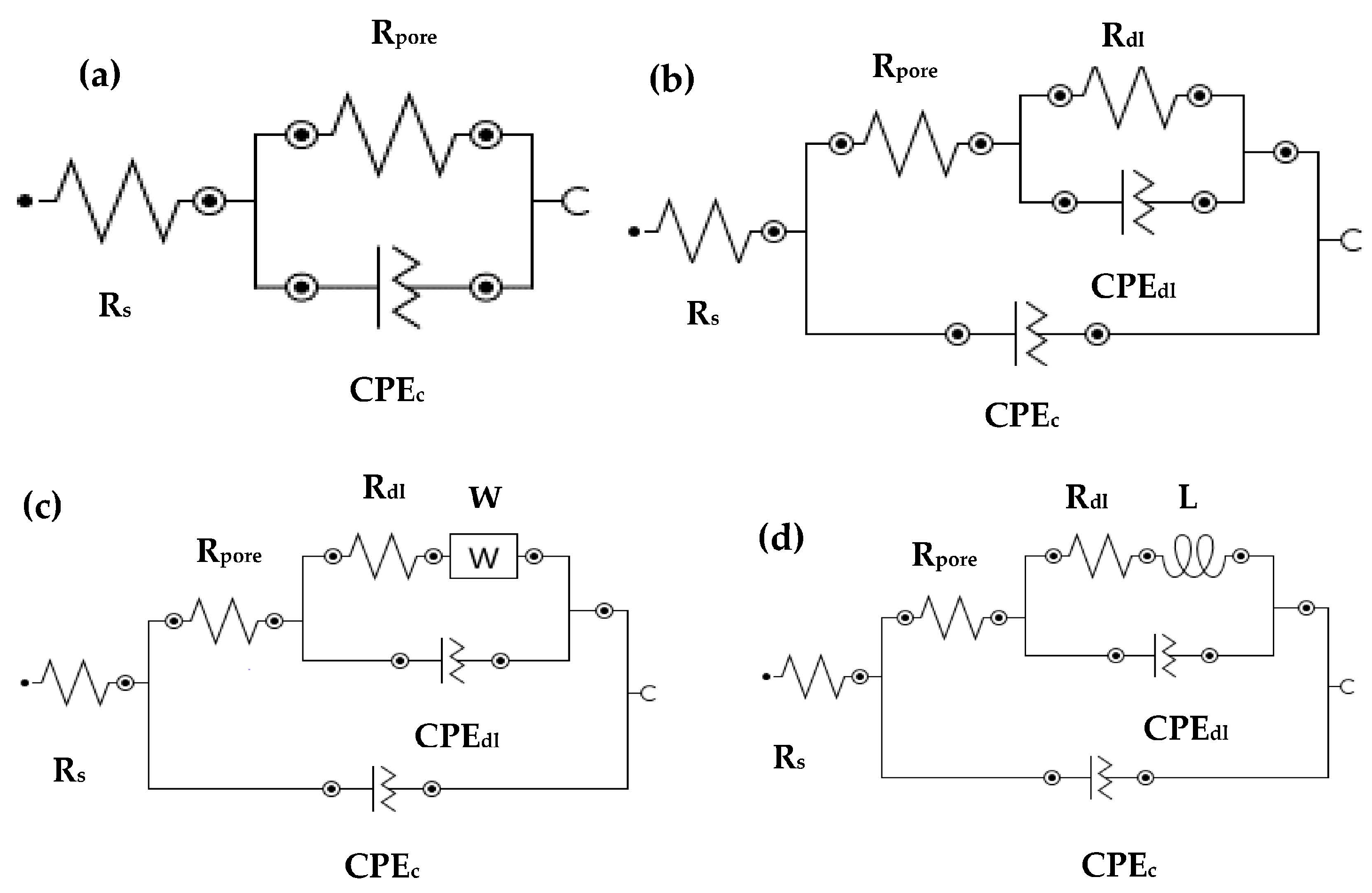
3.2.1. EIS Experiments for Different pH Solutions
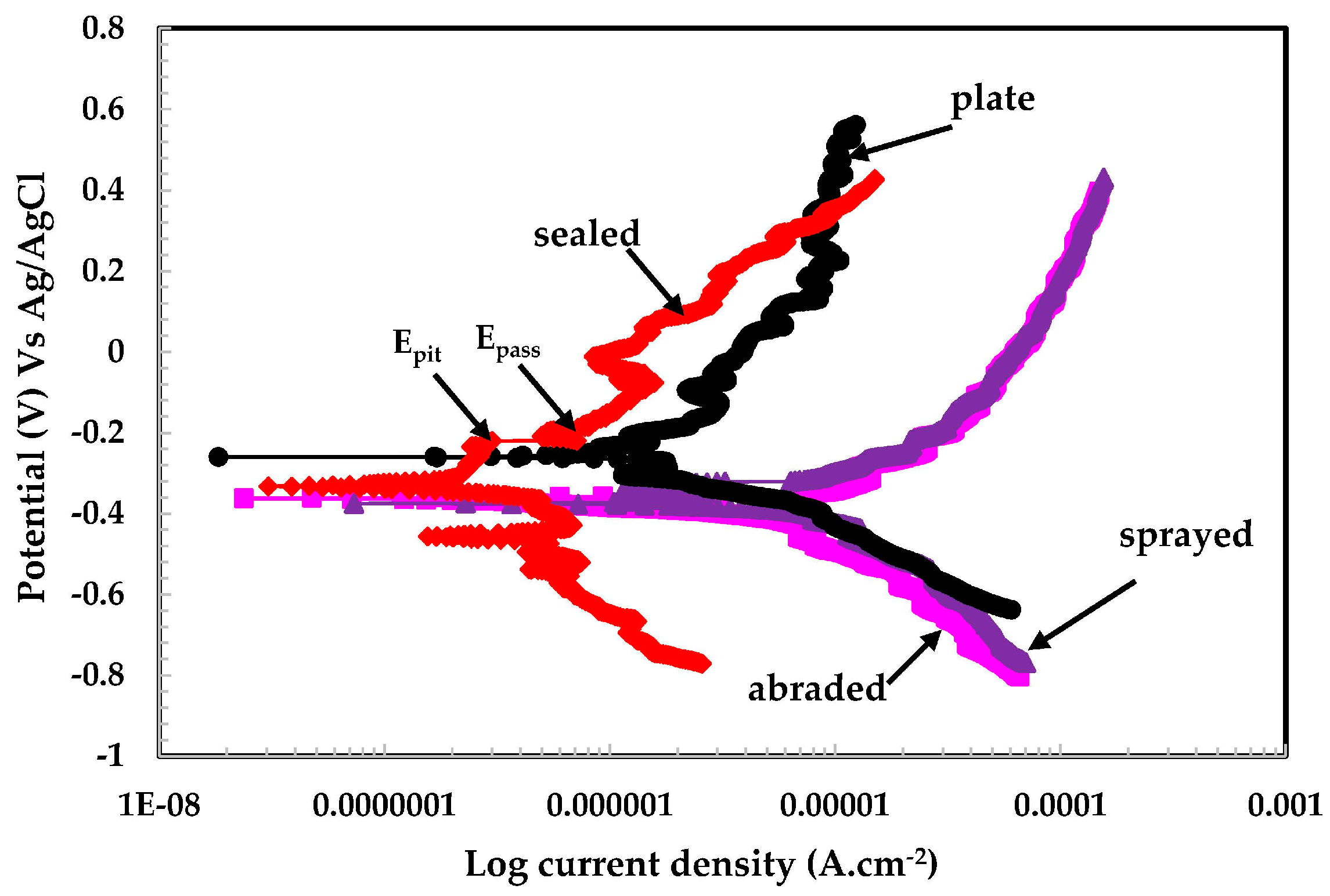
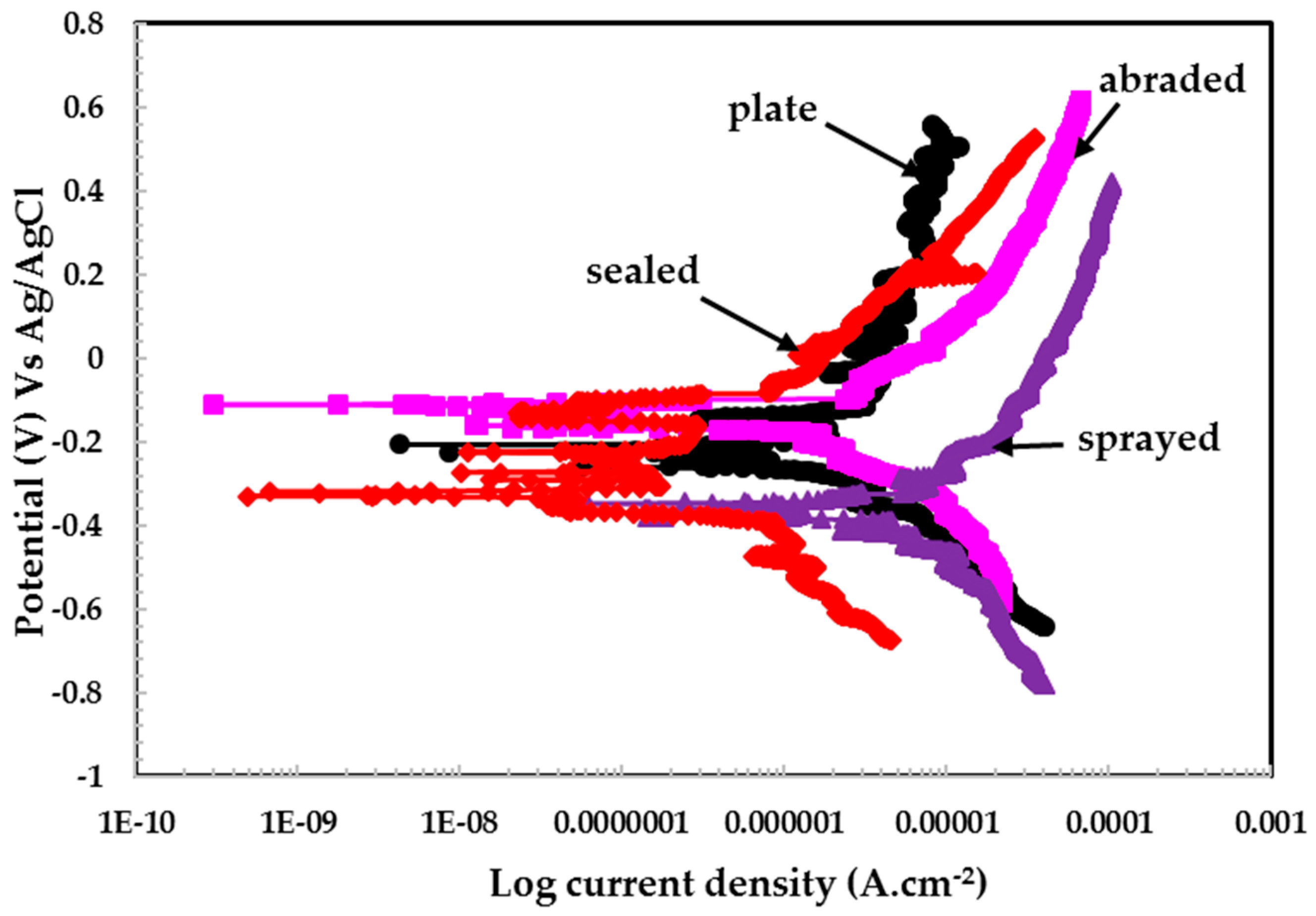
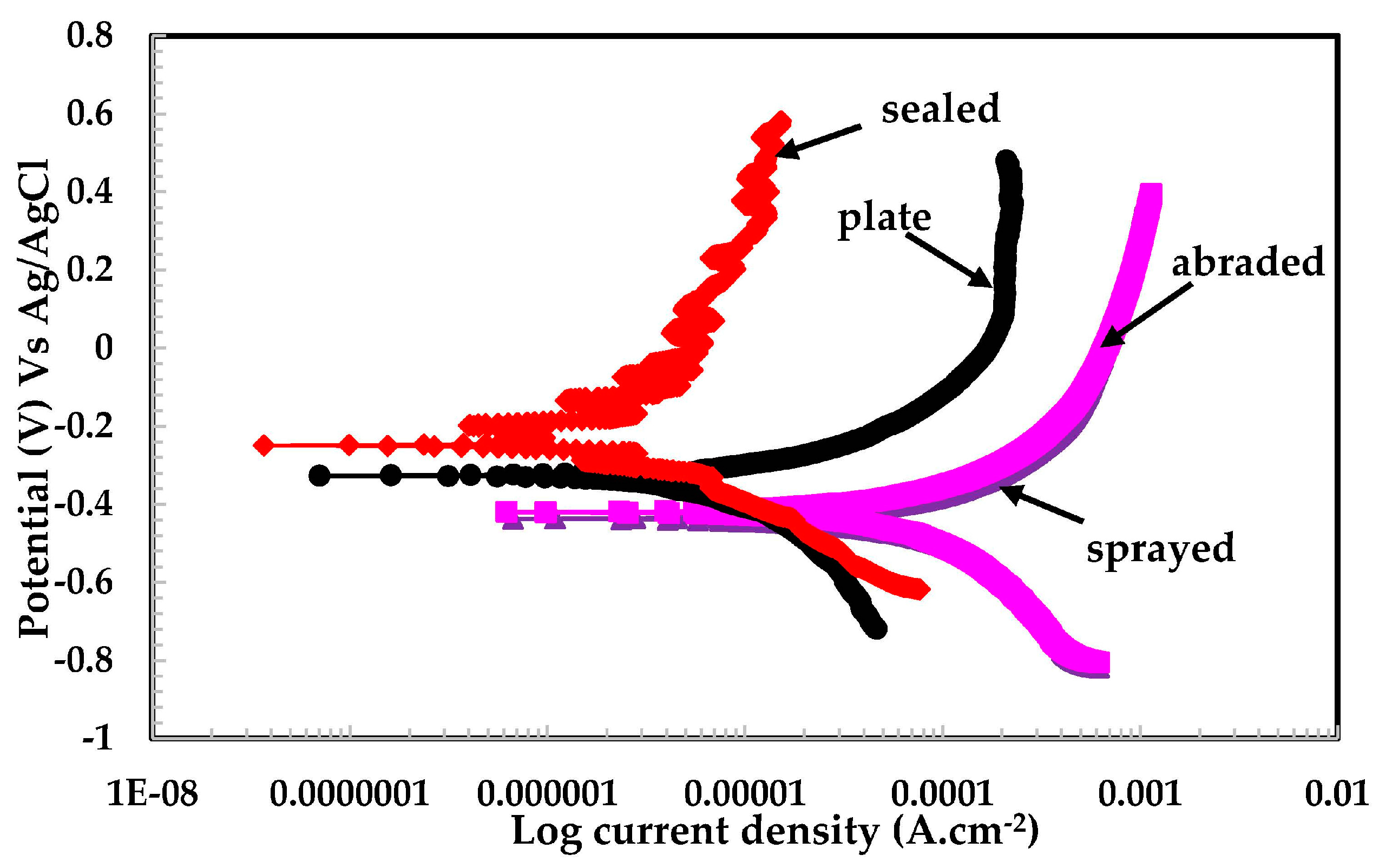
3.2.2. Potentiodynamic Experiments for Different pH Solutions
4. Discussion
5. Conclusions
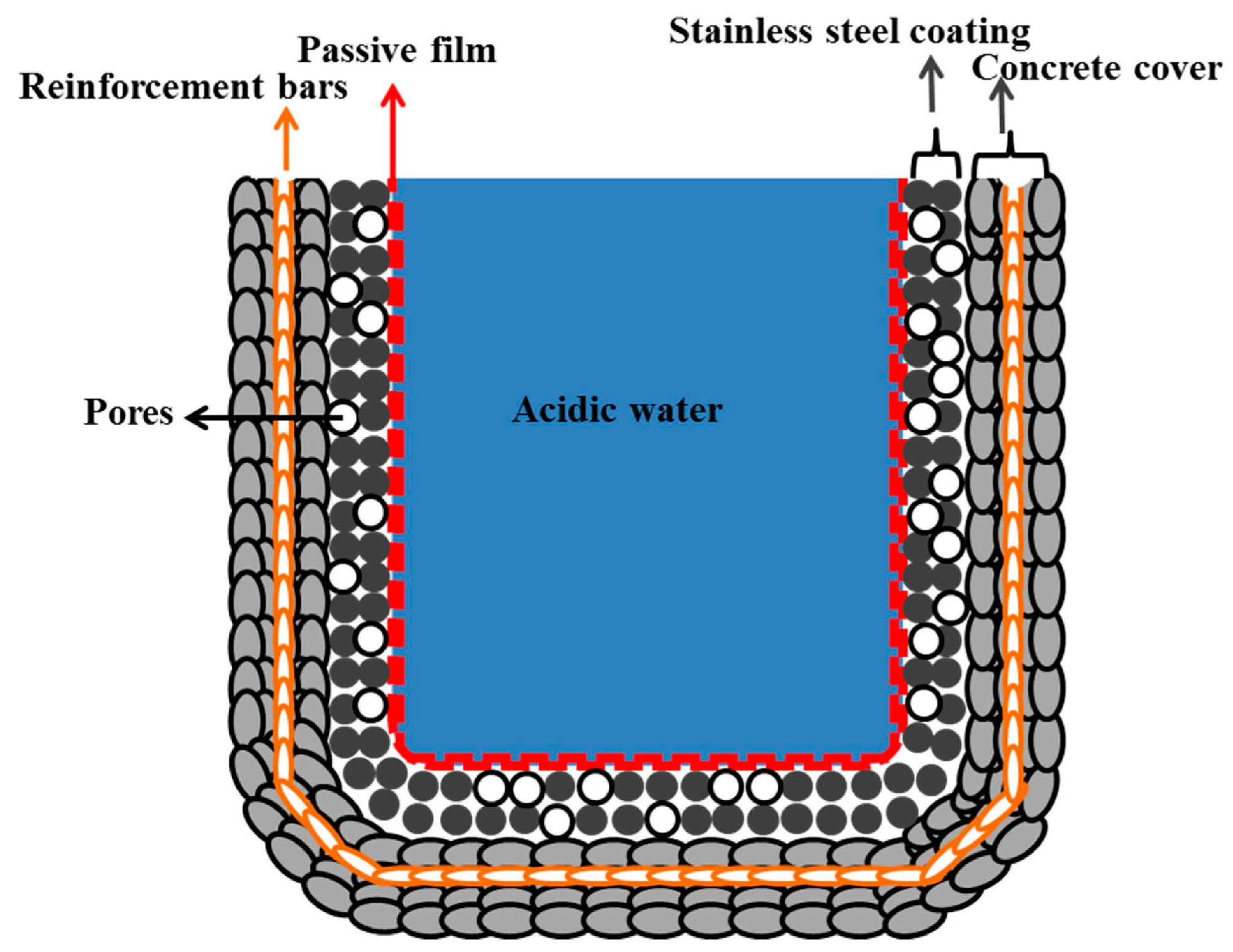
- The 316L stainless steel coating was applied to the concrete surface of a waste water reservoir, which provides protection across a variety of acidic pH solutions.
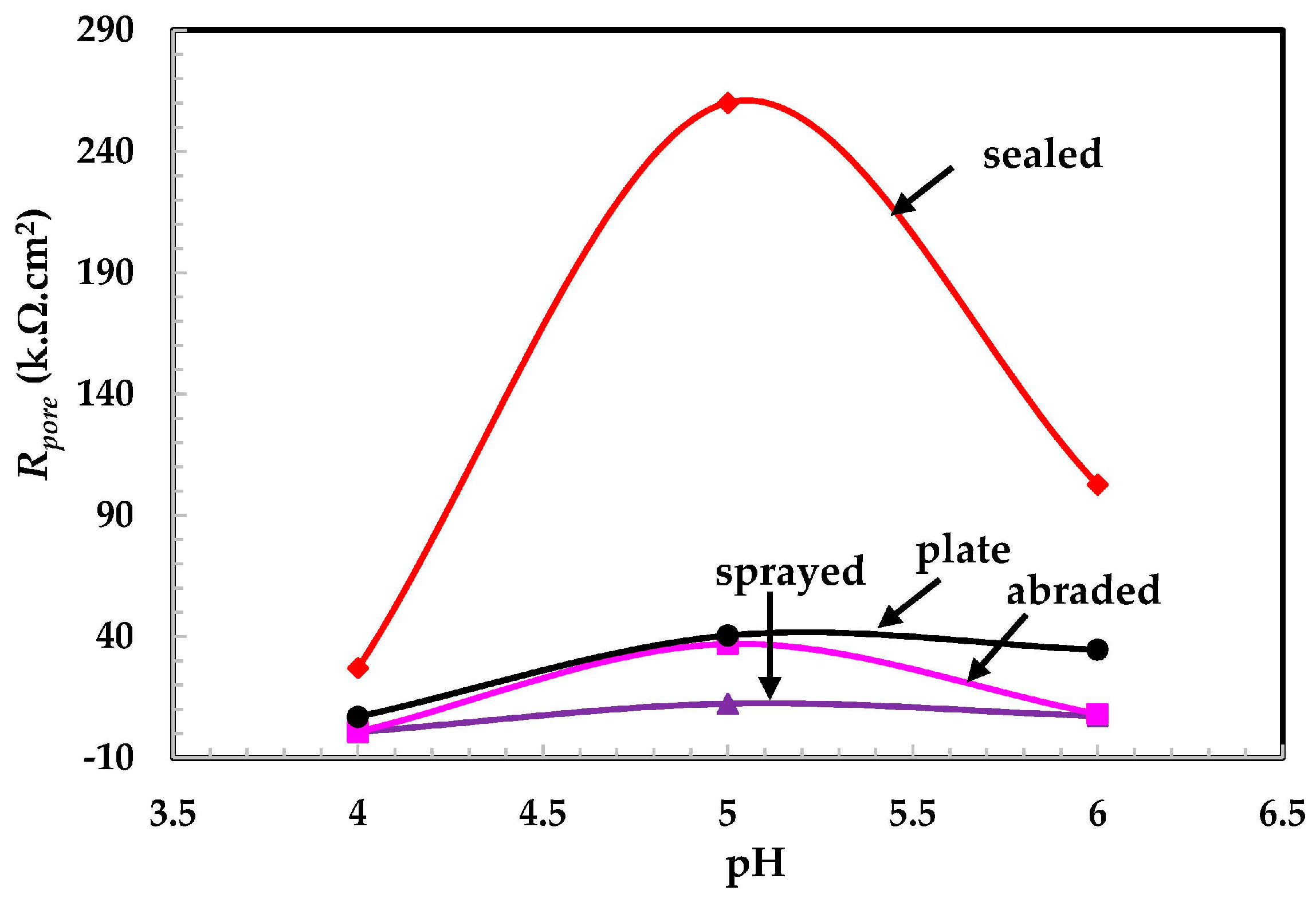
- The alkyl epoxide sealed coating provided the best protection against contamination because it worked as a barrier against the penetration of acidic solutions.
- The scattered impedance plots in the acidic pH solutions tested were due to the formation of capacitance and defective passive films at intermediate frequencies.
- The different values of pH of the aqueous H2SO4 acid solution passivated the 316L stainless steel coating because of the formation of Cr-enriched phases on the top surface.
- The lowest polarization resistance of the coatings in pH 4 was because of the aggressiveness of the solution, which leads to the dissolution of the coating.
- Defects in the surface of the coating made the coating susceptible to corrosion.
- The presence of macro cells in the sprayed and abraded coatings resulted in the formation of pits and galvanic cells, which caused the coating to corrode in the acidic solution.
- In the pH 5 solution, the abraded coating exhibited almost the same Rpore value as the 316L stainless steel plate coating owing to the formation of a stable passive film. This passive film is formed in the presence of un-dissociated water molecules of the aqueous H2SO4 solution.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Giergiczny, Z.; Krol, A. Immobilization of heavy metals (Pb, Cu, Cr, Zn, Cd, Mn) in the mineral additions containing concrete composites. J. Hazard. Mater. 2008, 160, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Lupsea, M.; Tiruta-Barna, L.; Schiopu, N. Leaching of hazardous substances from a composite construction product—An experimental and modelling approach for fibre-cement sheets. J. Hazard. Mater. 2014, 264, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q. Increases of lead and chromium in drinking water from using cement-mortar-lined pipes: Initial modeling and assessment. J. Hazard. Mater. 1997, 56, 181–213. [Google Scholar] [CrossRef]
- Jensen, H.S.; Lens, P.N.L.; Nielsen, J.L.; Bester, K.; Nielsen, A.; Haaning, H.-J.; Thorkild, V.J. Growth kinetics of hydrogen sulfide oxidizing bacteria in corroded concrete from sewers. J. Hazard. Mater. 2011, 189, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Owaki, E.; Okamoto, R.; Nagashio, D. Deterioration of concrete in an advanced water treatment plant. In Concrete under Severe Condition: Environment and Loading; Gjorv, E., Odd, E., Sakai, K., Banthia, N., Eds.; E & FN Spon: London, UK; New York, NY, USA, 1998; p. 438. [Google Scholar]
- Swamy, R.N.; Tanikawa, S. An external surface coating to protect the concrete and steel from aggressive environments. Mater. Struct. 1993, 26, 465–478. [Google Scholar] [CrossRef]
- Vera, R.; Apablaza, J.; Carvajal, A.; Vera, M. Enrique, Effect of Surface Coatings in the Corrosion of Reinforced Concrete in Acid Environments. Int. J. Electrochem. Sci. 2013, 8, 11832–11846. [Google Scholar]
- Crowe, D.; Nixon, R. Corrosion of Stainless Steels in Waste Water Applications. Available online: http://www.hwea.org/wp-content/uploads/2015/07/150204_Corrosion_of_Stainless_Steels_in_Wastewater_Applications.pdf (accessed on 19 January 2016).
- Bhalerao, B.B.; Arceivala, S.J. Application of Corrosion Control Techniques in Municipal Water and Waste Water Engineering. Available online: http://eprints.nmlindia.org/5825/1/129–139.PDF (accessed on 9 January 2016).
- Pawlowski, L. The Science and Engineering of Thermal Spray Coatings, 2nd ed.; John Wiley and Sons Ltd.: West Sussex, UK, 2008. [Google Scholar]
- Jandin, G.; Liao, H.; Feng, Z.Q.; Coddet, C. Correlations between operating conditions, microstructure and mechanical properties of twin wire arc sprayed steel Coatings. Mater. Sci. Eng. A 2003, 349, 298–305. [Google Scholar] [CrossRef]
- Chaliampalias, D.; Vourlias, G.; Pavlidou, E.; Stergioudis, G.; Skolianos, S.; Chrissafis, K. High temperature oxidation and corrosion in marine environments of thermal spray deposited coatings. Appl. Surf. Sci. 2008, 255, 3104–3111. [Google Scholar] [CrossRef]
- Choe, H.-B.; Lee, H.-S.; Shin, J.-H. Experimental study on the electrochemical anti corrosion properties of steel structures applying the arc thermal metal spraying method. Materials 2014, 7, 7722–7736. [Google Scholar] [CrossRef]
- Paredes, R.S.C.; Amico, S.C.; d’Oliveira, A.S.C.M. The effect of roughness and pre-heating of the substrate on the morphology of aluminium coatings deposited by thermal spraying. Surf. Coat. Technol. 2006, 200, 3049–3055. [Google Scholar] [CrossRef]
- Lee, H.S.; Singh, J.K.; Ismail, M.A.; Bhattacharya, C. Corrosion resistance properties of Aluminum coating applied by arc thermal metal spray in SAE J2334 solution with exposure periods. Metals 2016, 6, 1–15. [Google Scholar] [CrossRef]
- Guenbour, A.; Benbachir, A.; Kacemi, A. Evaluation of the corrosion performance of zinc-phosphate-painted carbon steel. Surf. Coat. Technol. 1999, 113, 36–43. [Google Scholar] [CrossRef]
- Cinca, N.; Lima, C.R.C.; Guilemany, J.M. An overview of intermetallics research and application: Status of thermal spray coatings. J. Mater. Res. Technol. 2013, 2, 75–86. [Google Scholar] [CrossRef]
- Bettridge, D.F.; Ubank, R.G. Quality control of high-temperature protective coatings. Mater. Sci. Technol. 1986, 2, 232–242. [Google Scholar] [CrossRef]
- Steffens, H.D.; Babiak, Z.; Wewel, M. Recent developments in arc spraying. IEEE Trans. Plasma Sci. 1990, 18, 974–975. [Google Scholar] [CrossRef]
- Muhamad, H.A.M.; Hayati, S.N.; Kiyai, A.S.; Binti, M.S.N. Critical process and performance parameter of thermal arc spray coatings. Int. J. Mater. Eng. Innov. 2014, 5, 12–27. [Google Scholar]
- Davis, J.R. Surface Engineering for Corrosion and Wear Resistance; ASM International USA: Materials Park, OH, USA, 2001. [Google Scholar]
- Lee, H.S.; Singh, J.K.; Park, J.H. Pore blocking characteristics of corrosion products formed on Aluminum coating produced by arc thermal metal spray process in 3.5 wt % NaCl solution. Constr. Build. Mater. 2016, 113, 905–916. [Google Scholar] [CrossRef]
- Standard Test Method for Pull-Off Strength of Coatings Using Portable Adhesion Testers; ASTM D4541; STM International: West Conshohocken, PA, USA, 2010.
- Maurice, V.; Yang, W.P.; Marcus, P. XPS and STM Study of Passive Films Formed on Fe-22Cr (110) Single-Crystal Surfaces. J. Electrochem. Soc. 1996, 143, 1182–1200. [Google Scholar] [CrossRef]
- Goossens, A.; Macdonald, D.D. A photoelectrochemical impedance spectroscopic study of passive tungsten. J. Electroanal. Chem. 1993, 352, 65–81. [Google Scholar] [CrossRef]
- Macdonald, D.D.; Sikora, E.; Engelhardt, G. Characterizing electrochemical systems in the frequency domain. Electrochim. Acta 1998, 43, 87–107. [Google Scholar] [CrossRef]
- Song, H.; Macdonald, D.D. Photoelectrochemical Impedance Spectroscopy I. Validation of the Transfer Function by Kramers-Kronig Transformation. J. Electrochem. Soc. 1991, 138, 1408–1410. [Google Scholar] [CrossRef]
- Ismail, K.M.; El-Moneim, A.A.; Badawy, W.A. Stability of Sputter-Deposited Amorphous Mn-Ta Alloys in Chloride-Free and Chloride-Containing H2SO4 Solutions. J. Electrochem. Soc. 2001, 148, C81–C87. [Google Scholar] [CrossRef]
- Fattah-alhosseini, A.; Vafaeian, S. Passivation behavior of a ferritic stainless steel in concentrated alkaline solutions. J. Mater. Res. Technol. 2015, 4, 423–428. [Google Scholar] [CrossRef]
- Banas, J.; Mazurkiewicz, B.; Stypula, B. Passivity of metals in concentrated and anhydrous solutions of sulphuric acid. Electrochim. Acta 1992, 37, 1069–1073. [Google Scholar] [CrossRef]
- Yadav, M.; Kumar, S. Experimental, thermodynamic and quantum chemical studies on adsorption and corrosion inhibition performance of synthesized pyridine derivatives on N80 steel in HCl solution. Surf. Interface Anal. 2014, 46, 254–268. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, D.; Yadav, P.; Yadav, M. Experimental and quantum chemical studies on corrosion inhibition effect of synthesized organic on N80 steel in hydrochloric acid. Ind. Eng. Chem. Res. 2013, 52, 14019–14029. [Google Scholar] [CrossRef]
- Boissy, C.; Alemany-Dumont, C.; Normand, B. EIS evaluation of steady-state characteristic of 316L stainless steel passive film grown in acidic solution. Electrochem. Commun. 2013, 26, 10–12. [Google Scholar] [CrossRef]
- Singh, J.K.; Singh, D.D.N. The nature of rusts and corrosion characteristics of low alloy and plain carbon steels in three kinds of concrete pore solution with salinity and different pH. Corros. Sci. 2012, 56, 129–142. [Google Scholar] [CrossRef]
- Singh, V.B.; Ray, M. Effect of H2SO4 addition on the corrosion behaviour of AISI 304 austenitic stainless steel in methanol-HCl solution. Int. J. Electrochem. Sci. 2007, 2, 329–340. [Google Scholar]
- Lai, W.Y.; Zhao, W.Z.; Yin, Z.F.; Zhang, J. EIS and XPS studies on passive film of AISI 304 stainless steel in dilute sulfuric acid solution. Surf. Interface Anal. 2012, 44, 418–425. [Google Scholar] [CrossRef]
- Marcus, P.; Olefjord, I. A round robin on combined electrochemical and AES/ESCA characterization of the passive films on Fe-Cr and Fe-Cr-Mo alloys. Corros. Sci. 1988, 28, 589–602. [Google Scholar] [CrossRef]
- Asami, K.; Hosimoto, K.; Shimodaria, S. An XPS study of the passivity of a series of iron-chromium alloys in sulphuric acid. Corros. Sci. 1978, 18, 151–160. [Google Scholar] [CrossRef]
- Olsson, C.O.A.; Landolt, D. Passive films on stainless steels-chemistry, structure and growth. Electrochem. Acta 2003, 48, 1093–1104. [Google Scholar] [CrossRef]
- Elsener, B.; Rossi, A. Effect of pH on Electrochemical Behaviour and Passive Film Composition of Stainless Steels. Mater. Sci. Forum 1995, 192, 225–236. [Google Scholar] [CrossRef]
- Mischler, S.; Vogel, A.; Mathieu, H.; Landolt, D. The chemical composition of the passive film on Fe-24Cr and Fe-24Cr-11Mo studied by AES, XPS and SIMS. Corros. Sci. 1991, 32, 925–944. [Google Scholar] [CrossRef]
- Fattah-alhosseini, A.; Vafaeian, S. Effect of solution pH on the electrochemical behaviour of AISI304 austenitic and AISI 430 ferritic stainless steels in concentrated acidic media. Egypt. J. Pet. 2015, 24, 333–341. [Google Scholar] [CrossRef]
- Dean, S.W. Electrochemical methods of corrosion testing. In Electrochemical Techniques for Corrosion; Baboian, R., Ed.; NACE: Houston, TX, USA, 1977; pp. 52–60. [Google Scholar]
| Elements (wt %) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| C | Mn | Si | Cr | Ni | Mo | P | S | N | Fe |
| 0.03 | 1.940 | 0.700 | 16.790 | 9.600 | 1.800 | 0.040 | 0.025 | 0.010 | balance |
| Sample Details | Bond Strength (MPa) | Average (MPa) | Standard Deviation (MPa) | |||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||
| Sprayed | 3.57 | 3.56 | 3.29 | 3.14 | 3.39 | 0.21 |
| Abraded | 3.33 | 3.22 | 3.19 | 3.49 | 3.31 | 0.14 |
| Sealed | 4.12 | 4.09 | 4.02 | 3.99 | 4.06 | 0.06 |
| pH | Sample Details | RS | Electrochemical Parameters | W | L | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| CPEc | CPEdl | |||||||||
| kΩ·cm2 | Rpore kΩ·cm2 | Yo1 (1 × 10−4) (Ω·cm−2·s−n) | α1 | Rdl kΩ·cm2 | Yo2 (1 × 10−6) (Ω·cm2·s−0.5) | α2 | (1 × 10−4) | (H·cm2) | ||
| 6 | Sprayed | 1.32 | 1.47 | 7.94 | 0.74 | - | - | - | - | - |
| Abraded | 1.27 | 2.01 | 7.80 | 0.79 | - | - | - | - | - | |
| Sealed | 1.09 | 607.00 | 0.01 | 0.94 | - | - | - | - | - | |
| Plate | 1.02 | 69.42 | 1.45 | 0.83 | - | - | - | - | - | |
| 5 | Sprayed | 1.37 | 1.97 | 1.54 | 0.95 | - | - | - | - | - |
| Abraded | 2.18 | 4.37 | 0.01 | 0.95 | - | - | - | - | - | |
| Sealed | 1.94 | 787.00 | 0.01 | 0.99 | 356.00 | 0.90 | 0.90 | - | - | |
| Plate | 1.25 | 570.00 | 0.11 | 0.98 | 110.00 | 1.32 | 0.89 | 1.10 | - | |
| 4 | Sprayed | 0.35 | 0.37 | 22.69 | 0.70 | - | - | - | - | - |
| Abraded | 0.30 | 0.35 | 28.32 | 0.70 | - | - | - | - | - | |
| Sealed | 0.55 | 563.00 | 0.21 | 0.90 | 173.00 | 1.10 | 0.89 | - | - | |
| Plate | 0.32 | 103.00 | 0.21 | 0.89 | 88.00 | 1.19 | 0.88 | - | 25.63 | |
| pH | Sample Details | Electrochemical Parameters | Corrosion Rate (μm·year−1) | |
|---|---|---|---|---|
| Ecorr (V) Vs Ag/AgCl | Icorr (μA·cm−2) | |||
| (Error, V) | (Error, μA·cm−2) | |||
| 6 | Sprayed | −0.380 (0.011) | 17.56 (0.60) | 200.43 |
| Abraded | −0.366 (0.020) | 8.52 (0.40) | 97.24 | |
| Sealed | −0.336 (0.013) | 0.32 (0.01) | 3.65 | |
| Plate | −0.244 (0.012) | 1.56 (0.07) | 17.80 | |
| 5 | Sprayed | −0.369 (0.019) | 8.27 (0.10) | 94.39 |
| Abraded | −0.156 (0.010) | 1.33 (0.03) | 15.18 | |
| Sealed | −0.278 (0.011) | 0.11 (0.006) | 1.25 | |
| Plate | −0.219 (0.011) | 0.74 (0.02) | 8.44 | |
| 4 | Sprayed | −0.444 (0.020) | 1124.5 (20.50) | 12,834.76 |
| Abraded | −0.413 (0.020) | 504.83 (8.09) | 5762.00 | |
| Sealed | −0.255 (0.015) | 3.31 (0.15) | 37.77 | |
| Plate | −0.369 (0.020) | 11.75 (0.54) | 134.11 | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.-S.; Park, J.-H.; Singh, J.K.; Ismail, M.A. Protection of Reinforced Concrete Structures of Waste Water Treatment Reservoirs with Stainless Steel Coating Using Arc Thermal Spraying Technique in Acidified Water. Materials 2016, 9, 753. https://doi.org/10.3390/ma9090753
Lee H-S, Park J-H, Singh JK, Ismail MA. Protection of Reinforced Concrete Structures of Waste Water Treatment Reservoirs with Stainless Steel Coating Using Arc Thermal Spraying Technique in Acidified Water. Materials. 2016; 9(9):753. https://doi.org/10.3390/ma9090753
Chicago/Turabian StyleLee, Han-Seung, Jin-Ho Park, Jitendra Kumar Singh, and Mohamed A. Ismail. 2016. "Protection of Reinforced Concrete Structures of Waste Water Treatment Reservoirs with Stainless Steel Coating Using Arc Thermal Spraying Technique in Acidified Water" Materials 9, no. 9: 753. https://doi.org/10.3390/ma9090753