Antibacterial Metallic Touch Surfaces
Abstract
:1. Introduction
2. Standards for Antimicrobial Assessment
3. Factors Responsible for Antimicrobial Behaviour
3.1. Adhesion of Bacteria to Antimicrobial Surfaces
3.2. Effect of Humidity on Antimicrobial Performance
3.3. Effect of Geometry, Chemistry and Physical Properties
3.3.1. Bulk Samples
3.3.2. Coatings
3.3.3. Physical Properties of Metallic Coatings
3.4. Oxides
4. Operational Challenges, Cleaning and Disinfection
4.1. Active Substances
- (a)
- Hydrogen peroxide solutions
- (b)
- Chlorine-releasing compounds (hypochlorite)
- (c)
- Alcohols (ethanol) and aldehydes (formaldehyde)
- (d)
- Quaternary ammonium compounds (benzalkonium chloride)
4.1.1. Hydrogen Peroxide
4.1.2. Chlorine-Releasing Compounds
4.1.3. Alcohols and Aldehydes
4.1.4. Quaternary Ammonium Compounds
4.2. Water and Sweat
4.3. The Influence of pH on Corrosion
5. Conclusions and Future Directions
- -
- There is a need for appropriate standardised antimicrobial tests for touch surfaces.
- -
- There is a lack of information about the effect of corrosion products on the antimicrobial behaviour of touch surfaces (there is either information about the effect of direct contact between cleaning products and microbes or the effect of cleaning products on the chemistry change of cleaned surfaces).
- -
- Very few antimicrobial tests have been performed under real conditions with long-term exposure to recreate hospital and other healthcare environments. Differences in humidity and temperature conditions across the globe may result in different outcomes.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- European Centre for Disease Prevention and Control. Point Prevalence Survey of Healthcare Associated Infections and Antimicrobial Use in European Acute Care Hospitals; ECDC: Stockholm, Sweden, 2013. [Google Scholar]
- European Centre for Disease Prevention and Control. Antimicrobial Resistance Surveillance in Europe 2014. Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net); ECDC: Stockholm, Sweden, 2015. [Google Scholar]
- Dollwet, H.H.A.; Sorenson, J.R.J. Historic Uses of Copper-Compounds in Medicine. Trace Elem. Med. 1985, 2, 80–87. [Google Scholar]
- Wilks, S.A.; Michels, H.; Keevil, C.W. The survival of Escherichia coli O157 on a range of metal surfaces. Int. J. Food Microbiol. 2005, 105, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Santo, C.E.; Lam, E.W.; Elowsky, C.G.; Quaranta, D.; Domaille, D.W.; Chang, C.J.; Grass, G. Bacterial Killing by Dry Metallic Copper Surfaces. Appl. Environ. Microbiol. 2011, 77, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Warnes, S.L. Laboratory Studies to Investigate the Efficacy and Mechanism of Action of Copper Alloys to Kill a Range of Bacterial Pathogens and Inactive Norovirus. Ph.D. Thesis, University of Southampton, Faculty for Natural and Environmental Sciences, Southampton, UK, 2014. [Google Scholar]
- Warnes, S.L.; Highmore, C.J.; Keevil, C.W. Horizontal transfer of antibiotic resistance genes on abiotic touch surfaces: Implications for public health. mBio 2012, 3, e00489. [Google Scholar] [CrossRef] [PubMed]
- Warnes, S.L.; Keevil, C.W. Inactivation of norovirus on dry copper alloy surfaces. PLoS ONE 2013, 8, e75017. [Google Scholar] [CrossRef] [PubMed]
- Borkow, G.; Gabbay, J. Copper, An Ancient Remedy Returning to Fight Microbial, Fungal and Viral Infections. Curr. Chem. Biol. 2009, 3, 272–278. [Google Scholar] [CrossRef]
- Grass, G.; Rensing, C.; Solioz, M. Metallic Copper as an Antimicrobial Surface. Appl. Environ. Microbiol. 2011, 77, 1541–1547. [Google Scholar] [CrossRef] [PubMed]
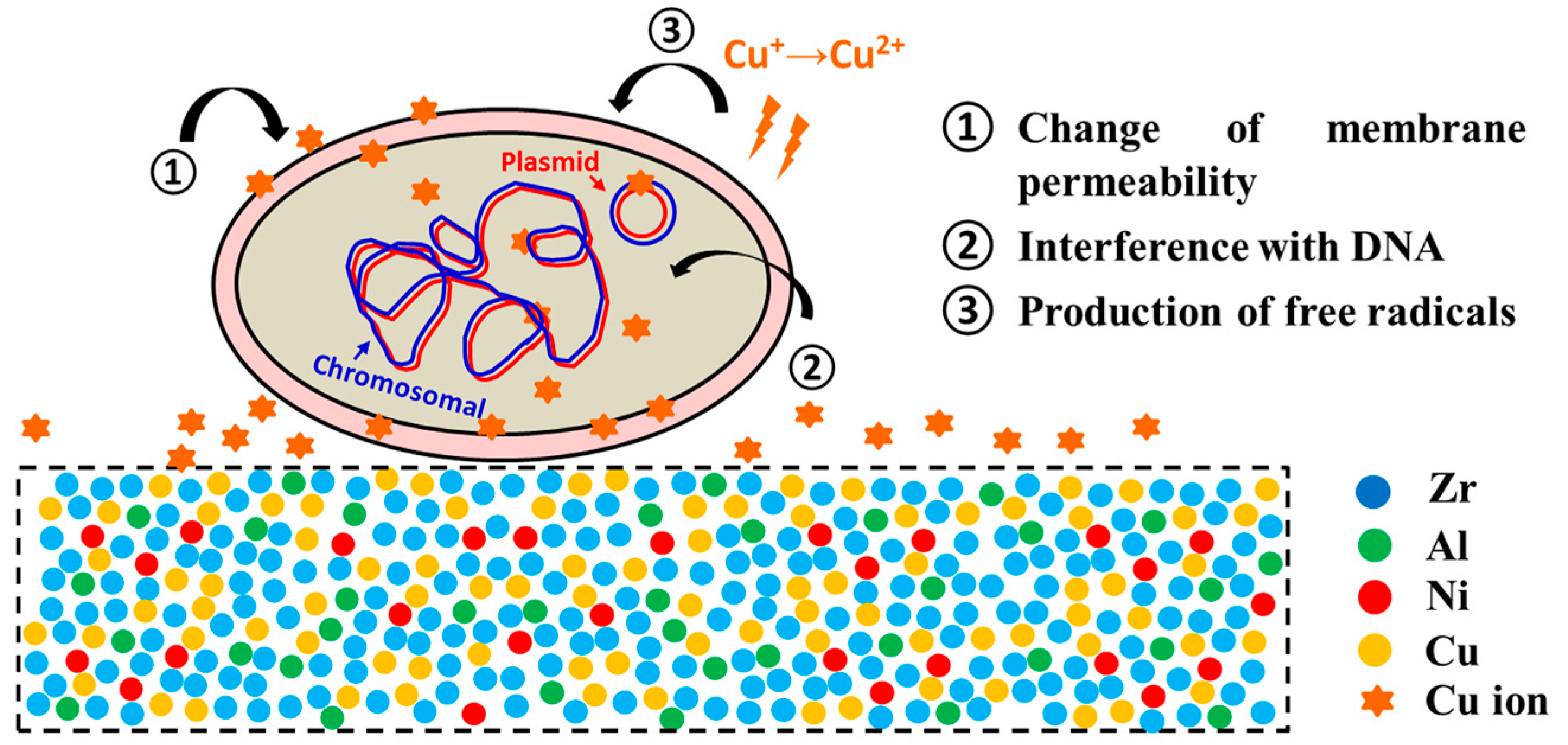
- Huang, L.; Fozo, E.M.; Zhang, T.; Liaw, P.K.; He, W. Antimicrobial behavior of Cu-bearing Zr-based bulk metallic glasses. Mater. Sci. Eng. C Mater. 2014, 39, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Vincent, M.; Hartemann, P.; Engels-Deutsch, M. Antimicrobial applications of copper. Int. J. Hyg. Environ. Health 2016. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.Y.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G., Jr. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef] [PubMed]
- Croxen, M.A.; Finlay, B.B. Molecular mechanisms of Escherichia coli pathogenicity. Nat. Rev. Microbiol. 2010, 8, 26–38. [Google Scholar] [PubMed]
- Driscoll, J.A.; Brody, S.L.; Kollef, M.H. The epidemiology, pathogenesis and treatment of Pseudomonas aeruginosa infections. Drugs 2007, 67, 351–368. [Google Scholar] [CrossRef] [PubMed]
- Protocol for the Evaluation of Bactericidal Activity of Hard, Non-porous Copper Containing Surface Products; US Environmental Protection Agency: Washington, DC, USA, 2016.
- Chu, J.H.; Lee, J.; Chang, C.C.; Chan, Y.C.; Liou, M.L.; Lee, J.W.; Jang, J.S.C.; Duh, J.G. Antimicrobial characteristics in Cu-containing Zr-based thin film metallic glass. Surf. Coat. Technol. 2014, 259, 87–93. [Google Scholar] [CrossRef]
- Sharifahmadian, O.; Salimijazi, H.R.; Fathi, M.H.; Mostaghimi, J.; Pershin, L. Relationship between surface properties and antibacterial behavior of wire arc spray copper coatings. Surf. Coat. Technol. 2013, 233, 74–79. [Google Scholar] [CrossRef]
- Metal-Related Antimicrobials Showcase Event; Durham University: Durham, UK, 2015.
- Katsikogianni, M.G.; Missirlis, Y.F. Concise review of mechanisms of bacterial adhesion to biomaterials and of techniques used in estimating bacteria-material interactions. Eur. Cells Mater. 2004, 8, 37–57. [Google Scholar]
- Krishnan, M.; Seema, S.; Tiwari, B.; Sharma, H.S.; Londhe, S.; Arora, V. Surface characterization of nickel titanium orthodontic arch wires. Med. J. Armed Forces India 2015, 71, S340–S345. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, S.; Abidi, Z.; Wilson, T.G., Jr.; Valderrama, P.; Wadhwani, C.; Palmer, K.; Rodrigues, D.C. In vitro evaluation of the effects of multiple oral factors on dental implants surfaces. J. Oral Implantol. 2016, 42, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Verardi, G.; Cenci, M.S.; Maske, T.T.; Webber, B.; Santos, L.R. Antiseptics and microcosm biofilm formation on titanium surfaces. Braz. Oral Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Scheuerman, T.R.; Camper, A.K.; Hamilton, M.A. Effects of substratum topography on bacterial adhesion. J. Colloid Interf. Sci. 1998, 208, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Edwards, K.J.; Rutenberg, A.D. Microbial response to surface microtopography: The role of metabolism in localized mineral dissolution. Chem. Geol. 2001, 180, 19–32. [Google Scholar] [CrossRef]
- Ojeil, M.; Jermann, C.; Holah, J.; Denyer, S.P.; Maillard, J.Y. Evaluation of new in vitro efficacy test for antimicrobial surface activity reflecting UK hospital conditions. J. Hosp. Infect. 2013, 85, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Casey, A.L.; Adams, D.; Karpanen, T.J.; Lambert, P.A.; Cookson, B.D.; Nightingale, P.; Miruszenko, L.; Shillam, R.; Christian, P.; Elliott, T.S.J. Role of copper in reducing hospital environment contamination. J. Hosp. Infect. 2010, 74, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Karpanen, T.J.; Casey, A.L.; Lambert, P.A.; Cookson, B.D.; Nightingale, P.; Miruszenko, L.; Elliott, T.S.J. The antimicrobial efficacy of copper alloy furnishing in the clinical environment: A crossover study. Infect. Cont. Hosp. Epidemiol. 2012, 33, 3–9. [Google Scholar] [CrossRef] [PubMed]
- O’Gorman, J.; Humphreys, H. Application of copper to prevent and control infection. Where are we now? J. Hosp. Infect. 2012, 81, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Robine, E.; Boulangé-Petermann, L.; Derangère, D. Assessing bactericidal properties of materials: The case of metallic surfaces in contact with air. J. Microbiol. Methods 2002, 49, 225–234. [Google Scholar] [CrossRef]
- Cervantes, H.I.; Alvarez, J.A.; Munoz, J.M.; Arreguin, V.; Mosqueda, J.L.; Macias, A.E. Antimicrobial activity of copper against organisms in aqueous solution: A case for copper-based water pipelines in hospitals? Am. J. Infect. Control 2013, 41, E115–E118. [Google Scholar] [CrossRef] [PubMed]
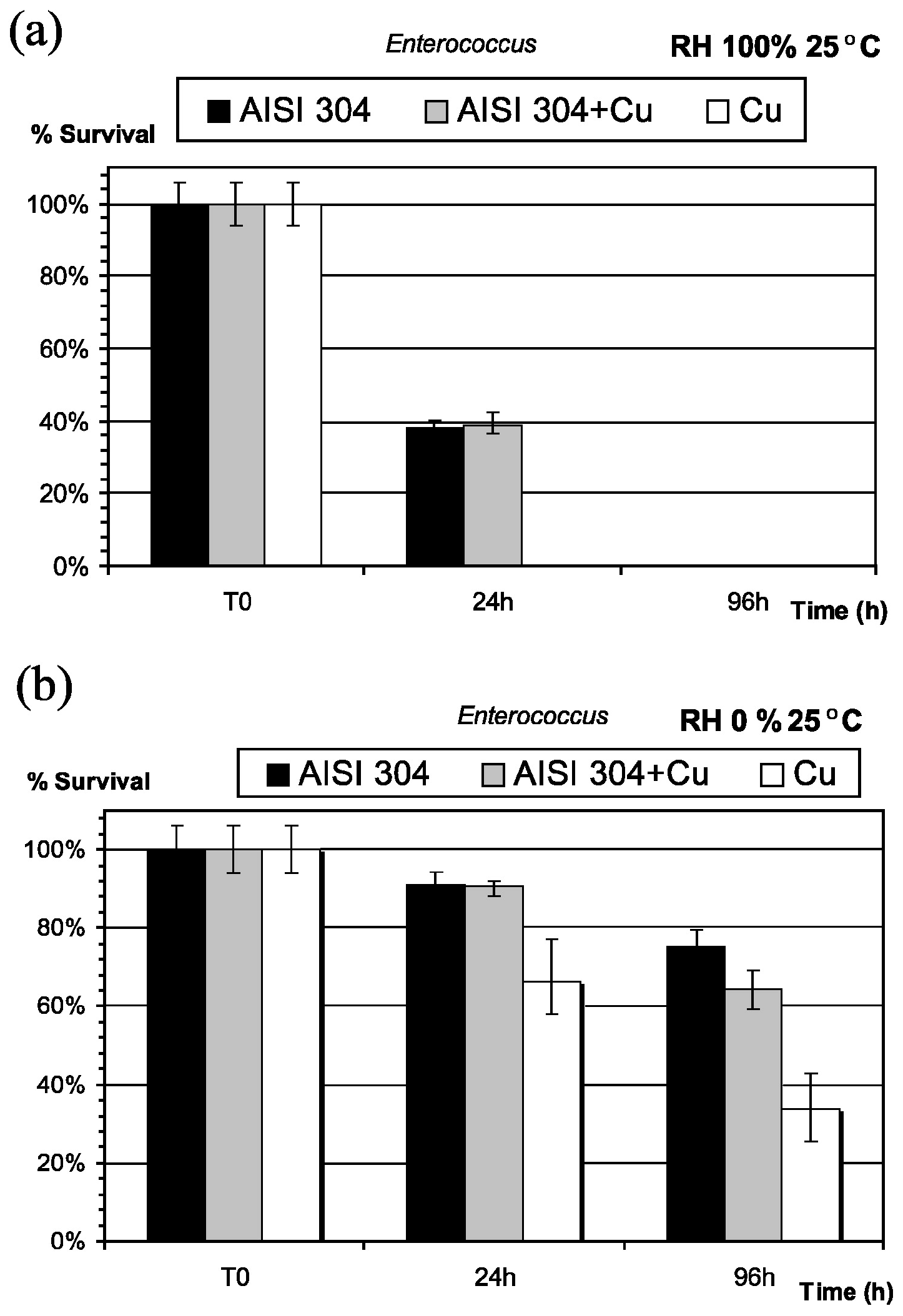
- Michels, H.T.; Noyce, J.O.; Keevil, C.W. Effects of temperature and humidity on the efficacy of methicillin-resistant Staphylococcus aureus challenged antimicrobial materials containing silver and copper. Lett. Appl. Microbiol. 2009. [Google Scholar] [CrossRef] [PubMed]
- Santo, C.S.; Taudte, N.; Nies, D.H.; Grass, G. Contribution of copper ion resistance to survival of Escherichia coli on metallic copper surfaces. Appl. Environ. Microbiol. 2008, 74, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Mathews, S.; Hans, M.; Mucklich, F.; Solioz, M. Contact Killing of Bacteria on Copper Is Suppressed if Bacterial-Metal Contact Is Prevented and Is Induced on Iron by Copper Ions. Appl. Environ. Microbiol. 2013, 79, 2605–2611. [Google Scholar] [CrossRef] [PubMed]
- Molteni, C.; Abicht, H.K.; Solioz, M. Killing of bacteria by copper surfaces involves dissolved copper. Appl. Environ. Microbiol. 2010, 76, 4099–4101. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Mu, R.; Yang, L.F.; Bian, X.F. Antibacterial effect of metallic glasses. Chin. Sci. Bull. 2012, 57, 1069–1072. [Google Scholar] [CrossRef]
- Jin, K.; Loffler, J.F. Bulk metallic glass formation in Zr-Cu-Fe-Al alloys. Appl. Phys. Lett. 2005, 86, 241909. [Google Scholar] [CrossRef]
- Faúndez, G.; Troncoso, M.; Navarrete, P.; Figueroa, G. Antimicrobial activity of copper surfaces against suspensions of Salmonella enterica and Campylobacter jejuni. BMC Microbiol. 2004, 4, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nie, Y.; Kalapos, C.; Nie, X.; Murphy, M.; Hussein, R.; Zhang, J. Superhydrophilicity and antibacterial property of a Cu-dotted oxide coating surface. Ann. Clin. Microbiol. Antimicrob. 2010, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Stranak, V.; Wulff, H.; Ksirova, P.; Zietz, C.; Drache, S.; Cada, M.; Hubicka, Z.; Bader, R.; Tichy, M.; Helm, C.A.; et al. Ionized vapor deposition of antimicrobial Ti-Cu films with controlled copper release. Thin Solid Films 2014, 550, 389–394. [Google Scholar] [CrossRef]
- Stranak, V.; Wulff, H.; Rebl, H.; Zietz, C.; Arndt, K.; Bogdanowicz, R.; Nebe, B.; Bader, R.; Podbielski, A.; Hubicka, Z.; et al. Deposition of thin titanium-copper films with antimicrobial effect by advanced magnetron sputtering methods. Mater. Sci. Eng. C Mater. 2011, 31, 1512–1519. [Google Scholar] [CrossRef]
- Wojcieszak, D.; Kaczmarek, D.; Antosiak, A.; Mazur, M.; Rybak, Z.; Rusak, A.; Osekowska, M.; Poniedzialek, A.; Gamian, A.; Szponar, B. Influence of Cu-Ti thin film surface properties on antimicrobial activity and viability of living cells. Matr. Sci. Eng. C Mater. 2015, 56, 48–56. [Google Scholar] [CrossRef]
- Arnell, R.D.; Kelly, P.J. Recent advances in magnetron sputtering. Surf. Coat. Technol. 1999, 112, 170–176. [Google Scholar] [CrossRef]
- Kelly, P.J.; Arnell, R.D. Magnetron sputtering: A review of recent developments and applications. Vacuum 2000, 56, 159–172. [Google Scholar] [CrossRef]
- Chen, H.W.; Hsu, K.C.; Chan, Y.C.; Duh, J.G.; Lee, J.W.; Jang, J.S.C.; Chen, G.J. Antimicrobial properties of Zr-Cu-Al-Ag thin film metallic glass. Thin Solid Films 2014, 561, 98–101. [Google Scholar] [CrossRef]
- Subramanian, B. In vitro corrosion and biocompatibility screening of sputtered Ti40Cu36Pd14Zr10 thin film metallic glasses on steels. Mater. Sci. Eng. C Mater. 2015, 47, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.B.; Elguindi, J.; Rensing, C.; Ravishankar, S. Antimicrobial activity of different copper alloy surfaces against copper resistant and sensitive Salmonella enterica. Food Microbiol. 2012, 30, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.Y.; Lin, Y.S.; Chang, C.M.; Liu, J.K.; Chen, C.H.; Huang, J.C. Promising antimicrobial capability of thin film metallic glasses. Mater. Sci. Eng. C Mater. 2014, 36, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Ferreri, I.; Calderon, V.S.; Galindo, R.E.; Palacio, C.; Henriques, M.; Piedade, A.P.; Carvalho, S. Silver activation on thin films of Ag-ZrCN coatings for antimicrobial activity. Mater. Sci. Eng. C Mater. 2015, 55, 547–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rebelo, R.; Manninen, N.K.; Fialho, L.; Henriques, M.; Carvalho, S. Morphology and oxygen incorporation effect on antimicrobial activity of silver thin films. Appl. Surf. Sci. 2016, 371, 1–8. [Google Scholar] [CrossRef]
- Wojcieszak, D.; Mazur, M.; Kaczmarek, D.; Mazur, P.; Szponar, B.; Domaradzki, J.; Kepinski, L. Influence of the surface properties on bactericidal and fungicidal activity of magnetron sputtered Ti-Ag and Nb-Ag thin films. Mater. Sci. Eng. C Mater. 2016, 62, 86–95. [Google Scholar] [CrossRef] [PubMed]
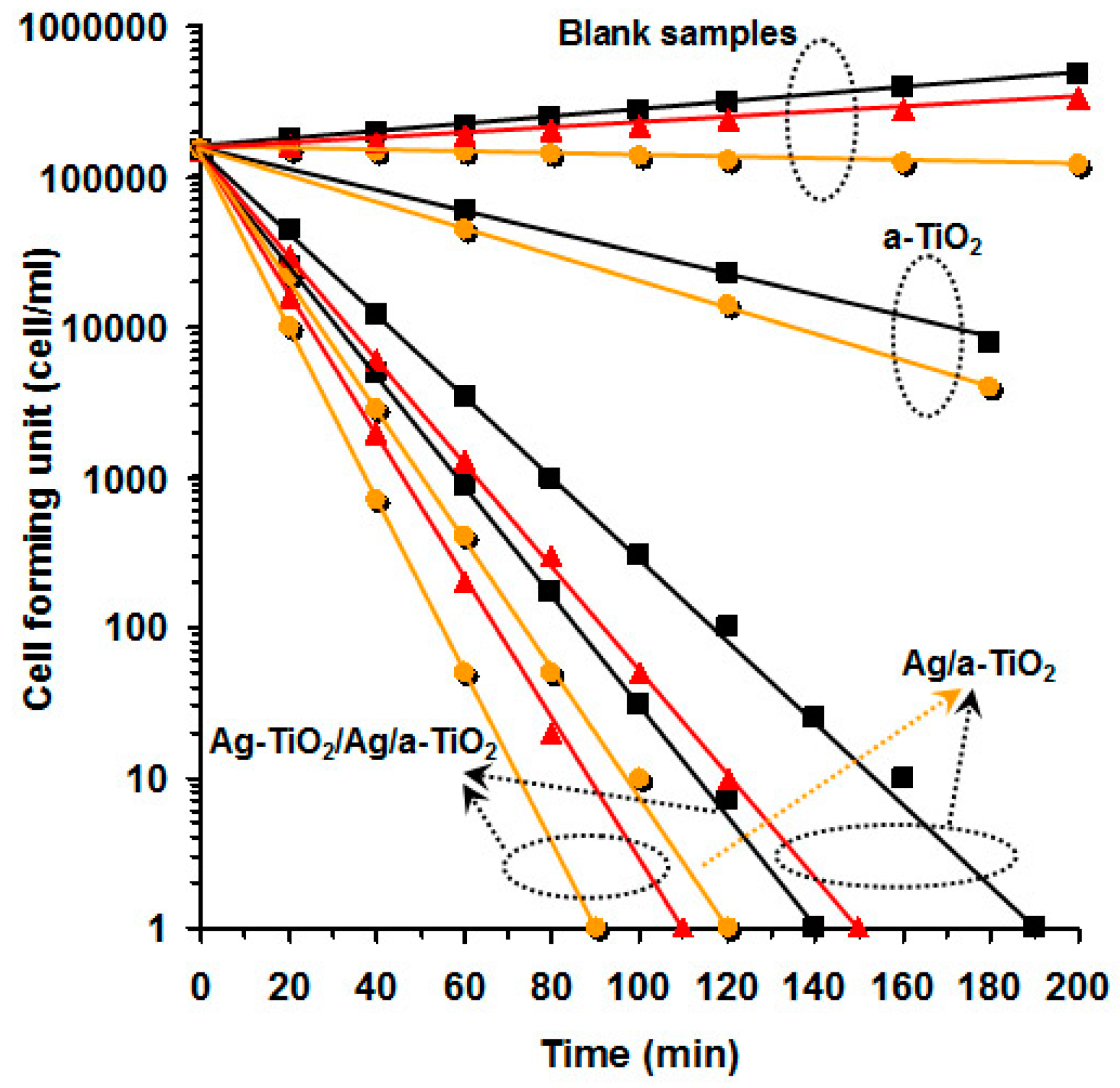
- Akhavan, O. Lasting antibacterial activities of Ag-TiO2/Ag/a-TiO2 nanocomposite thin film photocatalysts under solar light irradiation. J. Colloid Interf. Sci. 2009, 336, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Foster, H.A.; Sheel, D.W.; Sheel, P.; Evans, P.; Varghese, S.; Rutschke, N.; Yates, H.M. Antimicrobial activity of titania/silver and titania/copper films prepared by CVD. J. Photochem. Photobiol. A 2010, 216, 283–289. [Google Scholar] [CrossRef]
- Page, K.; Palgrave, R.G.; Parkin, I.P.; Wilson, M.; Savin, S.L.P.; Chadwick, A.V. Titania and silver-titania composite films on glass-potent antimicrobial coatings. J. Mater. Chem. 2007, 17, 95–104. [Google Scholar] [CrossRef]
- Yu, B.Y.; Leung, K.M.; Guo, Q.Q.; Lau, W.M.; Yang, J. Synthesis of Ag-TiO2 composite nano thin film for antimicrobial application. Nanotechnology 2011, 22, 115603. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, O.; Ghaderi, E. Bactericidal effects of Ag nanoparticles immobilized on surface of SiO2 thin film with high concentration. Curr. Appl. Phys. 2009, 9, 1381–1385. [Google Scholar] [CrossRef]
- Hans, M.; Erbe, A.; Mathews, S.; Chen, Y.; Solioz, M.; Mucklich, F. Role of Copper Oxides in Contact Killing of Bacteria. Langmuir 2013, 29, 16160–16166. [Google Scholar] [CrossRef] [PubMed]
- Hans, M.; Mathews, S.; Mucklich, F.; Solioz, M. Physicochemical properties of copper important for its antibacterial activity and development of a unified model. Biointerphases 2016, 11, 018902. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, P.S.M.; Sheeran, C.P.; Byrne, J.A.; McMahon, M.A.S.; Boyle, M.A.; McGuigan, K.G. Inactivation of clinically relevant pathogens by photocatalytic coatings. J. Photochem. Photobiol. A 2010, 216, 303–310. [Google Scholar] [CrossRef]
- Evans, P.; Sheel, D.W. Photoactive and antibacterial TiO2 thin films on stainless steel. Surf. Coat. Technol. 2007, 201, 9319–9324. [Google Scholar] [CrossRef]
- Yates, H.M.; Brook, L.A.; Ditta, I.B.; Evans, P.; Foster, H.A.; Sheel, D.W.; Steele, A. Photo-induced self-cleaning and biocidal behaviour of titania and copper oxide multilayers. J. Photochem. Photobiol. A 2008, 197, 197–205. [Google Scholar] [CrossRef]
- Reddy, M.P.; Venugopal, A.; Subrahmanyam, M. Hydroxyapatite-supported Ag-TiO2 as Escherichia coli disinfection photocatalyst. Water Res. 2007, 41, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Dancer, S.J. The role of environmental cleaning in the control of hospital-acquired infection. J. Hosp. Infect. 2009, 73, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Dancer, S.J. Hospital cleaning in the 21st century. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Dancer, S.J. How do we assess hospital cleaning? A proposal for microbiological standards for surface hygiene in hospitals. J. Hosp. Infect. 2004, 56, 10–15. [Google Scholar] [PubMed]
- Gillespie, E.; Wright, P.L.; Snook, K.; Ryan, S.; Vandergraaf, S.; Abernethy, M.; Lovegrove, A. The role of ultraviolet marker assessments in demonstrating cleaning efficacy. Am. J. Infect. Control 2015, 43, 1347–1349. [Google Scholar] [CrossRef] [PubMed]
- Griffith, C.J.; Cooper, R.A.; Gilmore, J.; Davies, C.; Lewis, M. An evaluation of hospital cleaning regimes and standards. J. Hosp. Infect. 2000, 45, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Malik, R.E.; Cooper, R.A.; Griffith, C.J. Use of audit tools to evaluate the efficacy of cleaning systems in hospitals. Am. J. Infect. Control 2003, 31, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.L.; Macbeth, D.A.; Derrington, P.; Gerrard, J.; Faloon, J.; Kenway, K.; Lavender, S.; Leonard, S.; Orr, A.; Tobin, D.; et al. An assessment of high touch object cleaning thoroughness using a fluorescent marker in two Australian hospitals. Healthc. Infect. 2012, 16, 156–163. [Google Scholar] [CrossRef]
- Airey, P.; Verran, J. Potential use of copper as a hygienic surface; problems associated with cumulative soiling and cleaning. J. Hosp. Infect. 2007, 67, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Copper Development Association. Available online: http://copperalliance.org.uk (accessed on 1 July 2016).
- Lalitha, A.; Ramesh, S.; Rajeswari, S. Surface protection of copper in acid medium by azoles and surfactants. Electrochim. Acta 2005, 51, 47–55. [Google Scholar] [CrossRef]
- Rutala, W.A.; Weber, D.J. Uses of inorganic hypochlorite (bleach) in health-care facilities. Clin. Microbiol. Rev. 1997, 10, 597–610. [Google Scholar] [PubMed]
- Lavorgna, M.; Russo, C.; D’Abrosca, B.; Parrella, A.; Isidori, M. Toxicity and genotoxicity of the quaternary ammonium compound benzalkonium chloride (BAC) using Daphnia magna and Ceriodaphnia dubia as model systems. Environ. Pollut. 2016, 210, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Wolkoff, P.; Schneider, T.; Kildeso, J.; Degerth, R.; Jaroszewski, M.; Schunk, H. Risk in cleaning: Chemical and physical exposure. Sci. Total Environ. 1998, 215, 135–156. [Google Scholar] [CrossRef]
- Cadnum, J.L.; Mana, T.S.C.; Jencson, A.; Thota, P.; Kundrapu, S.; Donskey, C.J. Effectiveness of a hydrogen peroxide spray for decontamination of soft surfaces in hospitals. Am. J. Infect. Control 2015, 43, 1357–1359. [Google Scholar] [CrossRef] [PubMed]
- Flores, M.J.; Brandi, R.J.; Cassano, A.E.; Labas, M.D. Chemical disinfection with H2O2—The proposal of a reaction kinetic model. Chem. Eng. J. 2012, 198, 388–396. [Google Scholar] [CrossRef]
- Labas, M.D.; Zalazar, C.S.; Brandi, R.J.; Cassano, A.E. Reaction kinetics of bacteria disinfection employing hydrogen peroxide. Biochem. Eng. J. 2008, 38, 78–87. [Google Scholar] [CrossRef]
- Bartels, M.D.; Kristoffersen, K.; Slotsbjerg, T.; Rohde, S.M.; Lundgren, B.; Westh, H. Environmental meticillin-resistant Staphylococcus aureus (MRSA) disinfection using dry-mist-generated hydrogen peroxide. J. Hosp. Infect. 2008, 70, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Piskin, N.; Celebi, G.; Kulah, C.; Mengeloglu, Z.; Yumusak, M. Activity of a dry mist-generated hydrogen peroxide disinfection system against methicillin-resistant Staphylococcus aureus and Acinetobacter baumannii. Am. J. Infect. Control 2011, 39, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Andersen, B.M.; Rasch, M.; Hochlin, K.; Jensen, F.H.; Wismar, P.; Fredriksen, J.E. Decontamination of rooms, medical equipment and ambulances using an aerosol of hydrogen peroxide disinfectant. J. Hosp. Infect. 2006, 62, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.Y.; Gent, P.; Kumar, V. Efficacy, efficiency and safety aspects of hydrogen peroxide vapour and aerosolized hydrogen peroxide room disinfection systems. J. Hosp. Infect. 2012, 80, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.L.; Milligan, T.W.; Joyner, R.E.; Jefferson, M.M. Antibacterial activity of hydrogen-peroxide and the lactoperoxidase-hydrogen peroxide-thiocyanate system against oral streptococci. Infect. Immun. 1994, 62, 529–535. [Google Scholar] [PubMed]
- McDonnell, G. The Use of Hydrogen Peroxide for Disinfection and Sterilization Applications. In PATAI’S Chemistry of Functional Groups; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2009. [Google Scholar]
- DeNardis, D.; Rosales-Yeomans, D.; Borucki, L.; Philipossian, A. Characterization of copper-hydrogen peroxide film growth kinetics. Thin Solid Films 2006, 513, 311–318. [Google Scholar] [CrossRef]
- Barillo, D.J.; Marx, D.E. Silver in medicine: A brief history BC 335 to present. Burns 2014, 40, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.Y.; et al. Antimicrobial effects of silver nanoparticles. Nanomedicine 2007, 3, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Armon, R.; Laot, N.; Lev, O.; Shuval, H.; Fattal, B. Controlling biofilm formation by hydrogen peroxide and silver combined disinfectant. Water Sci. Technol. 2000, 42, 187–192. [Google Scholar]
- Pasquet, J.; Chevalier, Y.; Pelletier, J.; Couval, E.; Bouvier, D.; Bolzinger, M.-A. The contribution of zinc ions to the antimicrobial activity of zinc oxide. Colloids Surf. A Physicochem. Eng. Asp. 2014, 457, 263–274. [Google Scholar] [CrossRef]
- Ercan, D.; Cossu, A.; Nitin, N.; Tikekar, R.V. Synergistic interaction of ultraviolet light and zinc oxide photosensitizer for enhanced microbial inactivation in simulated wash-water. Innov. Food Sci. Emerg. Technol. 2016, 33, 240–250. [Google Scholar] [CrossRef]
- Wöll, C. The chemistry and physics of zinc oxide surfaces. Prog. Surf. Sci. 2007, 82, 55–120. [Google Scholar] [CrossRef]
- Sawai, J.; Shoji, S.; Igarashi, H.; Hashimoto, A.; Kokugan, T.; Shimizu, M.; Kojima, H. Hydrogen peroxide as an antibacterial factor in zinc oxide powder slurry. J. Ferment. Bioeng. 1998, 86, 521–522. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Liu, X.; Zhao, L.; Ding, Y.; Povey, M.; Cang, D. The properties of ZnO nanofluids and the role of H2O2 in the disinfection activity against Escherichia coli. Water Res. 2013, 47, 4013–4021. [Google Scholar] [CrossRef] [PubMed]
- Roberge, P.R. Engineering Materials: Selection and Design Considerations. In Handbook of Corrosion Engineering, Second Edition; McGraw Hill Professional, Access Engineering: New York, NY, USA, 2012. [Google Scholar]
- Montes, J.C.; Hamdani, F.; Creus, J.; Touzain, S.; Correc, O. Impact of chlorinated disinfection on copper corrosion in hot water systems. Appl. Surf. Sci. 2014, 314, 686–696. [Google Scholar] [CrossRef]
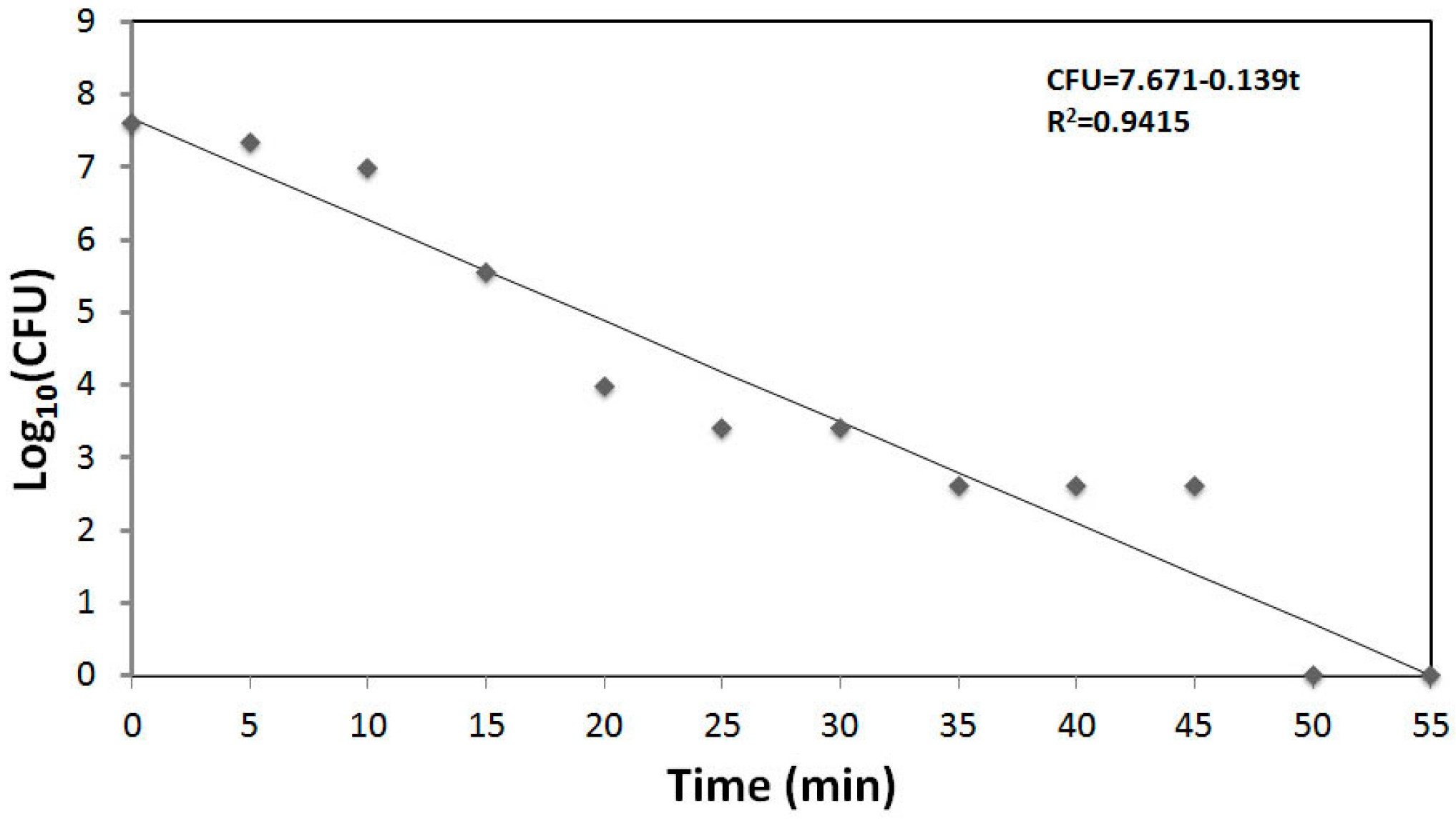
- Sierra, M.; Sanhueza, A.; Alcántara, R.; Sánchez, G. Antimicrobial evaluation of copper sulfate (II) on strains of Enterococcus faecalis. In vitro study. J. Oral Res. 2013, 2, 114–118. [Google Scholar] [CrossRef]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Sierra, J.F.; Ruiz, F.; Cruz Pena, D.C.; Martínez-Gutiérrez, F.; Martínez, A.E.; de Jesús Pozos Guillén, A.; Tapia-Pérez, H.; Martínez Castañón, G. The antimicrobial sensitivity of Streptococcus mutans to nanoparticles of silver, zinc oxide, and gold. Nanomedicine 2008, 4, 237–240. [Google Scholar] [CrossRef]
- Hsu, M.S.; Wu, M.Y.; Huang, Y.T.; Liao, C.H. Efficacy of chlorine dioxide disinfection to non-fermentative Gram-negative bacilli and non-tuberculous mycobacteria in a hospital water system. J. Hosp. Infect. 2016, 93, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Cooper, I.R.; Hanlon, G.W. Resistance of Legionella pneumophila serotype 1 biofilms to chlorine-based disinfection. J. Hosp. Infect. 2010, 74, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Pitts, B.; Stewart, P.S.; Camper, A.; Yoon, J. Comparison of the Antimicrobial Effects of Chlorine, Silver Ion, and Tobramycin on Biofilm. Antimicrob. Agents Chemother. 2008, 52, 1446–1453. [Google Scholar] [CrossRef] [PubMed]
- Landeen, L.K.; Yahya, M.T.; Gerba, C.P. Efficacy of copper and silver ions and reduced levels of free chlorine in inactivation of Legionella pneumophila. Appl. Environ. Microbiol. 1989, 55, 3045–3050. [Google Scholar] [PubMed]
- McDonnell, G.; Russell, A.D. Antiseptics and disinfectants: Activity, action, and resistance. Clin. Microbiol. Rev. 1999, 12, 147–179. [Google Scholar] [PubMed]
- Kampf, G.; Rudolf, M.; Labadie, J.C.; Barrett, S.P. Spectrum of antimicrobial activity and user acceptability of the hand disinfectant agent Sterillium Gel. J. Hosp. Infect. 2002, 52, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Brady, M.J.; Lisay, C.M.; Yurkovetskiy, A.V.; Sawan, S.P. Persistent silver disinfectant for the environmental control of pathogenic bacteria. Am. J. Infect. Control 2003, 31, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Guthery, E.; Seal, L.A.; Anderson, E.L. Zinc pyrithione in alcohol-based products for skin antisepsis: Persistence of antimicrobial effects. Am. J. Infect. Control 2005, 33, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Seal, L.A.; Rizer, R.L.; Maas-Irslinger, R. A unique water optional health care personnel handwash provides antimicrobial persistence and residual effects while decreasing the need for additional products. Am. J. Infect. Control 2005, 33, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.J.; Wren, M.W.; Jeanes, A.; Gant, V.A. A comparison of the antibacterial efficacy and cytotoxicity to cultured human skin cells of 7 commercial hand rubs and Xgel, a new copper-based biocidal hand rub. Am. J. Infect. Control 2009, 37, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Zaragoza Dörwald, F. Quaternary Ammonium Salts. In Lead Optimization for Medicinal Chemists; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012; pp. 118–119. [Google Scholar]
- Nakagawa, Y.; Hayashi, H.; Tawaratani, T.; Kourai, H.; Horie, T.; Shibasaki, I. Disinfection of water with quaternary ammonium salts insolubilized on a porous glass surface. Appl. Environ. Microbiol. 1984, 47, 513–518. [Google Scholar] [PubMed]
- Shirai, A.; Aihara, M.; Takahashi, A.; Maseda, H.; Omasa, T. Synergistic antimicrobial activity based on the combined use of a gemini-quaternary ammonium compound and ultraviolet-A light. J. Photochem. Photobiol. B Biol. 2014, 130, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Francavilla, C.; Low, E.; Nair, S.; Kim, B.; Shiau, T.P.; Debabov, D.; Celeri, C.; Alvarez, N.; Houchin, A.; Xu, P.; et al. Quaternary ammonium N,N-dichloroamines as topical, antimicrobial agents. Bioorganic Med. Chem. Lett. 2009, 19, 2731–2734. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, C.J.; Hanlon, G.W.; Denyer, S.P. Action of Disinfectant Quaternary Ammonium Compounds against Staphylococcus aureus. Antimicrob. Agents Chemother. 2007, 51, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Makvandi, P.; Ghaemy, M.; Mohseni, M. Synthesis and characterization of photo-curable bis-quaternary ammonium dimethacrylate with antimicrobial activity for dental restoration materials. Eur. Polym. J. 2016, 74, 81–90. [Google Scholar] [CrossRef]
- Harrison, J.J.; Turner, R.J.; Joo, D.A.; Stan, M.A.; Chan, C.S.; Allan, N.D.; Vrionis, H.A.; Olson, M.E.; Ceri, H. Copper and Quaternary Ammonium Cations Exert Synergistic Bactericidal and Antibiofilm Activity against Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2008, 52, 2870–2881. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, M.A.; Nazeer, A.A.; Shalabi, K. Electrochemical studies on the inhibition behavior of copper corrosion in pickling acid using quaternary ammonium salts. J. Mol. Liq. 2015, 209, 419–427. [Google Scholar] [CrossRef]
- Collins, K.J. The corrosion of metal by palmar sweat. Br. J. Ind. Med. 1957, 14, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Bond, J.W. Visualization of latent fingerprint corrosion of metallic surfaces. J. Forensic Sci. 2008, 53, 812–822. [Google Scholar] [CrossRef] [PubMed]
- Fredj, N.; Kolar, J.S.; Prichard, D.M.; Burleigh, T.D. Study of relative color stability and corrosion resistance of commercial copper alloys exposed to hand contact and synthetic hand sweat. Corros. Sci. 2013, 76, 415–423. [Google Scholar] [CrossRef]
- Tu, M.-E.; Wu, Y.-H. Multiple allergies to metal alloys. Dermatol. Sin. 2011, 29, 41–43. [Google Scholar] [CrossRef]
- Goodwin, F.E. Corrosion of Zinc and its Alloys. In Shreir’s Corrosion; Cottis, B., Graham, M., Lindsay, R., Lyon, S., Richardson, T., Scantlebury, D., Stott, H., Eds.; Elsevier: Oxford, UK, 2010; Volume 3, pp. 2078–2093. [Google Scholar]
- Lyon, S.B. Corrosion of Noble Metals. In Shreir’s Corrosion; Cottis, B., Graham, M., Lindsay, R., Lyon, S., Richardson, T., Scantlebury, D., Stott, H., Eds.; Elsevier: Oxford, UK, 2010; Volume 3, pp. 2205–2223. [Google Scholar]
- Tuck, C.D.S.; Powell, C.A.; Nuttall, J. Corrosion of Copper and its Alloys. In Shreir’s Corrosion; Cottis, B., Graham, M., Lindsay, R., Lyon, S., Richardson, T., Scantlebury, D., Stott, H., Eds.; Elsevier: Oxford, UK, 2010; Volume 3, pp. 1937–1973. [Google Scholar]
- Daniels, S.L.; Sprunger, P.T.; Kizilkaya, O.; Lytle, D.A.; Garno, J.C. Nanoscale surface characterization of aqueous copper corrosion: Effects of immersion interval and orthophosphate concentration. Appl. Surf. Sci. 2013, 285, 823–831. [Google Scholar] [CrossRef]
- Mann, E.E.; Manna, D.; Mettetal, M.R.; May, R.M.; Dannemiller, E.M.; Chung, K.K.; Brennan, A.B.; Reddy, S.T. Surface micropattern limits bacterial contamination. Antimicrob. Resist. Infect. Control 2014, 3, 28. [Google Scholar] [CrossRef] [PubMed]


| Standard | Name | Country of Application | Pathogens Analysed | Antibacterial Benchmark |
|---|---|---|---|---|
| JIS Z2801:2010 | Antibacterial products—Test for antibacterial activity and efficacy. | Japan | S. aureus, E. coli | No |
| ISO 22196:2011 | Measurement of antibacterial activity on plastics and other non-porous surfaces. | Europe | S. aureus, E. coli | No |
| US EPA 1 | Protocol for the Evaluation of Bactericidal Activity of Hard, Non-Porous Copper Containing Surface Products | USA | S. aureus, P. aeruginosa | Yes 2 |
| Component | Concentration (w/w %) | Examples of Compounds |
|---|---|---|
| Disinfectant (active substance) | 0.1–10 | Benzalkonium chloride, Sodium hypochlorite |
| Surfactant | 0.1–10 | Benzenesulphonic acid, dodecyl-, sodium salt |
| Base | 0.1–25 | Sodium hydroxide, Potassium triphosphate |
| Complexing agent | 5.0–30 | Pentasodium triphosphate, EDTA |
| Corrosion inhibitor | 1.0–10 | Disodium metasilicate |
| Solvent | 0.1–10 | 2-Propanol |
| Perfume | 0.002–1 | Citrus oils, eucalyptus oil |
| Pigment | 0.01–2 | |
| Acid | 0.1–35 | Phosphoric acid, Citric acid |
| Diluent | 15.0–95 | Water |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villapún, V.M.; Dover, L.G.; Cross, A.; González, S. Antibacterial Metallic Touch Surfaces. Materials 2016, 9, 736. https://doi.org/10.3390/ma9090736
Villapún VM, Dover LG, Cross A, González S. Antibacterial Metallic Touch Surfaces. Materials. 2016; 9(9):736. https://doi.org/10.3390/ma9090736
Chicago/Turabian StyleVillapún, Victor M., Lynn G. Dover, Andrew Cross, and Sergio González. 2016. "Antibacterial Metallic Touch Surfaces" Materials 9, no. 9: 736. https://doi.org/10.3390/ma9090736





